Identifying Early-Stage Changes in Volatile Organic Compounds of Ceratocystis fimbriata Ellis & Halsted-Infected Sweet Potatoes (Ipomoea batatas L. Lam) Using Headspace Gas Chromatography-Ion Mobility Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Malondialdehyde, Starch, Soluble Protein, and Pyruvate Content Assays
2.3. Lipoxygenase, Pyruvate Decarboxylase, Alcohol Dehydrogenase, and Phenylalanine Ammonia Lyase Activity Assays
2.4. HS-GC-IMS Analysis
2.5. Statistical Analysis
3. Results and Discussion
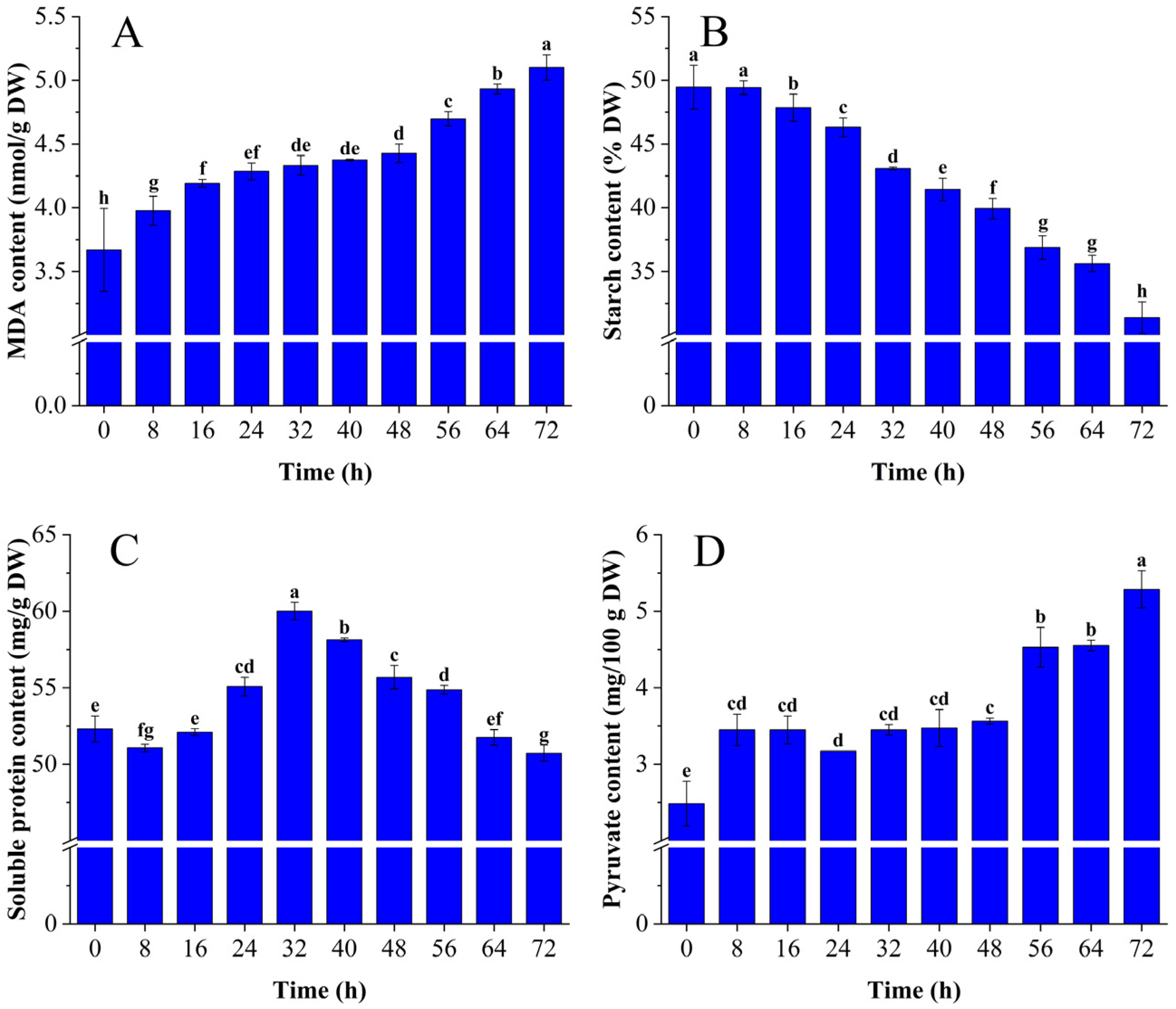
3.1. The Effects of C. fimbriata-infected Sweet Potatoes on Physiological Indicators Related to VOCs
3.1.1. Changes in MDA, Starch, Soluble Protein, and Pyruvate Content
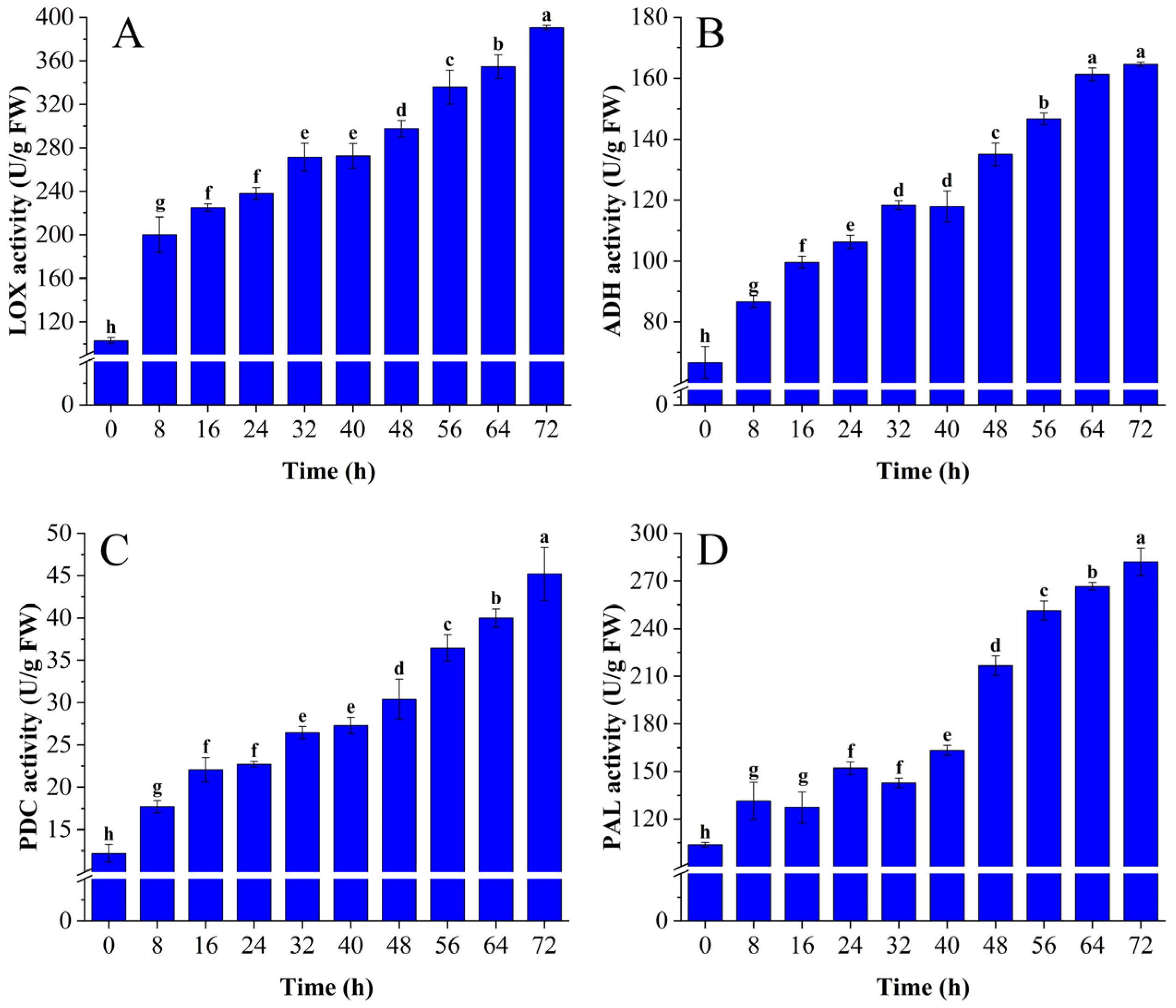
3.1.2. Changes in LOX, PDC, ADH, and PAL Activity
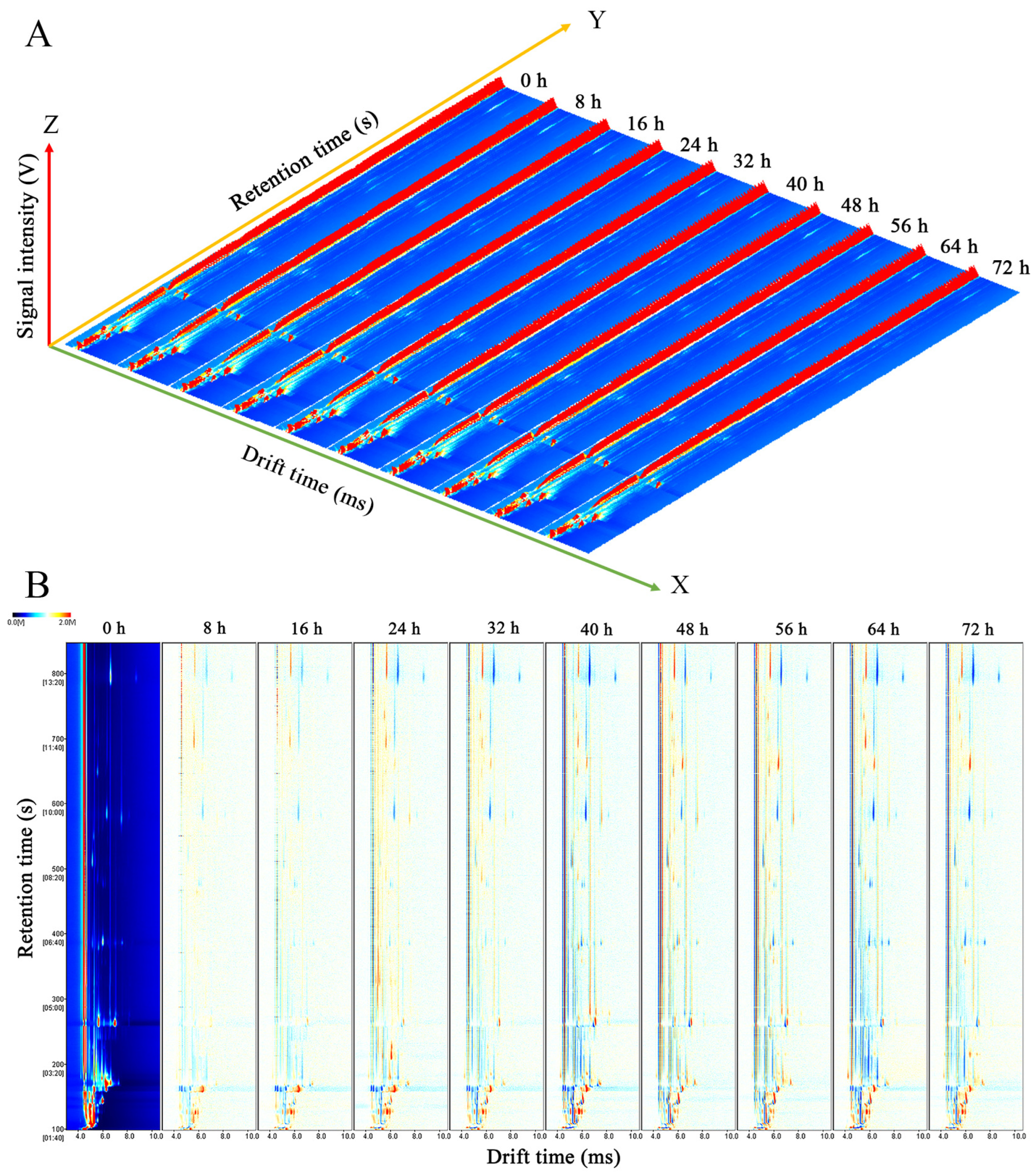
3.2. HS-GC-IMS Analysis of C. fimbriata-Infected Sweet Potatoes in the Early Stage
3.2.1. Topographic Plots of C. fimbriata-Infected Sweet Potatoes
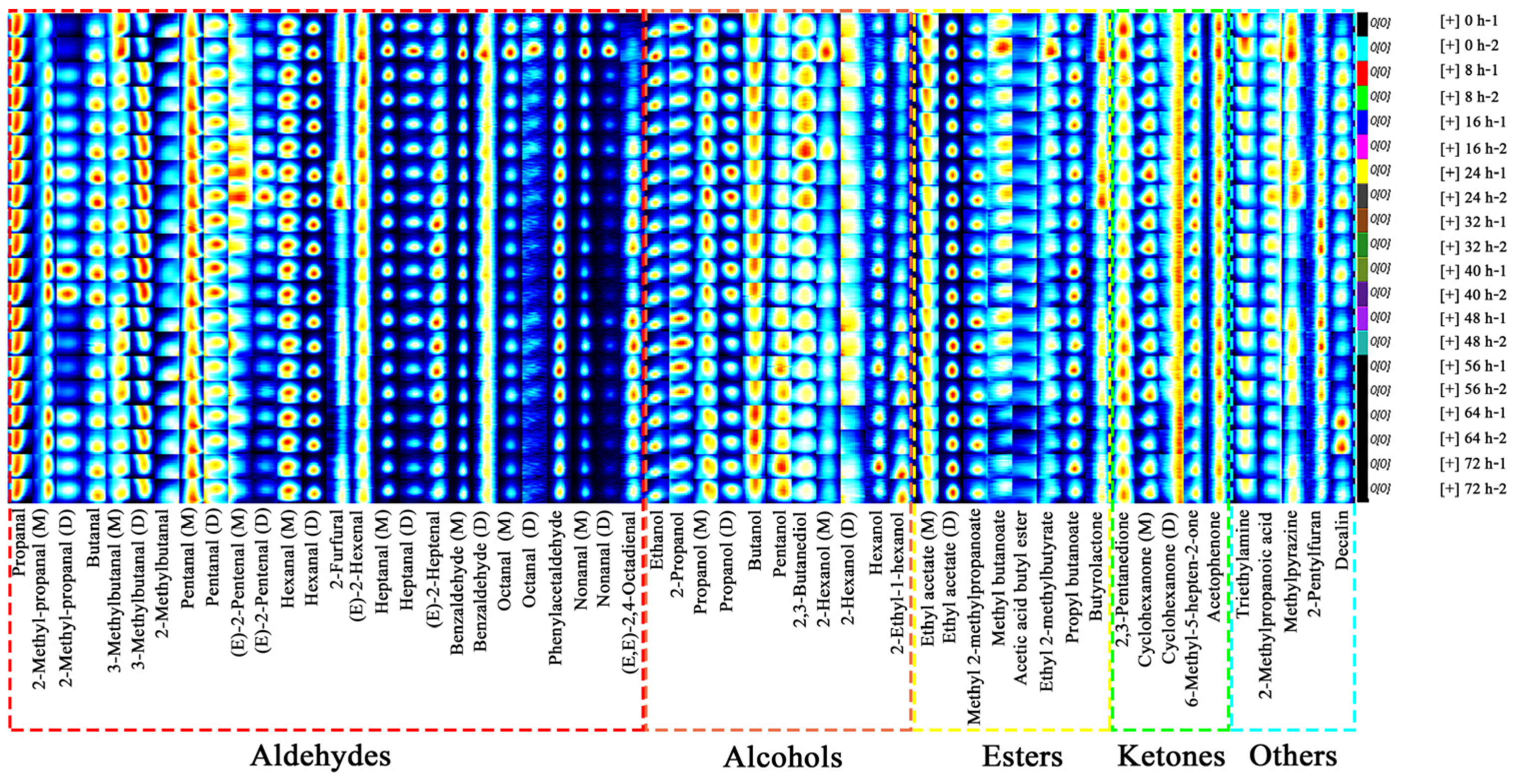
3.2.2. Fingerprints of C. fimbriata-Infected Sweet Potatoes
3.2.3. Changes of VOC Content in C. fimbriata-Infected Sweet Potatoes
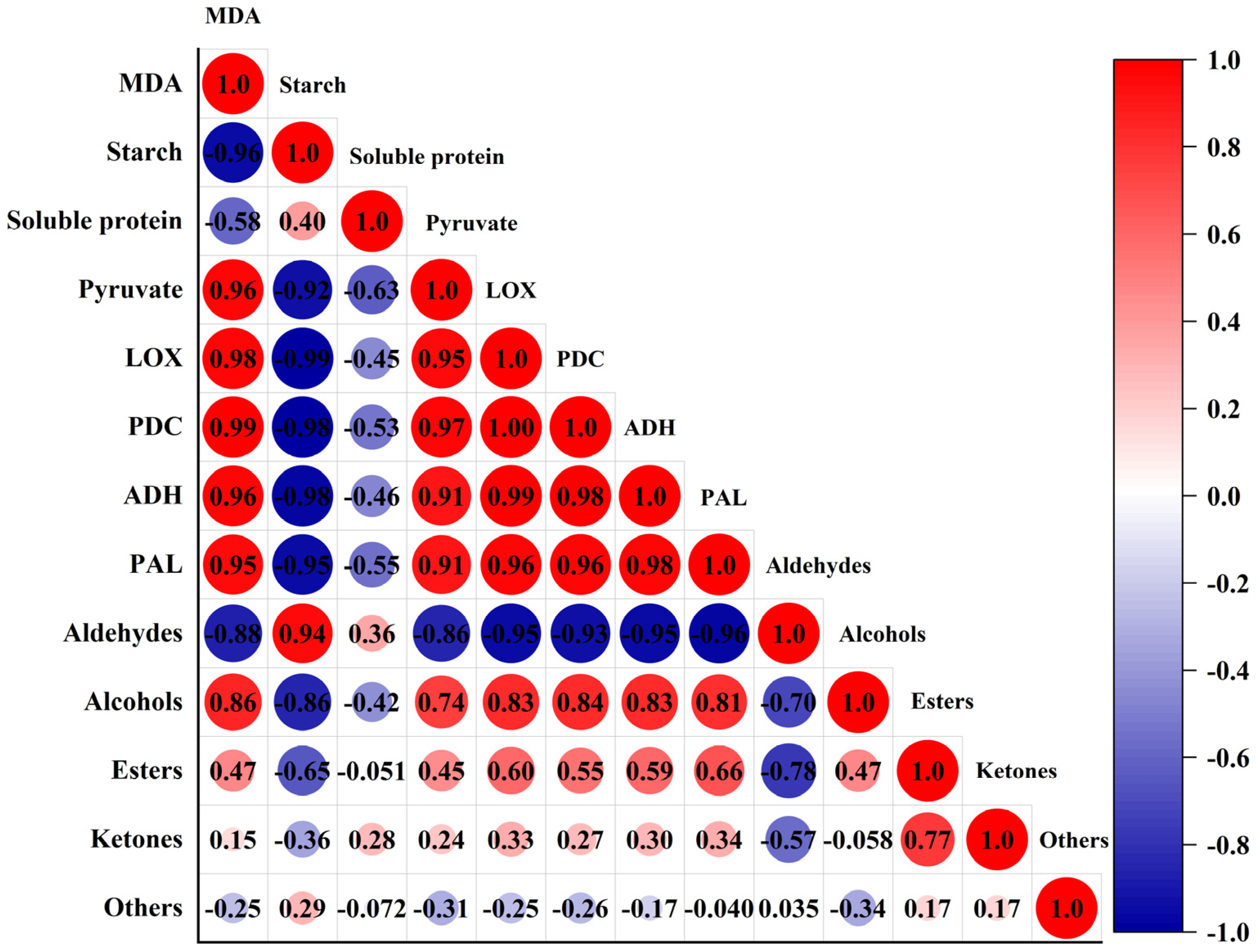
3.3. Correlation between VOCs and Physiological Indicators
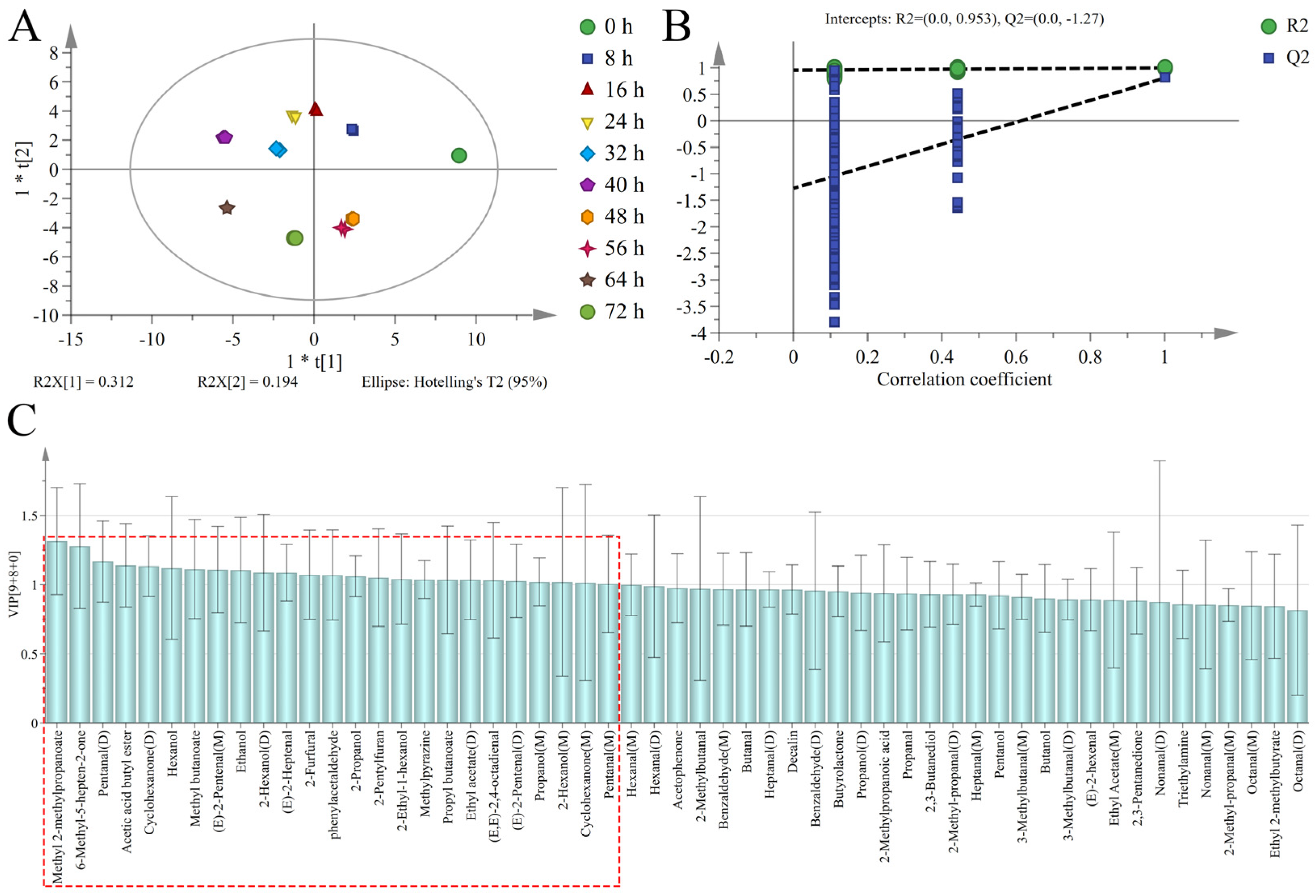
3.4. Discrimination of Sweet Potatoes with Different Infection Times Based on PCA
3.5. Discrimination of Sweet Potatoes with Different Infection Times Based on OPLS-DA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, Y.J.; Lin, L.Y.; Yang, K.M.; Chiang, Y.C.; Chen, M.H.; Chiang, P.Y. Effects of Roasting Sweet Potato (Ipomoea batatas L. Lam.): Quality, Volatile Compound Composition, and Sensory Evaluation. Foods 2021, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Liu, M.; Huang, T.G.; Yang, K.L.; Zhou, S.H.; Li, Y.X.; Tian, J. Antifungal effect of nerol via transcriptome analysis and cell growth repression in sweet potato spoilage fungi Ceratocystis fimbriata. Postharvest Biol. Technol. 2021, 171, 111343. [Google Scholar] [CrossRef]
- Inoue, H.; Ôba, K.; Ando, M.; Uritani, I. Enzymatic reduction of dehydroipomeamarone to ipomeamarone in sweet potato root tissue infected by Ceratocystis fimbriata. Physiol. Plant Pathol. 1984, 25, 1–8. [Google Scholar] [CrossRef]
- Tiwari, S.; Kate, A.; Mohapatra, D.; Tripathi, M.K.; Ray, H.; Akuli, A.; Ghosh, A.; Modhera, B. Volatile organic compounds (VOCs): Biomarkers for quality management of horticultural commodities during storage through e-sensing. Trends Food Sci. Technol. 2020, 106, 417–433. [Google Scholar] [CrossRef]
- Wu, J.; Cao, J.P.; Chen, J.B.; Huang, L.X.; Wang, Y.; Sun, C.; Sun, C.D. Detection and classification of volatile compounds emitted by three fungi-infected citrus fruit using gas chromatography-mass spectrometry. Food Chem. 2023, 412, 135524. [Google Scholar] [CrossRef]
