Effects of Concentration and Type of Lipids on the Droplet Size, Encapsulation, Colour and Viscosity in the Oil-in-Water Emulsions Stabilised by Rapeseed Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients
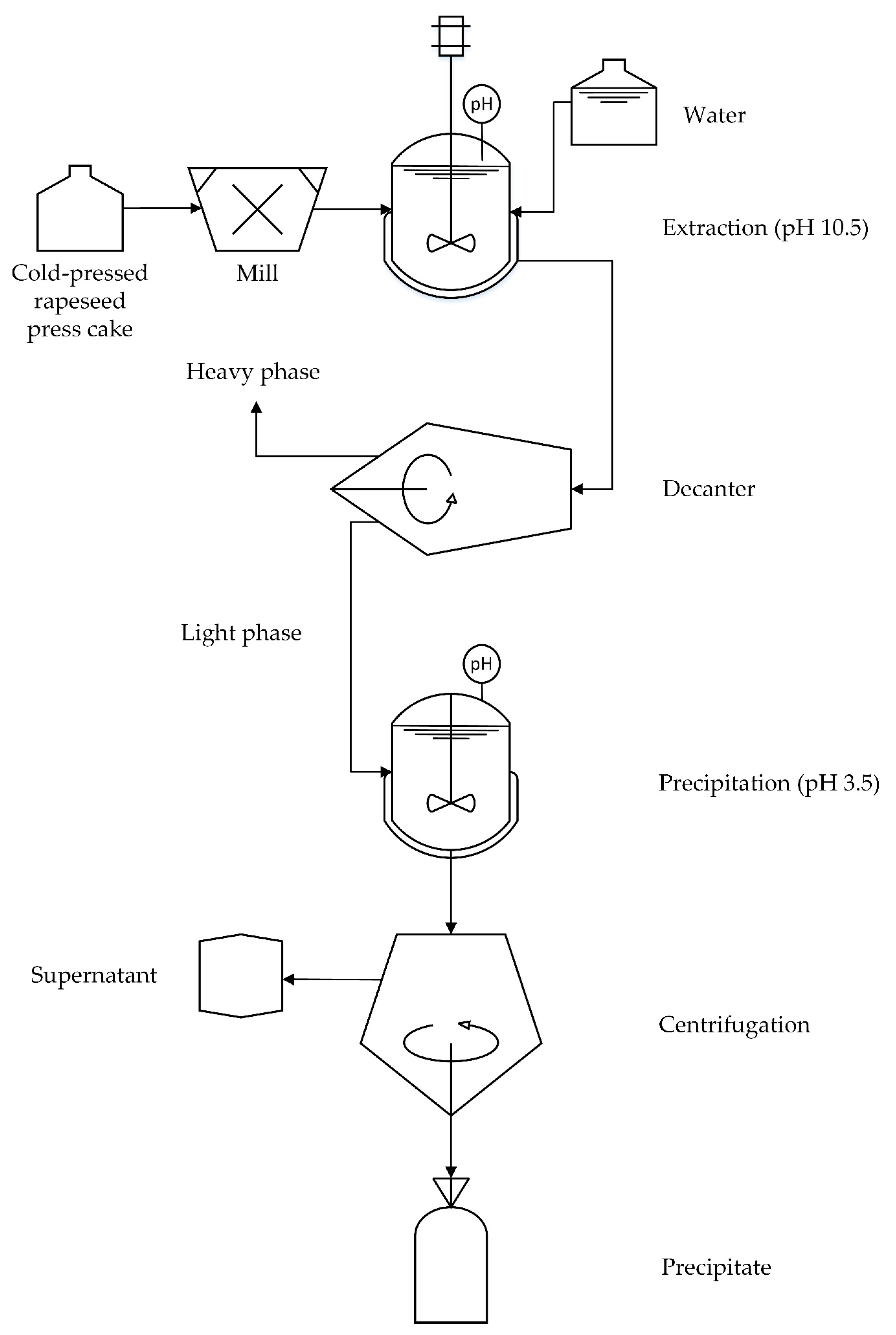
2.2. Preparation of Rapeseed Protein Concentrate
2.3. Proximate Analysis of the Rapeseed Protein Concentrate
