Development of Magnetic Lateral Flow and Direct Competitive Immunoassays for Sensitive and Specific Detection of Halosulfuron-Methyl Using a Novel Hapten and Monoclonal Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Buffers, and Instruments
2.2. Synthesis of the HM Hapten
2.3. Preparation of the Protein-Hapten Conjugate
2.4. Immunization and Monoclonal Antibody Production
2.5. Establishment and Optimization of the dcELISA
2.6. Functionalization of MNPs with Antibodies
2.7. Establishment of the MLFIA
2.8. Sample Extraction and Analysis
3. Results and Discussion
3.1. Characterization of the HM Hapten and Its Bioconjugates
3.2. Antibody Generation and Characterization
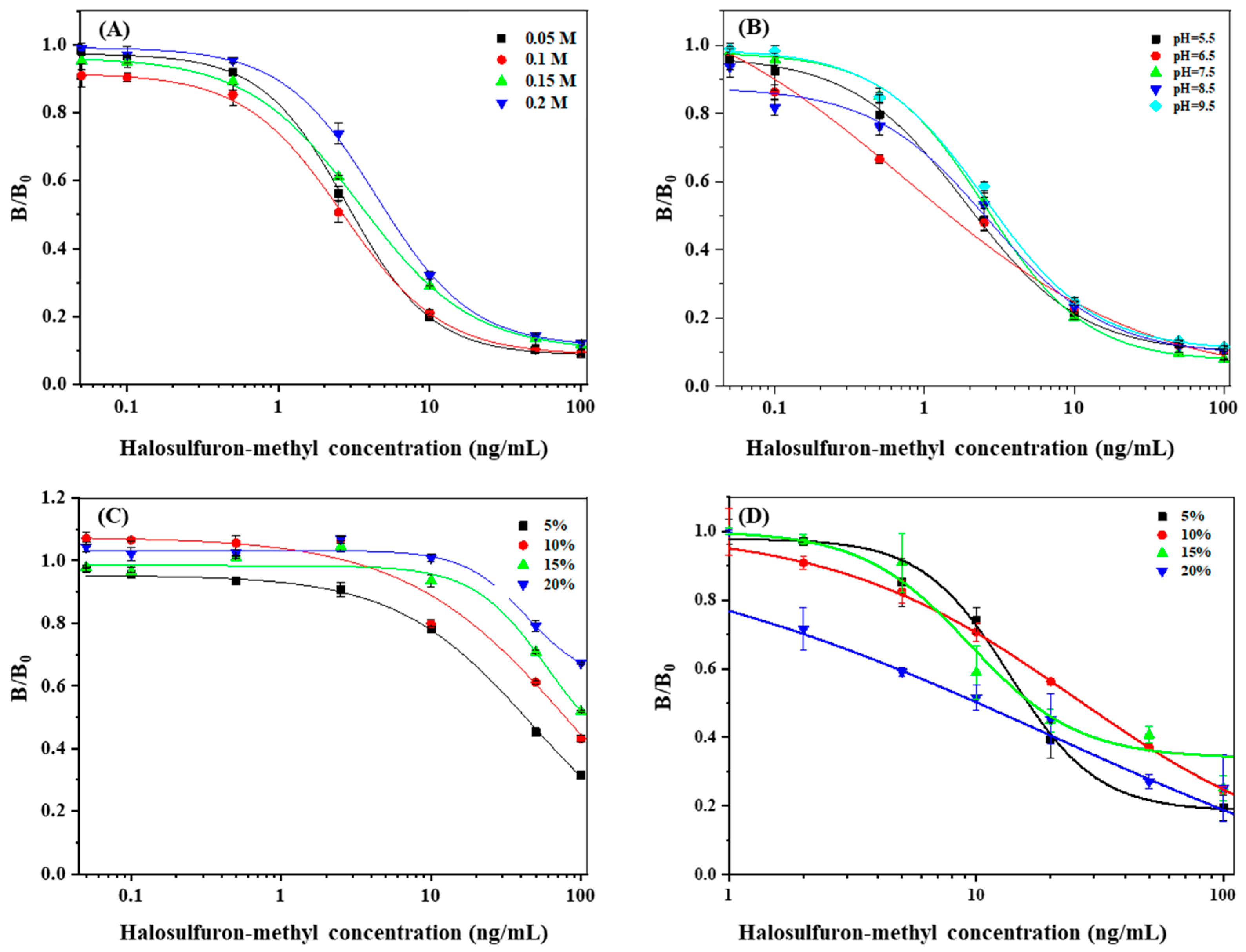
3.3. Optimization of dcELISA Conditions
3.4. Optimization of the MLFIA
3.4.1. The EDC/NHS Ratio
3.4.2. HM Antibodies Content and pH of the Conjugation Solution
3.4.3. Optimization of MNP Probe Enrichment Time and Volume
3.5. Evaluation and Specificity of MLFIA and dcELISA
3.6. Food Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ying, Y.; Cao, Z.; Li, H.; He, J.; Zheng, L.; Jin, M.; Wang, J. An optimized LC-MS/MS workflow for evaluating storage stability of fluroxypyr and halosulfuron-methyl in maize samples. J. Environ. Sci. Health B 2021, 56, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, H.; Zhang, H.; He, Q.; Huang, S.; Qin, M.; Chai, S.; Gao, H.; Ma, Y. Analysis of four sulfonylurea herbicides in cereals using modified Quick, Easy, Cheap, Effective, Rugged, and Safe sample preparation method coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1537, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.; dos Santos, V.B.; Vidal, D.T.R.; do Lago, C.L. Determination of halosulfuron-methyl herbicide in sugarcane juice and tomato by capillary electrophoresis-tandem mass spectrometry. Food Chem. 2015, 175, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Yu, Y.; Li, X.; Tan, H. Integrated proteomics, metabolomics and physiological analyses for dissecting the toxic effects of halosulfuron-methyl on soybean seedlings (Glycine max merr.). Plant Physiol. Biochem. 2020, 157, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, T.; Dong, B.; Hu, J. QuEChERS-based study on residue determination and dissipation of three herbicides in corn fields using HPLC-MS/MS. Toxicol. Environ. Chem. 2015, 98, 216–225. [Google Scholar] [CrossRef]