- Radványi, D. Smelling the difference: Separation of healthy and infected button mushrooms via microbial volatile organic compounds. Heliyon 2023, 9, e12703. [Google Scholar] [CrossRef]
- Tiwari, S.; Goswami, U.; Kate, A.; Modhera, B.; Tripathi, M.K.; Mohapatra, D. Biological relevance of VOCs emanating from red onions infected with Erwinia (Pectobacterium) carotovora under different storage conditions. Postharvest Biol. Technol. 2022, 184, 111761. [Google Scholar] [CrossRef]
- Zheng, A.R.; Wei, C.K.; Liu, D.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. GC-MS and GC×GC-ToF-MS analysis of roasted/broth flavors produced by Maillard reaction system of cysteine-xylose-glutamate. Curr. Res. Food Sci. 2023, 6, 100445. [Google Scholar] [CrossRef]
- Yin, X.L.; Fu, W.J.; Chen, Y.; Zhou, R.F.; Sun, W.; Ding, B.; Peng, X.T.; Gu, H.W. GC-MS-based untargeted metabolomics reveals the key volatile organic compounds for discriminating grades of Yichang big-leaf green tea. LWT 2022, 171, 114148. [Google Scholar] [CrossRef]
- Balkan, T.; Karaağaçlı, H. Determination of 301 pesticide residues in tropical fruits imported to Turkey using LC–MS/MS and GC-MS. Food Control 2023, 147, 109576. [Google Scholar] [CrossRef]
- Oliveira, L.G.d.; Kurz, M.H.S.; Guimarães, M.C.M.; Martins, M.L.; Prestes, O.D.; Zanella, R.; Ribeiro, J.N.d.S.; Gonçalves, F.F. Development and validation of a method for the analysis of pyrethroid residues in fish using GC–MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, D.; Zanardi, S.; Dall’Asta, C.; Suman, M. Ion mobility spectrometry coupled to gas chromatography: A rapid tool to assess eggs freshness. Food Chem. 2019, 271, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.Y.; Xu, H.Y.; Jiang, X.; Sun, T.T.; Luo, Y.X.; Shi, W.Z. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res. Int. 2022, 158, 111584. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Wu, J.W.; Pang, L.J.; Zhang, X.Q.; Lu, X.H.; Yin, L.Q.; Lu, G.Q.; Cheng, J.Y. Early Discrimination and Prediction of C. fimbriata-Infected Sweetpotatoes during the Asymptomatic Period Using Electronic Nose. Foods 2022, 11, 1919. [Google Scholar] [CrossRef]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative analysis of the responses to water stress in eggplant (Solanum melongena) cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef]
- GB 5009.9-2016; National Food Safety Standard Determination of Starch in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- Chu, E.P.; Tavares, A.R.; Kanashiro, S.; Giampaoli, P.; Yokota, E.S. Effects of auxins on soluble carbohydrates, starch and soluble protein content in Aechmea blanchetiana (Bromeliaceae) cultured in vitro. Sci. Hortic. 2010, 125, 451–455. [Google Scholar] [CrossRef]
- Bacon, J.R.; Moates, G.K.; Ng, A.; Rhodes, M.J.C.; Smith, A.C.; Waldron, K.W. Quantitative analysis of flavour precursors and pyruvate levels in different tissues and cultivars of onion (Allium cepa). Food Chem. 1999, 64, 257–261. [Google Scholar] [CrossRef]