2.4. Preparation of Emulsions
2.5. Characterisation
2.5.1. pH Measurements
2.5.2. Droplet Size
2.5.3. Microstructure
2.5.4. Rheology
2.5.5. Colour Coordinates
2.5.6. Centrifugal Encapsulation
2.5.7. FTIR Analysis
3. Results and Discussion
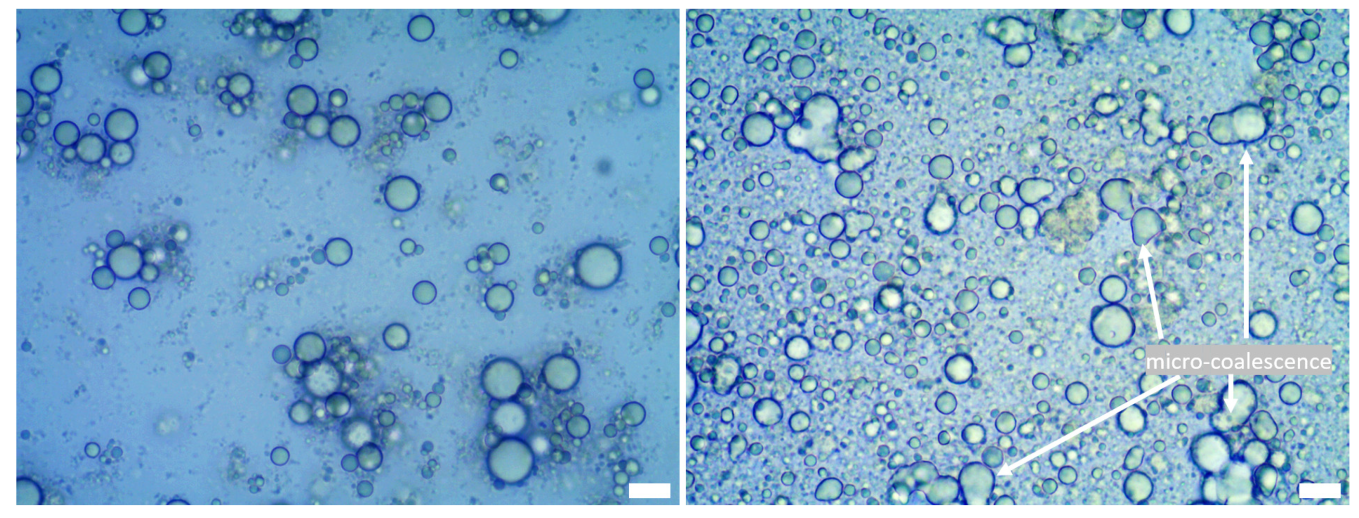
3.1. Microstructure and Encapsulation of Emulsions
