Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
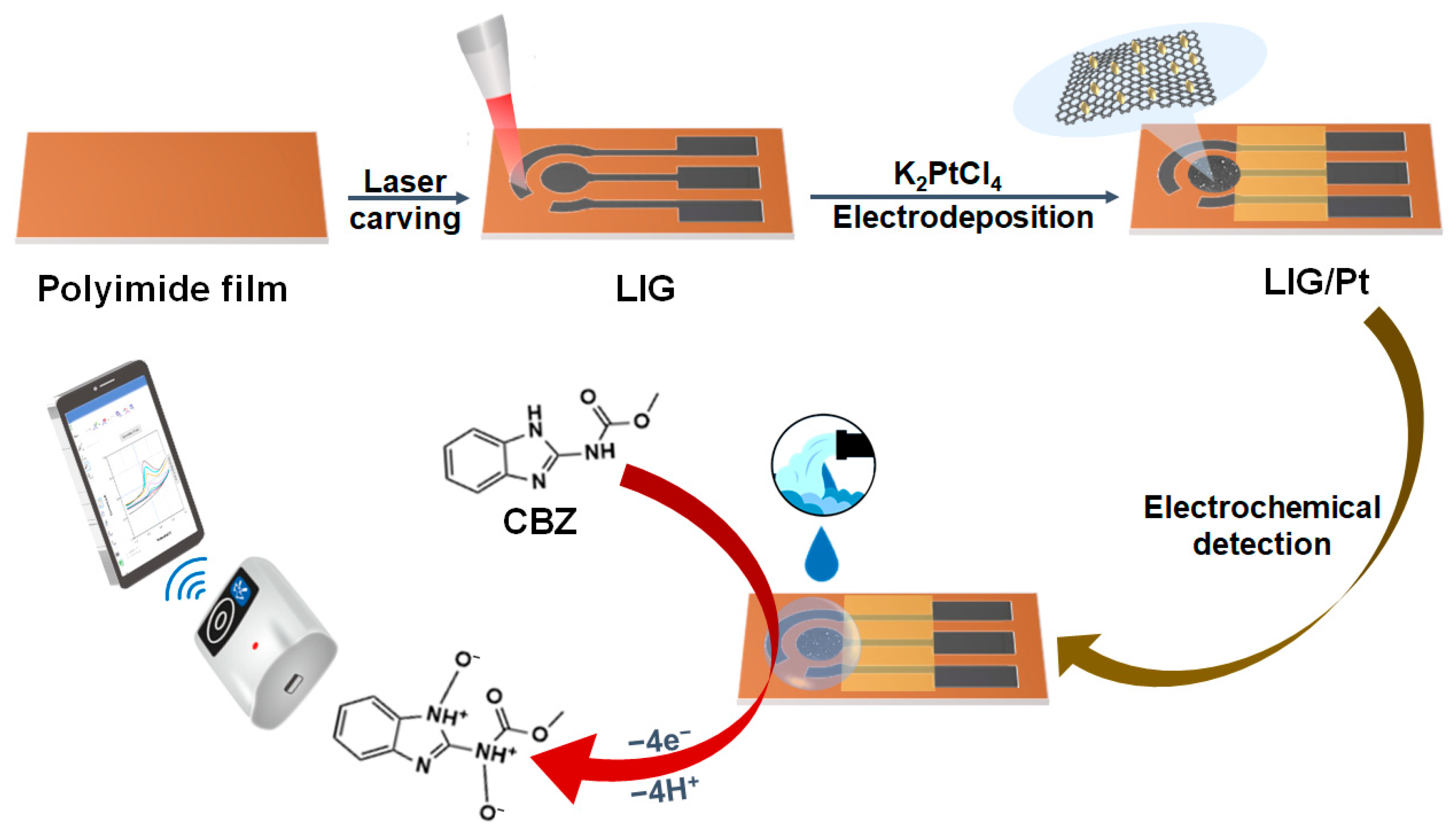
2.2. Preparation of the LIG/Pt Sensor
2.3. Electrochemical Detection of CBZ
2.4. Real Sample Analysis
3. Results and Discussion
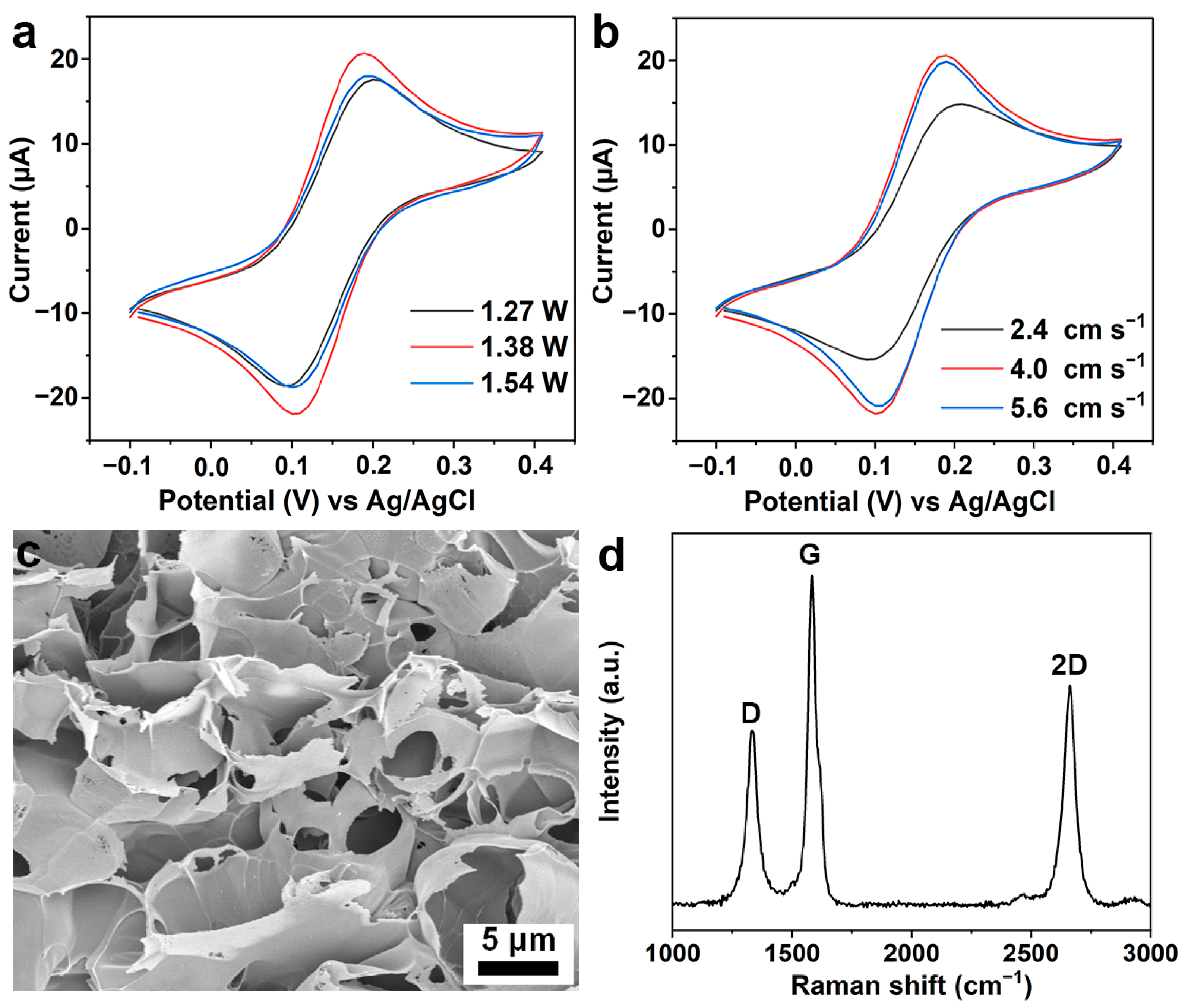
3.1. Characterization of the LIG Electrode
3.2. Characterization of the LIG/Pt Sensor
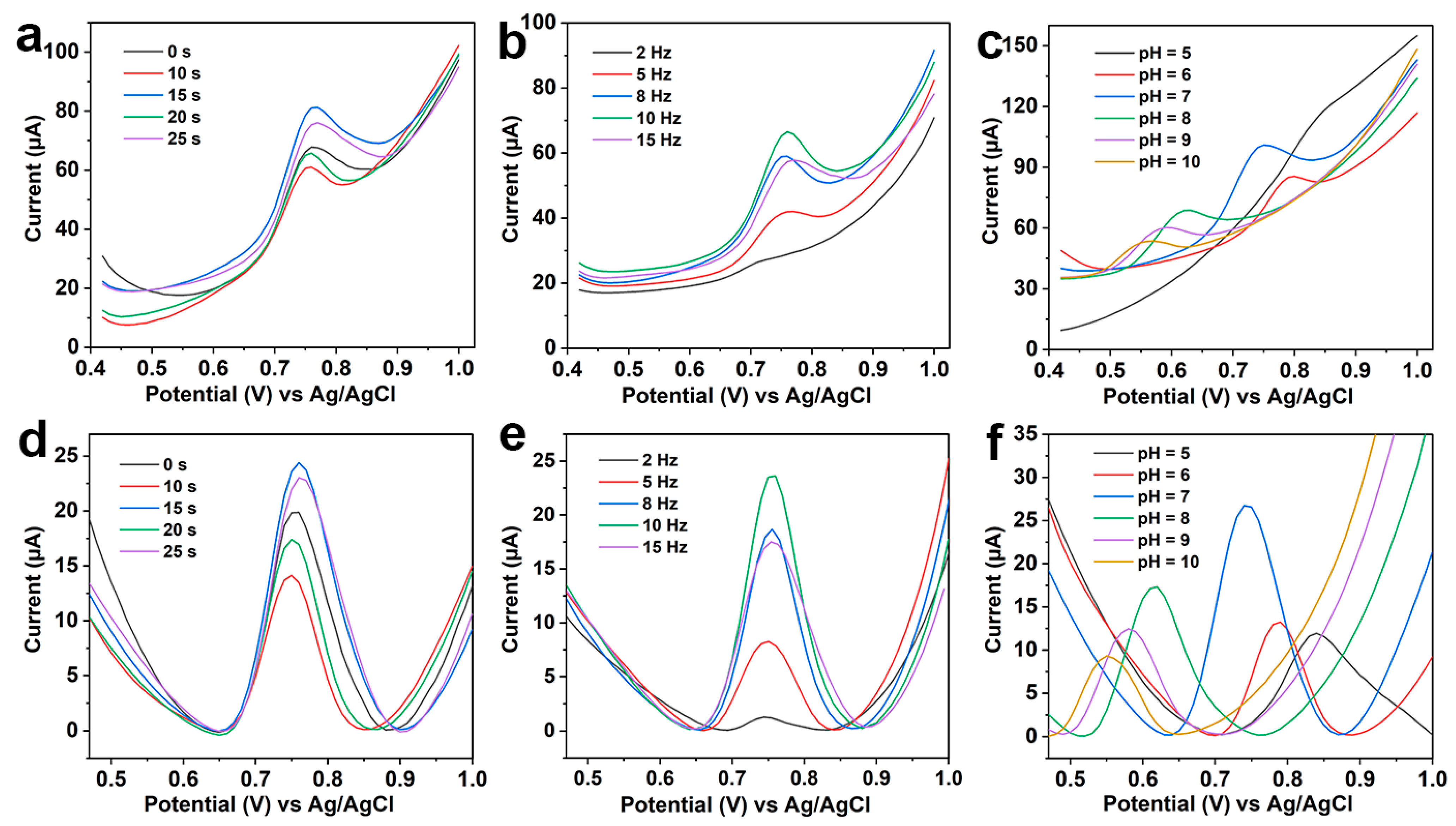
3.3. Optimization of Experimental Parameters
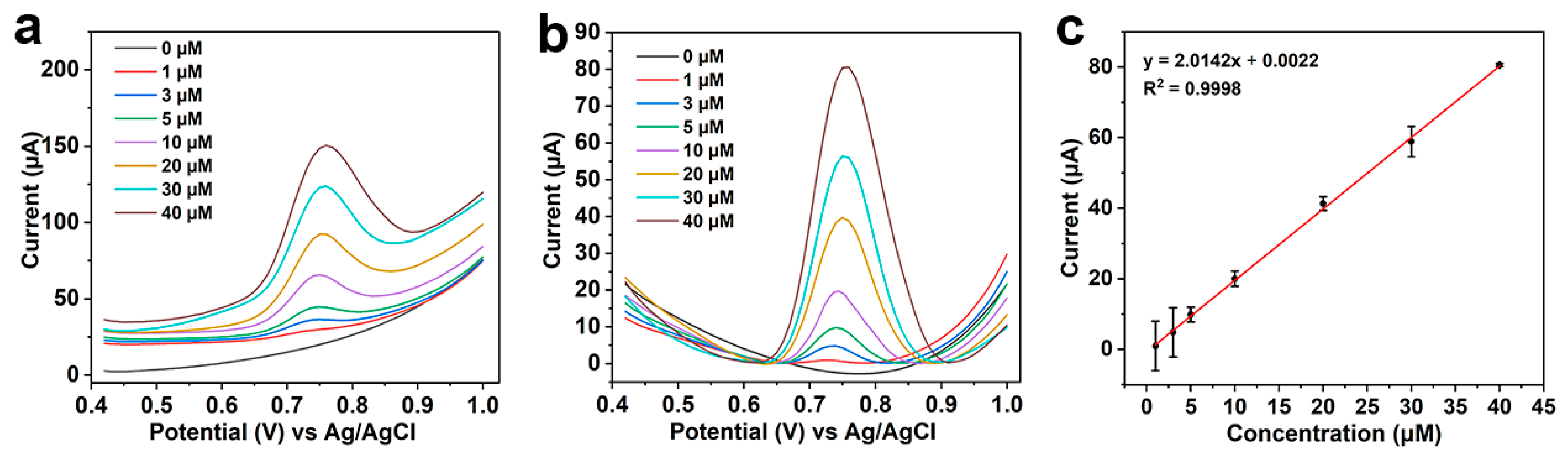
3.4. Electrochemical Detection of CBZ
3.5. Anti-Interference, Selectivity, Reproducibility, and Stability Analysis
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haipeng, G.; Ruiqi, Y.; Doudou, L.; Xiao, M.; Dingkun, Z.; Peng, L.; Jiabo, W.; Lidong, Z.; Weijun, K. Red-emissive carbon dots based fluorescent and smartphone-integrated paper sensors for sensitive detection of carbendazim. Microchem. J. 2023, 190, 108586. [Google Scholar]
- Martins, T.S.; Machado, S.A.S.; Oliveira, O.N., Jr.; Bott-Neto, J.L. Optimized paper-based electrochemical sensors treated in acidic media to detect carbendazim on the skin of apple and cabbage. Food Chem. 2023, 410, 135429. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.R.; Cardoso, D.N.; Cruz, A.; Mendo, S.; Soares, A.; Loureiro, S. Long-term exposure of Daphnia magna to carbendazim: How it affects toxicity to another chemical or mixture. Environ. Sci. Pollut. Res. 2019, 26, 16289–16302. [Google Scholar] [CrossRef] [PubMed]
- Yamuna, A.; Chen, T.W.; Chen, S.M. Synthesis and characterizations of iron antimony oxide nanoparticles and its applications in electrochemical detection of carbendazim in apple juice and paddy water samples. Food Chem. 2022, 373 Pt B, 131569. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, J.; Liu, Y.; Wang, Y.; Liu, H.; Pang, J.; Cui, Y.; Zhao, Q.; Wang, C.; Li, Z.; et al. Portable and quantitative detection of carbendazim based on the readout of a thermometer. Food Chem. 2021, 351, 129292. [Google Scholar] [CrossRef]