- Cai, H.F.; An, X.J.; Han, S.; Jiang, L.; Yu, M.L.; Ma, R.J.; Yu, Z.F. Effect of 1-MCP on the production of volatiles and biosynthesis-related gene expression in peach fruit during cold storage. Postharvest Biol. Technol. 2018, 141, 50–57. [Google Scholar] [CrossRef]
- Zuo, X.X.; Cao, S.F.; Ji, N.N.; Li, Y.F.; Zhang, J.L.; Jin, P.; Zheng, Y.H. High relative humidity enhances chilling tolerance of zucchini fruit by regulating sugar and ethanol metabolisms during cold storage. Postharvest Biol. Technol. 2022, 189, 111932. [Google Scholar] [CrossRef]
- Zhao, R.J.; Huo, C.Y.; Qian, Y.; Ren, D.F.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Yao, L.Y.; Sun, J.Y.; Liang, Y.; Feng, T.; Wang, H.T.; Sun, M.; Yu, W.G. Volatile fingerprints of Torreya grandis hydrosols under different downstream processes using HS-GC–IMS and the enhanced stability and bioactivity of hydrosols by high pressure homogenization. Food Control 2022, 139, 109058. [Google Scholar] [CrossRef]
- Koç, E.; Üstün, A.S.; İşlek, C.; Arıcı, Y.K. Defence responses in leaves of resistant and susceptible pepper (Capsicum annuum L.) cultivars infected with different inoculum concentrations of Phytophthora capsici Leon. Sci. Hortic. 2011, 128, 434–442. [Google Scholar] [CrossRef]
- Xu, F.X.; Lu, F.; Xiao, Z.G.; Li, Z. Influence of drop shock on physiological responses and genes expression of apple fruit. Food Chem. 2020, 303, 125424. [Google Scholar] [CrossRef]
- Wei, F.L.; Ma, N.N.; Haseeb, H.A.; Gao, M.X.; Liu, X.X.; Guo, W. Insights into structural and physicochemical properties of maize starch after Fusarium verticillioides infection. J. Food Compos. Anal. 2022, 114, 104819. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Li, Y.; Qi, H.Y.; Jin, Y.Z.; Tian, X.B.; Sui, L.L.; Qiu, Y. Role of ethylene in biosynthetic pathway of related-aroma volatiles derived from amino acids in oriental sweet melons (Cucumis melo var. makuwa Makino). Sci. Hortic. 2016, 201, 24–35. [Google Scholar] [CrossRef]
- Boeckx, J.; Hertog, M.; Geeraerd, A.; Nicolaï, B. Regulation of the fermentative metabolism in apple fruit exposed to low-oxygen stress reveals a high flexibility. Postharvest Biol. Technol. 2019, 149, 118–128. [Google Scholar] [CrossRef]
- Anthony, K.K.; George, D.S.; Baldev Singh, H.K.; Fung, S.M.; Santhirasegaram, V.; Razali, Z.; Somasundram, C. Reactive Oxygen Species Activity and Antioxidant Properties of Fusarium Infected Bananas. J. Phytopathol. 2017, 165, 213–222. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.Q.; Xiao, H.; Mariga, A.M.; Chen, Y.P.; Zhang, X.C.; Wang, L.; Li, D.; Li, L.; Luo, Z.S. Rethinking of botanical volatile organic compounds applied in food preservation: Challenges in acquisition, application, microbial inhibition and stimulation. Trends Food Sci. Technol. 2022, 125, 166–184. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhao, A.C.; Morcillo, R.J.L.; Yu, G.; Xue, H.; Rufian, J.S.; Sang, Y.Y.; Macho, A.P. A bacterial effector protein uncovers a plant metabolic pathway involved in tolerance to bacterial wilt disease. Mol. Plant 2021, 14, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Mu, Y.H.; Xie, S.; Meng, D.H.; Zheng, Y.Q.; Meng, X.S.; Lv, Z.L. Impact of UHT processing on volatile components and chemical composition of sea buckthorn (Hippophae rhamnoides) pulp: A prediction of the biochemical pathway underlying aroma compound formation. Food Chem. 2022, 390, 133142. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Bi, Y.; Zong, Y.Y.; Li, Y.C.; Sionov, E.; Prusky, D. Characterization and sources of volatile organic compounds produced by postharvest pathogenic fungi colonized fruit. Postharvest Biol. Technol. 2022, 188, 111903. [Google Scholar] [CrossRef]
- Bakhtawar, F.; Wang, X.K.; Manan, A.; Iftikhar, Y.; Atta, S.; Bashir, M.A.; Mubeen, M.; Sajid, A.; Hannan, A.; Hashem, M.; et al. Biochemical characterization of citrus bent leaf viroid infecting citrus cultivars. J. King Saud Univ.-Sci. 2022, 34, 101733. [Google Scholar] [CrossRef]
- Gonda, I.; Davidovich-Rikanati, R.; Bar, E.; Lev, S.; Jhirad, P.; Meshulam, Y.; Wissotsky, G.; Portnoy, V.; Burger, J.; Schaffer, A.A.; et al. Differential metabolism of L–phenylalanine in the formation of aromatic volatiles in melon (Cucumis melo L.) fruit. Phytochemistry 2018, 148, 122–131. [Google Scholar] [CrossRef]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Zhu, Z.S.; Lei, Y.; Huang, S.H.; Huang, M. Effect of ageing time on the flavour compounds in Nanjing water-boiled salted duck detected by HS-GC-IMS. LWT 2022, 155, 112870. [Google Scholar] [CrossRef]
- Xuan, X.T.; Sun, R.Y.; Zhang, X.Y.; Cui, Y.; Lin, X.D.; Sun, Y.; Deng, W.Y.; Liao, X.J.; Ling, J.G. Novel application of HS-GC-IMS with PCA for characteristic fingerprints and flavor compound variations in NFC Chinese bayberry (Myrica rubra) juice during storage. LWT 2022, 167, 113882. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.Q.; Song, S.Q.; Wang, H.T.; Yao, L.Y.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Griffith, R.; Hammond, E.G. Generation of Swiss Cheese Flavor Components by the Reaction of Amino Acids with Carbonyl Compounds1. J. Dairy Sci. 1989, 72, 604–613. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Li, Y.C.; Zong, Y.Y.; Han, Y.; Prusky, D. Both Penicillium expansum and Trichothecim roseum Infections Promote the Ripening of Apples and Release Specific Volatile Compounds. Front. Plant Sci. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Mayuoni-Kirshinbaum, L.; Daus, A.; Porat, R. Changes in sensory quality and aroma volatile composition during prolonged storage of ‘Wonderful’ pomegranate fruit. Int. J. Food Sci. Technol. 2013, 48, 1569–1578. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Guo, M.R.; Feng, J.Z.; Zhang, P.Y.; Jia, L.Y.; Chen, K.S. Postharvest treatment with trans-2-hexenal induced resistance against Botrytis cinerea in tomato fruit. Australas. Plant Pathol. 2015, 44, 121–128. [Google Scholar] [CrossRef]