3.2. Rheology and Encapsulation
3.3. Colour Characterisation
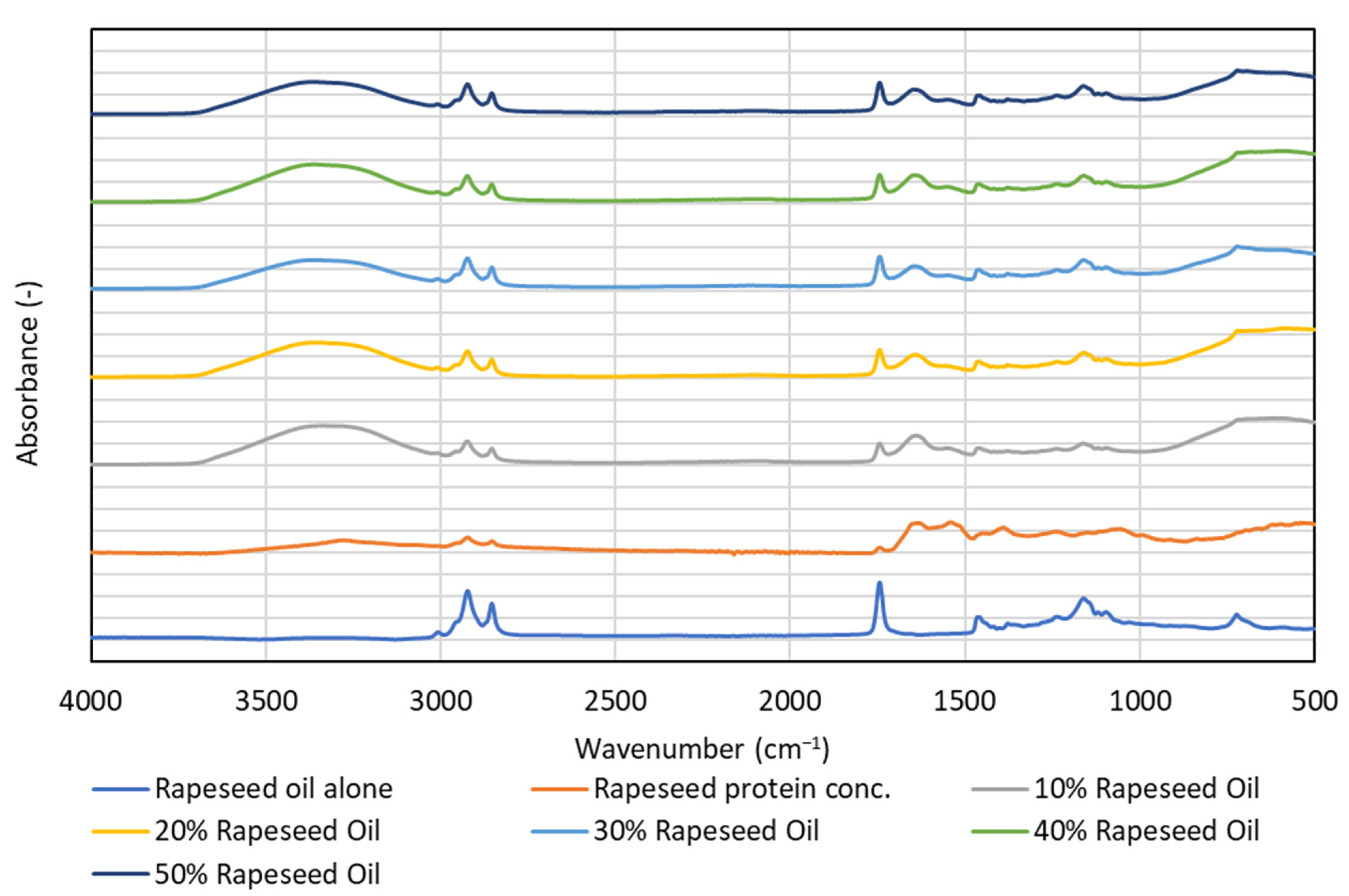
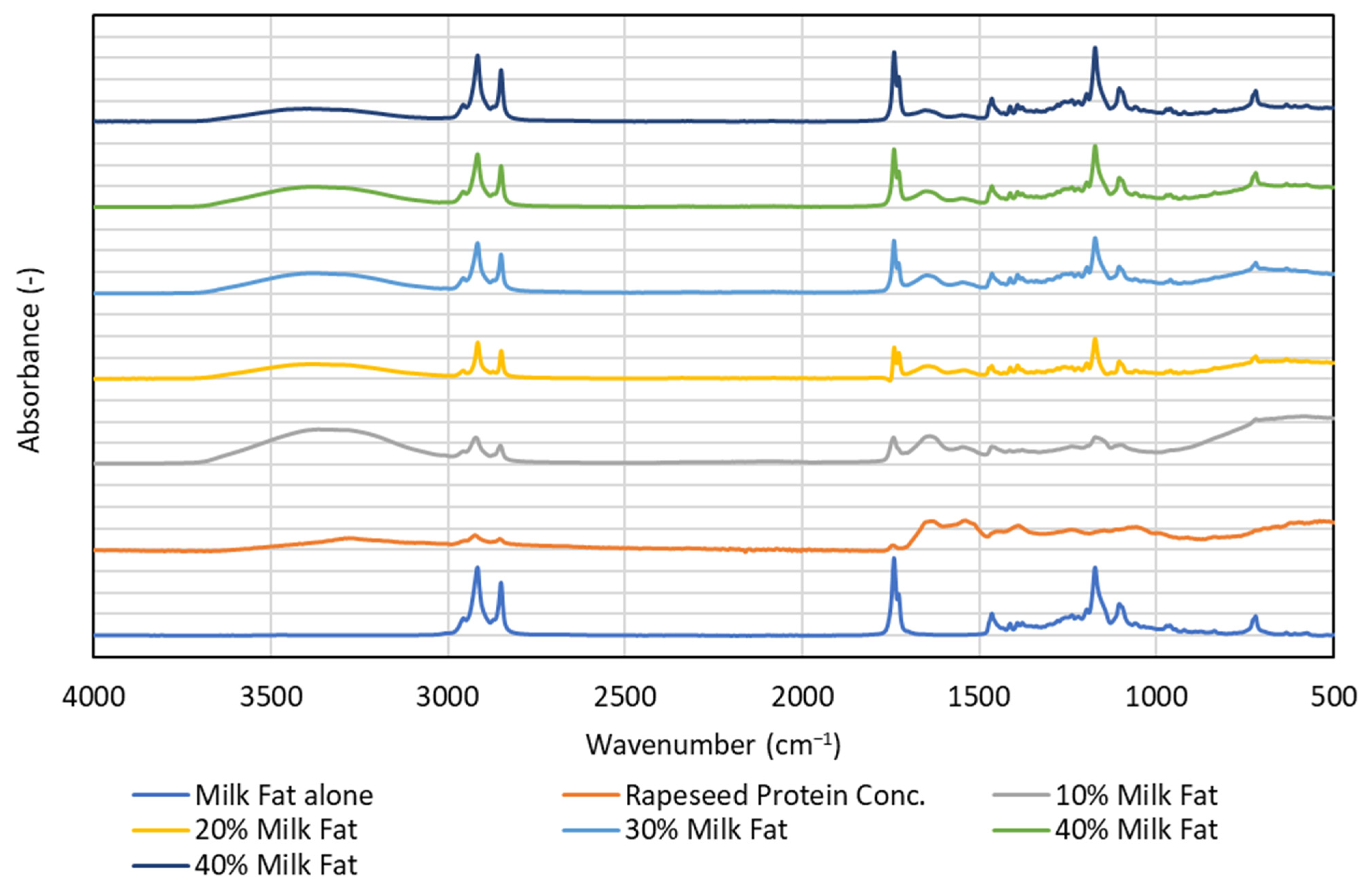
3.4. FTIR—Spectroscopy
4. Conclusions
- The examined emulsions remained stable without any oil loss for a period of 30 days at a temperature of 4 °C, regardless of the type and quantity of lipids present.