- Devi, R.; Duhan, A.; Punia, S.S.; Yadav, D.B. Degradation dynamics of halosulfuron-methyl in two textured soils. Bull. Environ. Contam. Toxicol. 2019, 102, 246–251. [Google Scholar] [CrossRef]
- Ceballos-Alcantarilla, E.; Agulló, C.; Abad-Fuentes, A.; Abad-Somovilla, A.; Mercader, J.V. Rational design of a fluopyram hapten and preparation of bioconjugates and antibodies for immunoanalysis. RSC Adv. 2015, 5, 51337–51341. [Google Scholar] [CrossRef]
- Goodrow, M.H.; Sanborn, J.R.; Stoutamire, D.W.; Gee, S.J.; Hammock, B.D. Strategies for immunoassay hapten design. In ACS Symposium Series; Immunoanalysis of Agrochemicals: Emerging Technologies; Nelson, J.O., Karu, A.E., Wong, R.B., Eds.; American Chemical Society: Washington, DC, USA, 1995; Volume 586, pp. 119–139. [Google Scholar]
- Kim, Y.J.; Cho, Y.A.; Lee, H.-S.; Lee, Y.T.; Gee, S.J.; Hammock, B.D. Synthesis of haptens for immunoassay of organophosphorus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity. Anal. Chim. Acta 2002, 475, 85–96. [Google Scholar] [CrossRef]
- Mercader, J.V.; Suarez-Pantaleon, C.; Agullo, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Production and characterization of monoclonal antibodies specific to the strobilurin pesticide pyraclostrobin. J. Agric. Food Chem. 2008, 56, 7682–7690. [Google Scholar] [CrossRef]
- Schlaeppi, J.M.A.; Meyer, W.; Ramsteiner, K.A. Determination of Triasulfuron in Soil by Monoclonal Antibody-Based Enzyme Immunoassay. J. Agrlc. Food Chem. 1992, 40, 1093–1098. [Google Scholar] [CrossRef]
- Welzig, E.; Pichler, H.; Krska, R.; Knopp, D.; Niessner, R. Development of an Enzyme Immunoassay for the Determination of the Herbicide Metsulfuron-Methyl Based on Chicken Egg Yolk Antibodies. Int. J. Environ. Anal. Chem. 2000, 78, 279–288. [Google Scholar] [CrossRef]
- Zhao, J.; Yi, G.-X.; He, S.-P.; Wang, B.-M.; Yu, C.-X.; Li, G.; Zhai, Z.-X.; Li, Z.-H.; Li, Q.X. Development of a Monoclonal Antibody-Based Enzyme-Linked Immunosorbent Assay for the Herbicide Chlorimuron-ethyl. J. Agric. Food Chem. 2006, 5, 4948–4953. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yates, S.R.; Papiernik, S.K. Transformation kinetics and mechanism of the sulfonylurea herbicides pyrazosulfuron ethyl and halosulfuron methyl in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Chen, M.-L.; He, Y.-J.; Chen, X.-W.; Wang, J.-H. Quantum dots conjugated with Fe3O4-filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar] [CrossRef]