- Günther, C.S.; Marsh, K.B.; Winz, R.A.; Harker, R.F.; Wohlers, M.W.; White, A.; Goddard, M.R. The impact of cold storage and ethylene on volatile ester production and aroma perception in ‘Hort16A’ kiwifruit. Food Chem. 2015, 169, 5–12. [Google Scholar] [CrossRef]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Perego, P.; Fabiano, B.; Cavicchioli, M.; Del Borghi, M. Cocoa Quality and Processing: A Study by Solid-phase Microextraction and Gas Chromatography Analysis of Methylpyrazines. Food Bioprod. Process. 2004, 82, 291–297. [Google Scholar] [CrossRef]
- Song, J.X.; Shao, Y.; Yan, Y.M.; Li, X.H.; Peng, J.; Guo, L. Characterization of volatile profiles of three colored quinoas based on GC-IMS and PCA. LWT 2021, 146, 111292. [Google Scholar] [CrossRef]
- Lörchner, C.; Horn, M.; Berger, F.; Fauhl-Hassek, C.; Glomb, M.A.; Esslinger, S. Quality control of spectroscopic data in non-targeted analysis—Development of a multivariate control chart. Food Control 2022, 133, 108601. [Google Scholar] [CrossRef]
- Kang, C.D.; Zhang, Y.Y.; Zhang, M.Y.; Qi, J.; Zhao, W.T.; Gu, J.; Guo, W.P.; Li, Y.Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Wang, H.R.; Shi, X.; Chen, H.; Suo, R. Goat milk authentication based on amino acid ratio and chemometric analysis. J. Food Compos. Anal. 2022, 111, 104636. [Google Scholar] [CrossRef]
- He, F.; Duan, J.W.; Zhao, J.W.; Li, H.H.; Sun, J.Y.; Huang, M.Q.; Sun, B.G. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. 2021, 365, 130430. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Wu, W.D.; Tang, X.Y.; Ge, Q.Q.; Zhan, J.L. Screening out important substances for distinguishing Chinese indigenous pork and hybrid pork and identifying different pork muscles by analyzing the fatty acid and nucleotide contents. Food Chem. 2021, 350, 129219. [Google Scholar] [CrossRef]

| No. | Compounds | CAS | Signal Intensity (mV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 8 h | 16 h | 24 h | 32 h | 40 h | 48 h | 56 h | 64 h | 72 h | |||
| Aldehydes (26) | ||||||||||||
| 1 | Propanal | C123386 | 3835.6 ± 166.6 a | 3812.1 ± 38.7 ab | 3806.3 ± 0.9 ab | 3737.3 ± 15.5 ab | 3657.1 ± 25.8 bc | 3531.4 ± 18.6 cd | 3760.4 ± 48.2 ab | 3751.9 ± 5.4 ab | 3451.4 ± 15.4 d | 3696.7 ± 89.5 ab |
| 2 | 2-Methyl-propanal (M) | C78842 | 1327.8 ± 26.9 h | 1654.8 ± 15.8 f | 1727.6 ± 19.4 e | 1895.5 ± 29.8 c | 1839.6 ± 17.6 d | 2020.1 ± 16.8 b | 1510.1 ± 2.9 g | 1516.6 ± 33.1 g | 2085.9 ± 8.1 a | 1839.2 ± 25.0 d |
| 3 | 2-Methyl-propanal (D) | C78842 | 62.5 ± 7.7 f | 114.8 ± 9.9 d | 157.1 ± 6.4 c | 188.1 ± 1.4 b | 155.9 ± 0.1 c | 261.5 ± 6.0 a | 102.7 ± 13.5 de | 90.7 ± 0.7 e | 192.4 ± 7.6 b | 163.6 ± 0.2 c |
| 4 | Butanal | C123728 | 161.4 ± 12.1 e | 190.5 ± 2.0 d | 210.2 ± 6.7 bc | 214.0 ± 0.2 ab | 207.5 ± 2.5 bc | 225.6 ± 3.2 a | 196.7 ± 10.7 cd | 198.2 ± 2.9 cd | 162.7 ± 0.8 e | 171.1 ± 1.2 e |