- The size of the oil droplets was influenced by the content and type of lipids used in the emulsions.
- All emulsions exhibited shear thinning behaviour, even though there was a general increase in apparent viscosity with higher lipid content.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbut, S.; Tiensa, B.E.; Marangoni, A.G. Partial Fat Replacement in Liver Pâté Using Canola Oil Organogel. LWT 2021, 139, 110428. [Google Scholar] [CrossRef]
- Nieto, G.; Lorenzo, J.M. Use of Olive Oil as Fat Replacer in Meat Emulsions. Curr. Opin. Food Sci. 2021, 40, 179–186. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in Natural Emulsifiers for Utilization in Food Emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Food Emulsions: Their Structures and Properties. In Food Emulsions; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-8247-4696-4. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-15403-4. [Google Scholar]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of PH on the Inter-Relationships between the Physicochemical, Interfacial and Emulsifying Properties for Pea, Soy, Lentil and Canola Protein Isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Houdijk, J.G.M.; Liddell, S.; Davis, K.; Olukosi, O.A.; Kightley, S.; White, G.A.; Wiseman, J. Rapeseed Napin and Cruciferin Are Readily Digested by Poultry. J. Anim. Physiol. Anim. Nutr. 2017, 101, 658–666. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Gray, D.A.; Decker, E.A. Physical and Oxidative Stability of Pre-Emulsified Oil Bodies Extracted from Soybeans. Food Chem. 2012, 132, 1514–1520. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Wijesundera, C.; Boiteau, T.; Xu, X.; Shen, Z.; Watkins, P.; Logan, A. Stabilization of Fish Oil-in-Water Emulsions with Oleosin Extracted from Canola Meal: Oleosin-Stabilized Fish Oil Emulsions. J. Food Sci. 2013, 78, C1340–C1347. [Google Scholar] [CrossRef]
- Iggman, D.; Gustafsson, I.-B.; Berglund, L.; Vessby, B.; Marckmann, P.; Risérus, U. Replacing Dairy Fat with Rapeseed Oil Causes Rapid Improvement of Hyperlipidaemia: A Randomized Controlled Study: Rapeseed Oil and Metabolic Risk. J. Intern. Med. 2011, 270, 356–364. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Houdijk, J.G.M.; Kightley, S.; Olukosi, O.A.; White, G.A.; Carre, P.; Wiseman, J. Effects of Rapeseed Variety and Oil Extraction Method on the Content and Ileal Digestibility of Crude Protein and Amino Acids in Rapeseed Cake and Softly Processed Rapeseed Meal Fed to Broiler Chickens. Anim. Feed Sci. Technol. 2016, 213, 90–98. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Houdijk, J.G.M.; Olukosi, O.A.; Appleyard, H.; Kightley, S.P.J.; Carre, P.; Wiseman, J. The Influence of Oil Extraction Process of Different Rapeseed Varieties on the Ileal Digestibility of Crude Protein and Amino Acids in Broiler Chickens. Anim. Feed Sci. Technol. 2017, 227, 68–74. [Google Scholar] [CrossRef]