- Wu, J.; Dong, M.; Zhang, C.; Wang, Y.; Xie, M.; Chen, Y. Magnetic Lateral Flow Strip for the Detection of Cocaine in Urine by Naked Eyes and Smart Phone Camera. Sensors 2017, 17, 1286. [Google Scholar] [CrossRef]
- Pang, L.; Quan, H.; Sun, Y.; Wang, P.; Ma, D.; Mu, P.; Chai, T.; Zhang, Y.; Hammock, B.D. A rapid competitive ELISA assay of Okadaic acid level based on epoxy-functionalized magnetic beads. Food Agric. Immunol. 2019, 30, 1286–1302. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, G.; Zhu, H.; Jiang, G. A Class-Specific Enzyme-Linked Immunosorbent Assay Based on Magnetic Particles for Multiresidue Organophosphorus Pesticides. J. Agric. Food Chem. 2010, 58, 2801–2806. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, Z.; Guo, S.; Zhang, W.; Tan, G.; Li, Z.; Wang, B. Hapten Synthesis and Monoclonal Antibody-Based Immunoassay Development for the Analysis of Thidiazuron. J. Plant Growth Regul. 2015, 35, 357–365. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, H.; Cui, Y.; Zhang, L.; Tan, G.; Wang, B.; Li, Q.X. Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of paclobutrazol residue in wheat kernel. J. Agric. Food Chem. 2014, 62, 1826–1831. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, K.; Cui, Y.; Zhang, W.; He, L.; Guo, S.; Chen, Y.; Li, Q.X.; Liu, S.; Wang, B. Development of a monoclonal antibody-based ELISA for the detection of the novel insecticide cyantraniliprole. RSC Adv. 2015, 5, 35874–35881. [Google Scholar] [CrossRef]
- Simon, E.; Knopp, D.; Carrasco, P.B.; Niessner, R. Development of an enzyme immunoassay for metsulfuron-methyl. Food Agric. Immunol. 2008, 10, 105–120. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Wang, B.-M.; Liu, W.; Nan, T.-G.; Zhai, Z.-X.; Li, Z.-H.; Li, Q.X. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of glycyrrhizic acid. Anal. Bioanal. Chem. 2006, 386, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Eremin, S.; Ryabova, I.; Yakovleva, J.; Yazynina, E.; Zherdev, A.; Dzantiev, B. Development of a rapid, specific fluorescence polarization immunoassay for the herbicide chlorsulfuron. Anal. Chim. Acta 2002, 468, 229–236. [Google Scholar] [CrossRef]







| Analytes and Structures | IC50 (ng/mL) | Cross Reactivity | |