| 5 | 3-Methylbutanal (M) | C590863 | 2529.7 ± 106.5 h | 3876.5 ± 5.7 d | 4355.2 ± 2.2 b | 4539.5 ± 22.8 a | 4038.0 ± 21.2 c | 4540.5 ± 10.5 a | 3071.0 ± 12.6 f | 2716.3 ± 16.5 g | 4050.5 ± 24.1 c | 3616.3 ± 40.1 e |
| 6 | 3-Methylbutanal (D) | C590863 | 1320.6 ± 53.8 a | 976.9 ± 36.6 c | 874.5 ± 3.0 d | 805.7 ± 14.2 e | 991.5 ± 8.3 c | 861.3 ± 1.5 de | 1180.6 ± 29.8 b | 1161.7 ± 17.1 b | 878.8 ± 2.2 d | 967.1 ± 35.0 c |
| 7 | 2-Methylbutanal | C96173 | 255.4 ± 5.2 ab | 234.5 ± 6.4 bc | 226.6 ± 14.0 bc | 208.2 ± 6.5 c | 238.1 ± 0.1 abc | 234.2 ± 2.4 bc | 254.3 ± 24.7 ab | 266.8 ± 25.9 a | 255.5 ± 4.9 ab | 240.1 ± 4.8 ab |
| 8 | Pentanal (M) | C110623 | 2741.6 ± 17.8 c | 2877.0 ± 69.0 a | 2956.1 ± 35.6 a | 2730.3 ± 5.1 c | 2766.8 ± 36.9 bc | 2866.0 ± 7.7 ab | 2582.5 ± 61.2 e | 2700.9 ± 41.4 cd | 2726.4 ± 10.7 c | 2599.3 ± 94.8 de |
| 9 | Pentanal (D) | C110623 | 452.8 ± 24.7 e | 483.7 ± 6.3 d | 509.0 ± 8.3 c | 511.1 ± 2.7 c | 542.1 ± 2.4 a | 533.6 ± 1.3 ab | 470.9 ± 12.9 de | 484.0 ± 3.1 d | 481.2 ± 1.0 d | 512.5 ± 2.3 bc |
| 10 | (E)-2-Pentenal (M) | C1576870 | 86.6 ± 0.4 bc | 76.0 ± 3.6 c | 76.6 ± 3.6 c | 86.6 ± 5.4 bc | 100.1 ± 2.8 ab | 89.4 ± 3.4 bc | 89.2 ± 12.3 bc | 81.5 ± 13.3 c | 106.0 ± 5.8 a | 83.0 ± 2.9 c |
| 11 | (E)-2-Pentenal (D) | C1576870 | 69.2 ± 13.1 de | 73.7 ± 0.6 cd | 84.7 ± 5.6 bc | 128.6 ± 1.9 a | 93.3 ± 0.2 b | 70.4 ± 0.2 de | 73.8 ± 2.2 cd | 62.6 ± 11.0 de | 68.3 ± 0.4 de | 57.0 ± 2.6 e |
| 12 | Hexanal (M) | C66251 | 3987.7 ± 205.8 cd | 4227.9 ± 1.3 ab | 4231.8 ± 101.6 ab | 4061.1 ± 33.1 bc | 4376.5 ± 70.9 a | 4331.9 ± 29.5 a | 3830.0 ± 48.6 de | 3809.2 ± 93.4 de | 3846.9 ± 2.5 cde | 3639.8 ± 124.9 e |
| 13 | Hexanal (D) | C66251 | 2382.2 ± 3.4 b | 2394.0 ± 22.5 b | 2465.3 ± 38.9 b | 2386.3 ± 69.5 b | 2441.2 ± 26.5 b | 2610.7 ± 21.8 a | 2390.7 ± 44.6 b | 2377.1 ± 3.4 b | 2454.9 ± 4.7 b | 2381.9 ± 138.1 b |
| 14 | 2-Furfural | C98011 | 376.5 ± 12.1 b | 307.8 ± 18.7 cd | 288.7 ± 2.9 d | 433.5 ± 12.2 a | 274.2 ± 24.5 de | 246.2 ± 1.7 ef | 326.6 ± 0.6 c | 330.2 ± 28.1 c | 217.5 ± 14.3 f | 326.6 ± 13.0 c |
| 15 | (E)-2-Hexenal | C6728263 | 733.0 ± 50.5 a | 728.2 ± 11.7 ab | 721.6 ± 23.5 ab | 668.0 ± 5.9 cd | 678.1 ± 22.2 bc | 641.8 ± 10.0 cde | 644.9 ± 21.2 cde | 647.5 ± 15.0 cde | 613.8 ± 4.7 de | 599.2 ± 23.7 e |
| 16 | Heptanal (M) | C111717 | 1461.3 ± 147.3 a | 1351.1 ± 16.2 ab | 1251.2 ± 68.7 bcd | 1229.5 ± 14.6 bcd | 1218.2 ± 18.3 bcd | 1158.5 ± 6.2 cd | 1266.8 ± 48.0 bc | 1130.2 ± 12.7 d | 992.3 ± 7.1 e | 997.2 ± 30.7 e |
| 17 | Heptanal (D) | C111717 | 265.9 ± 45.1 a | 242.4 ± 4.8 ab | 214.5 ± 23.4 bc | 200.7 ± 7.5 bc | 203.0 ± 15.2 bc | 191.9 ± 7.2 cd | 211.4 ± 14.4 bc | 170.9 ± 1.0 cd | 156.3 ± 9.2 d | 152.9 ± 12.2 d |
| 18 | (E)-2-Heptenal | C18829555 | 232.9 ± 5.7 ef | 210.4 ± 7.7 f | 263.1 ± 27.6 cd | 259.4 ± 1.9 cd | 316.1 ± 7.0 a | 267.0 ± 2.6 c | 304.7 ± 4.6 a | 295.9 ± 13.2 ab | 276.8 ± 9.0 bc | 239.8 ± 2.3 de |
| 19 | Benzaldehyde (M) | C100527 | 325.5 ± 41.6 a | 323.9 ± 14.0 a | 327.9 ± 3.5 a | 339.8 ± 3.1 a | 332.9 ± 7.9 a | 317.1 ± 3.0 a | 316.6 ± 15.4 a | 322.1 ± 18.5 a | 341.1 ± 1.6 a | 320.5 ± 1.5 a |