- Östbring, K.; Nilsson, K.; Ahlström, C.; Fridolfsson, A.; Rayner, M. Emulsifying and Anti-Oxidative Properties of Proteins Extracted from Industrially Cold-Pressed Rapeseed Press-Cake. Foods 2020, 9, 678. [Google Scholar] [CrossRef]
- Wu, J.; Muir, A.D. Comparative Structural, Emulsifying, and Biological Properties of 2 Major Canola Proteins, Cruciferin and Napin. J Food Sci. 2008, 73, C210–C216. [Google Scholar] [CrossRef]
- Ntone, E.; Bitter, J.H.; Nikiforidis, C.V. Not Sequentially but Simultaneously: Facile Extraction of Proteins and Oleosomes from Oilseeds. Food Hydrocoll. 2020, 102, 105598. [Google Scholar] [CrossRef]
- Ahlström, C.; Thuvander, J.; Rayner, M.; Mayer Labba, I.-C.; Sandberg, A.-S.; Östbring, K. Pilot-Scale Protein Recovery from Cold-Pressed Rapeseed Press Cake: Influence of Solids Recirculation. Processes 2022, 10, 557. [Google Scholar] [CrossRef]
- AOAC. 934.01 AOAC Official Method 934.01. Proximate Analysis and Calculations Moisture. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. 990.03 AOAC Official Method 990.03: Protein (Crude) in Animal Feed. Combustion Method. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. 923.03 AOAC Official Method 923.03. Ash of Flour Direct Method. In Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- Sjölin, M.; Thuvander, J.; Wallberg, O.; Lipnizki, F. Purification of Sucrose in Sugar Beet Molasses by Utilizing Ceramic Nanofiltration and Ultrafiltration Membranes. Membranes 2019, 10, 5. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High Internal Phase Emulsions (HIPE) Using Pea Protein and Different Polysaccharides as Stabilizers. Food Hydrocoll. 2020, 105, 105775. [Google Scholar] [CrossRef]
- Bin Sintang, M.D.; Rimaux, T.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Oil Structuring Properties of Monoglycerides and Phytosterols Mixtures. Eur. J. Lipid Sci. Technol. 2017, 119, 1500517. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical Profiling of the Major Components in Natural Waxes to Elucidate Their Role in Liquid Oil Structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Östbring, K.; Malmqvist, E.; Nilsson, K.; Rosenlind, I.; Rayner, M. The Effects of Oil Extraction Methods on Recovery Yield and Emulsifying Properties of Proteins from Rapeseed Meal and Press Cake. Foods 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Weiss, J.; Kohlus, R. Factors Determining the Surface Oil Concentration of Encapsulated Lipid Particles: Impact of the Emulsion Oil Droplet Size. Eur. Food Res. Technol. 2020, 246, 1933–1943. [Google Scholar] [CrossRef]
- Rayner, M. Current Status on Novel Ways for Stabilizing Food Dispersions by Oleosins, Particles and Microgels. Curr. Opin. Food Sci. 2015, 3, 94–109. [Google Scholar] [CrossRef]