|---|---|---|---|
| HM | 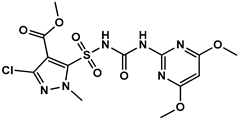 | 28.89 | 100% |
| HM hapten |  | 43.5 a | 0.06% a |
| Nicosulfuron |  | >20,000 | <0.02% |
| Chlosulfuron |  | >20,000 | <0.02% |
| Ethosulfuron |  | >20,000 | <0.02% |
| Chlorimuron-ethyl |  | >20,000 | <0.02% |
| Pyrazosulfuron-ethyl |  | >20,000 | <0.02% |
| Matrix | Spiked Halosulfuron Methyl (mg/kg) | Average Recovery and RSD (%) | |||
|---|---|---|---|---|---|
| dcELISA | RSD | LC-MS/MS | RSD | ||
| Tomato | 0.025 | 78.9 ± 0.02 | 9.1 | 77.2 ± 0.9 | 1.2 |
| 0.05 | 84.4 ± 0.01 | 1.1 | 87.2 ± 2.3 | 2.6 | |
| 0.1 | 87.9 ± 0.01 | 6.8 | 87.6 ± 1.3 | 1.8 | |
| Maize | 0.025 | 107.4 ± 5.6 | 6.4 | 92.8 ± 8.2 | 6.0 |
| 0.05 | 105.5 ± 7.3 | 7.7 | 93.0 ± 8.5 | 7.7 | |
| 0.1 | 103.0 ± 2.6 | 2.7 | 92.1 ± 6.0 | 7.6 | |
| Matrix | Spiked Halosulfuron Methyl (mg/kg) | Average Recovery and RSD (%) | |||
|---|---|---|---|---|---|
| MLFIA | RSD | LC-MS/MS | RSD | ||
| Paddy water | 0.025 | 81.5 | 8.3 | 93.5 | 1.8 |
| 0.05 | 92.5 | 9.7 | 98.9 | 2.3 | |
| 0.1 | 89.3 | 5.4 | 96.5 | 2.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Y.; Cui, X.; Li, H.; Pan, L.; Luo, T.; Cao, Z.; Wang, J. Development of Magnetic Lateral Flow and Direct Competitive Immunoassays for Sensitive and Specific Detection of Halosulfuron-Methyl Using a Novel Hapten and Monoclonal Antibody. Foods 2023, 12, 2764. https://doi.org/10.3390/foods12142764
Ying Y, Cui X, Li H, Pan L, Luo T, Cao Z, Wang J. Development of Magnetic Lateral Flow and Direct Competitive Immunoassays for Sensitive and Specific Detection of Halosulfuron-Methyl Using a Novel Hapten and Monoclonal Antibody. Foods. 2023; 12(14):2764. https://doi.org/10.3390/foods12142764
Chicago/Turabian StyleYing, Ying, Xueyan Cui, Hui Li, Lingyi Pan, Ting Luo, Zhen Cao, and Jing Wang. 2023. "Development of Magnetic Lateral Flow and Direct Competitive Immunoassays for Sensitive and Specific Detection of Halosulfuron-Methyl Using a Novel Hapten and Monoclonal Antibody" Foods 12, no. 14: 2764. https://doi.org/10.3390/foods12142764
APA StyleYing, Y., Cui, X., Li, H., Pan, L., Luo, T., Cao, Z., & Wang, J. (2023). Development of Magnetic Lateral Flow and Direct Competitive Immunoassays for Sensitive and Specific Detection of Halosulfuron-Methyl Using a Novel Hapten and Monoclonal Antibody. Foods, 12(14), 2764. https://doi.org/10.3390/foods12142764