- Scheel, G.L.; Teixeira Tarley, C.R. Simultaneous microextraction of carbendazim, fipronil and picoxystrobin in naturally and artificial occurring water bodies by water-induced supramolecular solvent and determination by HPLC-DAD. J. Mol. Liq. 2020, 297, 111897. [Google Scholar] [CrossRef]
- Passos, M.L.C.; Saraiva, M.F.S.M.L. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral Capillary Electrophoresis. Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Chen, H.; Hu, O.; Fan, Y.; Xu, L.; Zhang, L.; Lan, W.; Hu, Y.; Xie, X.; Ma, L.; She, Y.; et al. Fluorescence paper-based sensor for visual detection of carbamate pesticides in food based on CdTe quantum dot and nano ZnTPyP. Food Chem. 2020, 327, 127075. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, F.; Zou, J.; Liu, S.; Li, M.; Gao, Y.; Yu, Y.; Wang, X.; Lu, L. MXene@Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006. [Google Scholar] [CrossRef]
- Liu, R.; Chang, Y.; Li, F.; Dubovyk, V.; Li, D.; Ran, Q.; Zhao, H. Highly sensitive detection of carbendazim in juices based on mung bean-derived porous carbon@chitosan composite modified electrochemical sensor. Food Chem. 2022, 392, 133301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Hu, P.; Dai, R.; Wu, B.; Yang, L.; Huang, Y.; Zhuang, G. Recent developments in the spectrometry of fluorescence, ultraviolet visible and surface-enhanced Raman scattering for pesticide residue detection. Bull. Mater. Sci. 2022, 45, 202. [Google Scholar] [CrossRef]
- Rashmi Dilip, K.; Rangappa, S.K.; Nagaraju, D.H.; Srinivasa, B. State-of-the-art Electrochemical Sensors for Quantitative Detection of Pesticides. Appl. Organomet. Chem. 2023, 37, e7097. [Google Scholar]