- Tzen, J.T.; Lie, G.C.; Huang, A.H. Characterization of the Charged Components and Their Topology on the Surface of Plant Seed Oil Bodies. J. Biol. Chem. 1992, 267, 15626–15634. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effect of Oil Content and Composition on the Gelling Properties of Egg-SPI Proteins Stabilized Emulsion Gels. Food Biophys. 2020, 15, 473–481. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Cui, M.; Yuan, F.; Gao, Y. Effect of the Solid Fat Content on Properties of Emulsion Gels and Stability of β-Carotene. J. Agric. Food Chem. 2019, 67, 6466–6475. [Google Scholar] [CrossRef]
- Domagała, J.; Sady, M.; Grega, T.; Bonczar, G. Rheological Properties and Texture of Yoghurts When Oat-Maltodextrin Is Used as a Fat Substitute. Int. J. Food Prop. 2006, 9, 1–11. [Google Scholar] [CrossRef]
- Khushbu, S.; Sunil, C.K. Effect of Particle Size, Concentration, Temperature and PH on Rheological Properties of Shallots Flour. J Food Sci. Technol. 2020, 57, 3601–3610. [Google Scholar] [CrossRef]
- Deleu, M.; Vaca-Medina, G.; Fabre, J.-F.; Roïz, J.; Valentin, R.; Mouloungui, Z. Interfacial Properties of Oleosins and Phospholipids from Rapeseed for the Stability of Oil Bodies in Aqueous Medium. Colloids Surf. B Biointerfaces 2010, 80, 125–132. [Google Scholar] [CrossRef]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Emulsifying Properties of Proteins Extracted from Australian Canola Meal. LWT—Food Sci. Technol. 2014, 57, 376–382. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour and Appearance; Blackie Academic & Professional: London, UK, 1994; ISBN 978-0-7514-0176-9. [Google Scholar]
- Culver, C.A.; Wrolstad, R.E. (Eds.) Color Quality of Fresh and Processed Foods; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 983, ISBN 978-0-8412-7419-8. [Google Scholar]
- Hu, X.; McClements, D.J. Construction of Plant-Based Adipose Tissue Using High Internal Phase Emulsions and Emulsion Gels. Innov. Food Sci. Emerg. Technol. 2022, 78, 103016. [Google Scholar] [CrossRef]
- Tan, Y.; Lee, P.W.; Martens, T.D.; McClements, D.J. Comparison of Emulsifying Properties of Plant and Animal Proteins in Oil-in-Water Emulsions: Whey, Soy, and RuBisCo Proteins. Food Biophys. 2022, 17, 409–421. [Google Scholar] [CrossRef]
- Sosulski, F. Organoleptic and Nutritional Effects of Phenolic Compounds on Oilseed Protein Products: A Review. J. Am. Oil Chem. Soc. 1979, 56, 711–715. [Google Scholar] [CrossRef]