| 20 | Benzaldehyde (D) | C100527 | 1195.7 ± 208.4 a | 967.5 ± 12.9 bc | 969.0 ± 54.5 bc | 996.3 ± 24.3 b | 903.1 ± 1.6 bc | 938.4 ± 32.1 bc | 843.9 ± 35.0 bc | 803.9 ± 23.6 c | 801.8 ± 4.9 c | 804.2 ± 39.9 c |
| 21 | Octanal (M) | C124130 | 1087.2 ± 209.0 a | 750.5 ± 5.7 b | 752.2 ± 44.7 b | 673.2 ± 14.2 bc | 624.0 ± 29.1 bcd | 498.1 ± 26.7 d | 701.7 ± 28.6 bc | 643.8 ± 24.6 bcd | 490.4 ± 10.4 d | 534.7 ± 9.1 cd |
| 22 | Octanal (D) | C124130 | 139.0 ± 38.3 a | 100.5 ± 12.9 b | 93.1 ± 1.3 b | 79.4 ± 1.8 b | 76.0 ± 3.3 b | 64.0 ± 4.2 b | 97.3 ± 11.5 b | 92.1 ± 7.8 b | 76.0 ± 13.4 b | 91.8 ± 11.9 b |
| 23 | Phenylacetaldehyde | C122781 | 507.4 ± 16.0 de | 495.3 ± 16.3 e | 535.5 ± 16.2 cde | 546.7 ± 6.3 bcd | 562.4 ± 7.9 abc | 581.5 ± 24.6 ab | 584.5 ± 16.4 ab | 590.7 ± 26.6 a | 526.7 ± 1.5 cde | 497.7 ± 17.2 e |
| 24 | Nonanal (M) | C124196 | 3416.4 ± 805.3 a | 2360.4 ± 29.6 b | 2437.9 ± 121.9 b | 2011.8 ± 107.7 bcd | 1831.6 ± 8.9 bcd | 1530.5 ± 55.2 d | 2196.4 ± 135.3 bc | 2019.8 ± 59.5 bcd | 1693.9 ± 28.3 cd | 1659.5 ± 70.5 cd |
| 25 | Nonanal (D) | C124196 | 605.6 ± 293.1 a | 316.4 ± 41.7 b | 302.6 ± 36.9 b | 254.6 ± 16.9 b | 239.9 ± 19.5 b | 194.1 ± 22.7 b | 279.3 ± 15.5 b | 250.4 ± 19.1 b | 214.2 ± 8.2 b | 203.8 ± 8.7 b |
| 26 | (E,E)-2,4-Octadienal | C30361285 | 173.1 ± 57.8 e | 205.6 ± 30.0 e | 282.6 ± 55.0 d | 323.7 ± 9.5 bcd | 361.6 ± 8.2 abc | 278.0 ± 3.3 d | 412.3 ± 9.9 a | 375.4 ± 2.9 ab | 355.5 ± 12.2 abc | 300.3 ± 11.6 cd |
| Alcohols (11) | ||||||||||||
| 27 | Ethanol | C64175 | 1104.3 ± 164.5 ab | 1061.1 ± 9.0 b | 972.9 ± 20.6 b | 978.0 ± 19.3 b | 1015.2 ± 34.9 b | 1116.3 ± 23.7 ab | 989.2 ± 46.5 b | 972.1 ± 40.7 b | 1218.7 ± 3.6 a | 980.6 ± 86.3 b |
| 28 | 2-Propanol | C67630 | 100.3 ± 14.7 bcd | 86.7 ± 0.9 de | 93.7 ± 1.0 bcd | 88.9 ± 1.4 cde | 91.4 ± 2.4 cde | 108.3 ± 4.6 b | 127.0 ± 3.0 a | 121.4 ± 10.3 a | 76.9 ± 3.6 e | 102.9 ± 4.3 bc |
| 29 | Propanol (M) | C71238 | 1573.1 ± 7.9 cd | 1563.6 ± 65.3 cd | 1492.6 ± 8.4 d | 1627.2 ± 0.9 bc | 1520.5 ± 6.3 cd | 1555.9 ± 35.3 cd | 1623.3 ± 87.2 bc | 1559.0 ± 64.0 cd | 1709.3 ± 21.7 ab | 1793.9 ± 54.5 a |
| 30 | Propanol (D) | C71238 | 202.1 ± 26.0 e | 250.4 ± 3.3 bc | 273.6 ± 16.7 b | 305.4 ± 2.7 a | 255.9 ± 1.2 bc | 307.7 ± 9.6 a | 222.6 ± 10.7 de | 217.6 ± 11.8 de | 238.5 ± 3.0 cd | 253.1 ± 3.9 bc |
| 31 | Butanol | C71363 | 498.8 ± 24.1 de | 545.1 ± 5.8 bc | 543.3 ± 15.1 bc | 526.1 ± 5.0 cd | 548.3 ± 1.9 bc | 563.4 ± 7.3 ab | 488.1 ± 13.2 e | 502.4 ± 13.5 de | 584.5 ± 10.4 a | 527.6 ± 20.9 cd |
| 32 | Pentanol | C71410 | 205.1 ± 12.9 de | 205.9 ± 9.7 de | 188.2 ± 0.4 e | 193.2 ± 1.7 e | 233.4 ± 2.1 bc | 218.6 ± 6.2 cd | 250.1 ± 10.1 b | 269.2 ± 2.3 a | 249.8 ± 8.5 b | 277.4 ± 11.9 a |
| 33 | 2,3-Butanediol | C513859 | 319.5 ± 15.5 ab | 330.8 ± 1.5 a | 336.1 ± 26.4 a | 327.9 ± 1.9 a | 292.9 ± 3.7 bc | 288.5 ± 3.1 bc | 287.9 ± 16.3 bc | 294.8 ± 22.8 bc | 261.4 ± 3.1 c | 265.0 ± 12.5 c |