- Zheng, X.; Khaoulani, S.; Ktari, N.; Lo, M.; Khalil, A.M.; Zerrouki, C.; Fourati, N.; Chehimi, M.M. Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors. Sensors 2021, 21, 4300. [Google Scholar] [CrossRef]
- Suresh, I.; Selvaraj, S.; Nesakumar, N.; Rayappan, J.B.B.; Kulandaiswamy, A.J. Nanomaterials based non-enzymatic electrochemical and optical sensors for the detection of carbendazim: A review. Trends Environ. Anal. Chem. 2021, 31, e00137. [Google Scholar] [CrossRef]
- Anil Kumar, Y.; Koyyada, G.; Ramachandran, T.; Kim, J.H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review. Nanomaterials 2023, 13, 1049. [Google Scholar] [CrossRef]
- Nithya, V.D. A review on holey graphene electrode for supercapacitor. J. Energy Storage 2021, 44, 103380. [Google Scholar] [CrossRef]
- Aiswaria, P.; Mohamed, S.N.; Singaravelu, D.L.; Brindhadevi, K.; Pugazhendhi, A. A review on graphene / graphene oxide supported electrodes for microbial fuel cell applications: Challenges and prospects. Chemosphere 2022, 296, 133983. [Google Scholar]
- Yogesh Kumar, K.; Prashanth, M.K.; Parashuram, L.; Palanivel, B.; Alharti, F.A.; Jeon, B.H.; Raghu, M.S. Gadolinium sesquisulfide anchored N-doped reduced graphene oxide for sensitive detection and degradation of carbendazim. Chemosphere 2022, 296, 134030. [Google Scholar] [CrossRef]
- Sundaresan, R.; Mariyappan, V.; Chen, T.-W.; Chen, S.-M.; Akilarasan, M.; Liu, X.; Yu, J. One-dimensional rare-earth tungstate nanostructure encapsulated reduced graphene oxide electrocatalyst-based electrochemical sensor for the detection of organophosphorus pesticide. J. Nanostructure Chem. 2023. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Stefano, J.S.; Orzari, L.O.; Brazaca, L.C.; Carrilho, E.; Marcolino-Junior, L.H.; Bergamini, M.F.; Munoz, R.A.A.; Janegitz, B.C. Electrochemical Biosensor for SARS-CoV-2 cDNA Detection Using AuPs-Modified 3D-Printed Graphene Electrodes. Biosensors 2022, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Carey, T.; Alhourani, A.; Tian, R.Y.; Seyedin, S.; Arbab, A.; Maughan, J.; Siller, L.; Horvath, D.; Kelly, A.; Kaur, H.; et al. Cyclic production of biocompatible few-layer graphene ink with in-line shear-mixing for inkjet-printed electrodes and Li-ion energy storage. Npj 2d Mater. Appl. 2022, 6, 3. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, J. 3D Printed Thick Reduced Graphene Oxide: Manganese Oxide/Carbon Nanotube Hybrid Electrode with Highly Ordered Microstructures for Supercapacitors. Adv. Mater. Technol. 2022, 8, 2200263. [Google Scholar] [CrossRef]
- Qiwen, Z.; Fangyi, Z.; Xing, L.; Zengji, Y.; Xi, C.; Zhengfen, W. Doping of Laser-Induced Graphene and Its Applications. Adv. Mater. Technol. 2023, 2300244. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Settu, K.; Chiu, P.T.; Huang, Y.M. Laser-Induced Graphene-Based Enzymatic Biosensor for Glucose Detection. Polymers 2021, 13, 2795. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Huang, Y.; Wang, D.; Li, L.; Liu, Z. In situ laser synthesis of Pt nanoparticles embedded in graphene films for wearable strain sensors with ultra-high sensitivity and stability. Carbon 2022, 190, 245–254. [Google Scholar] [CrossRef]
- Zhang, C.; Ping, J.; Ying, Y. Evaluation of trans-resveratrol level in grape wine using laser-induced porous graphene-based electrochemical sensor. Sci. Total Environ. 2020, 714, 136687. [Google Scholar] [CrossRef]