- Xu, L.; Diosady, L.L. Removal of Phenolic Compounds in the Production of High-Quality Canola Protein Isolates. Food Res. Int. 2002, 35, 23–30. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, Á. Effects of Ultraviolet Radiation on Properties of Films from Whey Protein Concentrate Treated before or after Film Formation. Food Hydrocoll. 2016, 55, 189–199. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary Structure of Food Proteins by Fourier Transform Spectroscopy in the Mid-Infrared Region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Hou, W.; Long, J.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C.; Li, X. Formation and Characterization of Solid Fat Mimetic Based on Pea Protein Isolate/Polysaccharide Emulsion Gels. Front. Nutr. 2022, 9, 1053469. [Google Scholar] [CrossRef]



| Sample ID | Type of Lipid | Content of Lipids (%) | Content of Water Phase (%) Enriched with Rapeseed Protein | Protein-to-Lipid Ratio |
|---|---|---|---|---|
| 1 | Rapeseed oil | 10 | 90 | 0.47 |
| 2 | Rapeseed oil | 20 | 80 | 0.23 |
| 3 | Rapeseed oil | 30 | 70 | 0.13 |
| 4 | Rapeseed oil | 40 | 60 | 0.09 |
| 5 | Rapeseed oil | 50 | 50 | 0.06 |
| 6 | Milk fat | 10 | 90 | 0.47 |
| 7 | Milk fat | 20 | 80 | 0.23 |
| 8 | Milk fat | 30 | 70 | 0.13 |
| 9 | Milk fat | 40 | 60 | 0.09 |
| 10 | Milk fat | 50 | 50 | 0.06 |
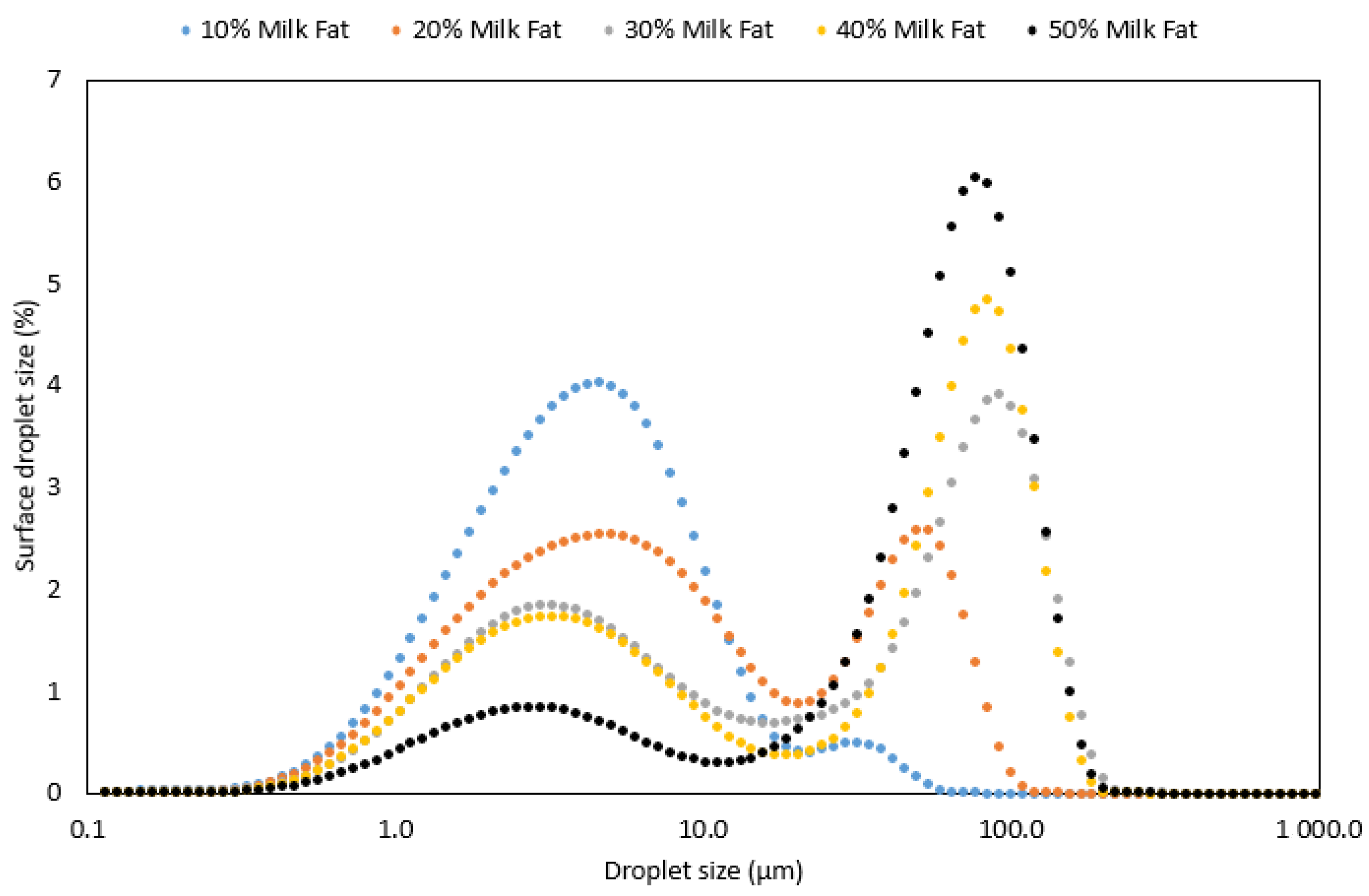
| Sample ID | Type of Lipid | Lipid Content (%) | D10 (µm) | D50 (µm) | D90 (µm) | Span | D4,3 | D3,2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Rapeseed oil | 10 | 1.2 (±0.01) | 4.3 (±0.04) | 11.8 (±0.09) | 2.5 (±0.01) | 6.0 (±0.04) | 2.6 (±0.01) |
| 2 | Rapeseed oil | 20 | 1.3 (±0.03) | 6.6 (±0.07) | 15.5 (±0.10) | 2.1 (±0.01) | 7.7 (±0.08) | 3.1 (±0.05) |
| 3 | Rapeseed oil | 30 | 1.4 (±0.07) | 9.5 (±0.55) | 19.2 (±0.71) | 1.9 (±0.04) | 10.0 (±0.53) | 3.7 (±0.20) |
| 4 | Rapeseed oil | 40 | 1.3 (±0.02) | 9.5 (±0.03) | 18.2 (±0.07) | 1.8 (±0.01) | 9.8 (±0.03) | 3.7 (±0.04) |
| 5 | Rapeseed oil | 50 | 1.3 (±0.01) | 8.1 (±0.07) | 15.3 (±0.12) | 1.7 (±0.00) | 8.6 (±0.11) | 3.5 (±0.04) |
| 6 | Milk fat | 10 | 1.2 (±0.01) | 4.1 (±0.19) | 12.6 (±1.28) | 2.7 (±0.19) | 6.6 (±0.78) | 2.6 (±0.06) |
| 7 | Milk fat | 20 | 1.4 (±0.01) | 6.6 (±0.04) | 54.5 (±1.28) | 8.1 (±0.16) | 17.9 (±0.42) | 3.4 (±0.02) |
| 8 | Milk fat | 30 | 1.6 (±0.06) | 26.6 (±4.81) | 113.6 (±3.80) | 4.3 (±0.66) | 44.8 (±2.31) | 4.5 (±0.18) |
| 9 | Milk fat | 40 | 1.7 (±0.01) | 41.7 (±1.57) | 107.3 (±1.42) | 2.5 (±0.07) | 46.0 (±0.81) | 4.8 (±0.06) |
| 10 | Milk fat | 50 | 2.7 (±0.04) | 59.1 (±0.53) | 111.8 (±0.97) | 1.8 (±0.00) | 58.9 (±0.61) | 8.4 (±0.09) |
| Sample ID | Type of Lipid | Content of Lipid (%) | Ostwald de Waele Model | ƞ(300) (Pa·s) | Encapsulation at Day 30 (%) | ||
|---|---|---|---|---|---|---|---|
| K (Pa·sn) | n (-) | R2 | |||||
| 1 | Rapeseed oil | 10 | 0.02 (±0.01) | 0.85 (±0.09) | 0.99 (±0.00) | 0.01 (±0.00) | 100 |
| 2 | Rapeseed oil | 20 | 0.07 (±0.01) | 0.76 (±0.00) | 0.99 (±0.00) | 0.02 (±0.00) | 100 |
| 3 | Rapeseed oil | 30 | 1.53 (±0.41) | 0.38 (±0.04) | 1.00 (±0.00) | 0.04 (±0.00) | 100 |
| 4 | Rapeseed oil | 40 | 7.10 (±0.66) | 0.26 (±0.03) | 0.99 (±0.00) | 0.10 (±0.01) | 100 |