- Hui, X.; Xuan, X.; Kim, J.; Park, J.Y. A highly flexible and selective dopamine sensor based on Pt-Au nanoparticle-modified laser-induced graphene. Electrochim Acta 2019, 328, 108586. [Google Scholar] [CrossRef]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Huang, L.; Chen, Y.; Li, Y. A laser-induced graphene electrochemical immunosensor for label-free CEA monitoring in serum. Analyst 2021, 146, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W.; et al. Recent Advances in Laser-Induced Graphene: Mechanism, Fabrication, Properties, and Applications in Flexible Electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Zhao, F.; He, J.; Li, X.; Bai, Y.; Ying, Y.; Ping, J. Smart plant-wearable biosensor for in-situ pesticide analysis. Biosens. Bioelectron. 2020, 170, 112636. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Sain, S.; Roy, S.; Mathur, A.; Rajesh, V.M.; Banerjee, D.; Sarkar, B.; Roy, S.S. Electrochemical Sensors Based on Flexible Laser-Induced Graphene for the Detection of Paraquat in Water. ACS Appl. Nano Mater. 2022, 5, 17516–17525. [Google Scholar] [CrossRef]
- Geng, H.; Vilms Pedersen, S.; Ma, Y.; Haghighi, T.; Dai, H.; Howes, P.D.; Stevens, M.M. Noble Metal Nanoparticle Biosensors: From Fundamental Studies toward Point-of-Care Diagnostics. Acc. Chem. Res. 2022, 55, 593–604. [Google Scholar] [CrossRef]
- Tian, C.H.; Zhang, S.F.; Wang, H.B.; Chen, C.; Han, Z.D.; Chen, M.L.; Zhu, Y.Y.; Cui, R.J.; Zhang, G.H. Three-dimensional nanoporous copper and reduced graphene oxide composites as enhanced sensing platform for electrochemical detection of carbendazim. J. Electroanal. Chem. 2019, 847, 113242. [Google Scholar] [CrossRef]
- Pham, T.S.H.; Hasegawa, S.; Mahon, P.; Guerin, K.; Dubois, M.; Yu, A.M. Graphene Nanocomposites Based Electrochemical Sensing Platform for Simultaneous Detection of Multi-drugs. Electroanalysis 2022, 34, 435–444. [Google Scholar] [CrossRef]