| 5 | Rapeseed oil | 50 | 47.87 (±2.42) | 0.11 (±0.01) | 1.00 (±0.00) | 0.30 (±0.00) | 100 |
| 6 | Milk fat | 10 | 0.06 (±0.01) | 0.79 (±0.03) | 0.99 (±0.00) | 0.02 (±0.00) | 100 |
| 7 | Milk fat | 20 | 0.33 (±0.24) | 0.54 (±0.19) | 0.99 (±0.00) | 0.02 (±0.00) | 100 |
| 8 | Milk fat | 30 | 1.64 (±0.39) | 0.32 (±0.04) | 0.98 (±0.00) | 0.03 (±0.00) | 100 |
| 9 | Milk fat | 40 | 8.74 (±0.30) | 0.27 (±0.07) | 0.74 (±0.30) | 0.15 (±0.02) | 100 |
| 10 | Milk fat | 50 | 37.55 (±1.10) | 0.17 (±0.00) | 1.00 (±0.00) | 0.32 (±0.00) | 100 |
| Sample ID | Type of Lipid | Content of Lipid (%) | L* (D65) | a* (D65) | b* (D65) |
|---|---|---|---|---|---|
| 1 | Rapeseed oil | 10 | 57.9 (±0.21) | 1.2 (±0.05) | 16.8 (±0.14) |
| 2 | Rapeseed oil | 20 | 59.5 (±0.24) | 1.4 (±0.05) | 16.5 (±0.19) |
| 3 | Rapeseed oil | 30 | 61.6 (±0.18) | 1.3 (±0.03) | 16.1 (±0.19) |
| 4 | Rapeseed oil | 40 | 64.0 (±0.06) | 1.0 (±0.02) | 15.2 (±0.13) |
| 5 | Rapeseed oil | 50 | 67.2 (±0.06) | 0.5 (±0.01) | 14.0 (±0.06) |
| 6 | Milk fat | 10 | 56.8 (±0.16) | 1.7 (±0.04) | 16.5 (±0.17) |
| 7 | Milk fat | 20 | 58.9 (±0.16) | 1.8 (±0.03) | 16.4 (±0.18) |
| 8 | Milk fat | 30 | 60.9 (±0.13) | 1.6 (±0.04) | 16.2 (±0.17) |
| 9 | Milk fat | 40 | 63.5 (±0.06) | 1.3 (±0.02) | 15.3 (±0.09) |
| 10 | Milk fat | 50 | 66.5 (±0.07) | 0.8 (±0.02) | 14.1 (±0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, M.M.; Jarzębski, M.; Smułek, W.; Berski, W.; Zając, M.; Östbring, K.; Ahlström, C.; Ptasznik, S.; Domagała, J. Effects of Concentration and Type of Lipids on the Droplet Size, Encapsulation, Colour and Viscosity in the Oil-in-Water Emulsions Stabilised by Rapeseed Protein. Foods 2023, 12, 2288. https://doi.org/10.3390/foods12122288
Kasprzak MM, Jarzębski M, Smułek W, Berski W, Zając M, Östbring K, Ahlström C, Ptasznik S, Domagała J. Effects of Concentration and Type of Lipids on the Droplet Size, Encapsulation, Colour and Viscosity in the Oil-in-Water Emulsions Stabilised by Rapeseed Protein. Foods. 2023; 12(12):2288. https://doi.org/10.3390/foods12122288
Chicago/Turabian StyleKasprzak, Mirosław M., Maciej Jarzębski, Wojciech Smułek, Wiktor Berski, Marzena Zając, Karolina Östbring, Cecilia Ahlström, Stanisław Ptasznik, and Jacek Domagała. 2023. "Effects of Concentration and Type of Lipids on the Droplet Size, Encapsulation, Colour and Viscosity in the Oil-in-Water Emulsions Stabilised by Rapeseed Protein" Foods 12, no. 12: 2288. https://doi.org/10.3390/foods12122288
APA StyleKasprzak, M. M., Jarzębski, M., Smułek, W., Berski, W., Zając, M., Östbring, K., Ahlström, C., Ptasznik, S., & Domagała, J. (2023). Effects of Concentration and Type of Lipids on the Droplet Size, Encapsulation, Colour and Viscosity in the Oil-in-Water Emulsions Stabilised by Rapeseed Protein. Foods, 12(12), 2288. https://doi.org/10.3390/foods12122288