- Özdokur, K.V.; Kuşcu, C.; Ertaş, F.N. Ultrasound Assisted Electrochemical Deposition of Polypyrrole-Carbon Nanotube Composite Film: Preparation, Characterization and Application to the Determination of Droxidopa. Curr. Anal. Chem. 2020, 16, 421–427. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ahmed, F.; Arshi, N. Fabrication of flower-like nickel cobalt-layered double hydroxide for electrochemical detection of carbendazim. Surf. Interfaces 2023, 36, 102570. [Google Scholar] [CrossRef]

| Sensor | Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| GCE/PPy-CNT | DPV | 4–20 μM | 1.3 μM | [39] |
| GCE/MBC@CTS | DPV | 0.1–20 µM | 0.02 μM | [11] |
| GCE/RGO-Pt | DPV | 25–115 μM | 2.96 μM | [38] |
| GCE/RGO/NP-Cu | DPV | 0.5–30 μM | 0.09 μM | [37] |
| GCE/NiCo-LDH | DPV | 0.006–14.1 μM | 0.001 μM | [40] |
| LIG/Pt | SWV | 1–40 μM | 0.67 μM | This work |
| Method | Added (µM) | Founded (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| This method | 10 | 9.95 ± 0.20 | 99.50 | 1.98 |
| 20 | 17.78 ± 1.61 | 88.92 | 4.12 | |
| 30 | 26.67 ± 1.10 | 88.89 | 5.11 | |
| LC–MS/MS | 10 | 8.51 ± 0.11 | 85.14 | 1.27 |
| 20 | 17.44 ± 0.55 | 87.22 | 3.14 | |
| 30 | 27.07 ± 1.69 | 90.24 | 6.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, M.; Li, B.; Wang, M.; Zhao, H.; Zhao, F. Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water. Foods 2023, 12, 2277. https://doi.org/10.3390/foods12122277
Wang L, Li M, Li B, Wang M, Zhao H, Zhao F. Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water. Foods. 2023; 12(12):2277. https://doi.org/10.3390/foods12122277
Chicago/Turabian StyleWang, Li, Mengyue Li, Bo Li, Min Wang, Hua Zhao, and Fengnian Zhao. 2023. "Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water" Foods 12, no. 12: 2277. https://doi.org/10.3390/foods12122277
APA StyleWang, L., Li, M., Li, B., Wang, M., Zhao, H., & Zhao, F. (2023). Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water. Foods, 12(12), 2277. https://doi.org/10.3390/foods12122277