| 34 | 2-Hexanol (M) | C626937 | 283.2 ± 95.1 a | 205.0 ± 36.6 ab | 233.3 ± 76.1 ab | 176.1 ± 18.8 ab | 174.7 ± 1.2 ab | 235.8 ± 12.0 ab | 207.8 ± 29.1 ab | 220.2 ± 14.4 ab | 186.9 ± 37.0 ab | 172.6 ± 10.0 b |
| 35 | 2-Hexanol (D) | C626937 | 58.8 ± 3.8 ab | 56.8 ± 3.9 abc | 48.7 ± 4.6 cd | 52.9 ± 2.5 bc | 57.1 ± 2.8 abc | 49.8 ± 0.8 c | 61.3 ± 5.9 a | 56.3 ± 2.0 abc | 41.2 ± 2.3 d | 53.1 ± 2.2 abc |
| 36 | Hexanol | C111273 | 117.6 ± 10.1 a | 151.3 ± 2.4 a | 120.8 ± 5.4 a | 123.6 ± 7.9 a | 119.0 ± 7.7 a | 137.9 ± 8.8 a | 128.1 ± 12.0 a | 144.0 ± 35.3 a | 144.3 ± 23.5 a | 136.8 ± 40.3 a |
| 37 | 2-Ethyl-1-hexanol | C104767 | 429.6 ± 86.4 bcd | 337.2 ± 9.1 e | 330.5 ± 17.5 e | 355.6 ± 5.5 e | 379.2 ± 3.0 de | 392.9 ± 4.9 cde | 454.1 ± 16.3 bc | 484.9 ± 7.9 ab | 387.5 ± 24.8 cde | 545.7 ± 12.8 a |
| Esters (8) | ||||||||||||
| 38 | Ethyl acetate (M) | C141786 | 1402.8 ± 61.0 a | 1339.7 ± 1.8 b | 1296.5 ± 15.8 bc | 1251.5 ± 13.6 cd | 1242.6 ± 0.2 cd | 1249.7 ± 0.2 cd | 1280.7 ± 4.0 cd | 1266.5 ± 26.1 cd | 1230.3 ± 4.3 d | 1290.4 ± 14.4 bc |
| 39 | Ethyl acetate (D) | C141786 | 3411.4 ± 66.4 e | 3607.1 ± 26.8 bc | 3231.7 ± 87.3 f | 3560.4 ± 12.1 cd | 3485.5 ± 3.8 de | 3710.7 ± 13.5 b | 4048.2 ± 11.0 a | 4066.4 ± 50.1 a | 3663.3 ± 45.4 bc | 4047.0 ± 48.5 a |
| 40 | Methyl 2-methylpropanoate | C547637 | 384.8 ± 0.4 e | 378.9 ± 2.3 e | 384.2 ± 3.4 e | 431.2 ± 14.7 c | 412.3 ± 5.5 d | 447.9 ± 1.2 ab | 457.6 ± 6.1 a | 382.8 ± 5.9 e | 436.8 ± 6.9 bc | 401.4 ± 5.2 d |
| 41 | Methyl butanoate | C623427 | 43.0 ± 1.3 abc | 35.9 ± 0.1 cde | 37.1 ± 5.0 bcde | 44.1 ± 0.4 ab | 41.0 ± 4.9 abcd | 47.1 ± 1.2 a | 40.0 ± 4.8 abcd | 34.3 ± 3.3 de | 32.5 ± 0.7 e | 34.0 ± 1.9 de |
| 42 | Acetic acid butyl ester | C123864 | 80.2 ± 1.9 bc | 87.1 ± 6.0 abc | 75.7 ± 5.3 bc | 72.1 ± 1.9 c | 91.3 ± 0.8 ab | 101.3 ± 2.5 a | 77.9 ± 6.2 bc | 81.2 ± 16.2 bc | 75.7 ± 1.3 bc | 70.8 ± 9.6 c |
| 43 | Ethyl 2-methylbutyrate | C7452791 | 218.9 ± 40.7 a | 169.7 ± 5.9 bc | 170.5 ± 13.7 bc | 146.8 ± 6.3 bcde | 158.7 ± 3.0 bcde | 132.6 ± 8.7 de | 178.4 ± 2.5 b | 167.4 ± 4.6 bcd | 131.1 ± 1.8 e | 140.3 ± 9.4 cde |
| 44 | Propyl butanoate | C105668 | 450.9 ± 72.8 a | 533.2 ± 9.7 a | 510.8 ± 41.7 a | 511.8 ± 29.9 a | 482.1 ± 19.2 a | 54.1 ± 16.9 a | 498.9 ± 4.9 a | 509.4 ± 63.2 a | 478.8 ± 25.5 a | 478.8 ± 72.3 a |
| 45 | Butyrolactone | C96480 | 233.0 ± 34.9 ab | 222.9 ± 0.3 abc | 217.5 ± 24.0 abc | 253.2 ± 2.8 a | 190.5 ± 0.1 cd | 188.2 ± 30.6 cd | 215.8 ± 0.2 abcd | 207.0 ± 2.6 bcd | 175.4 ± 1.7 d | 204.0 ± 0.1 bcd |
| Ketones (5) | ||||||||||||
| 46 | 2,3-Pentanedione | C600146 | 1837.3 ± 89.4 a | 1587.4 ± 109.7 cd | 1499.3 ± 34.2 de | 1379.5 ± 8.8 e | 1555.1 ± 8.0 cd | 1512.7 ± 0.9 de | 1764.8 ± 69.9 ab | 1789.7 ± 100.2 ab | 1526.3 ± 10.7 de | 1678.9 ± 53.2 bc |
| 47 | Cyclohexanone (M) | C108941 | 93.4 ± 4.5 ab | 90.7 ± 2.0 b | 95.0 ± 6.6 ab | 97.7 ± 0.5 ab | 98.9 ± 7.6 ab | 104.2 ± 6.4 a | 94.4 ± 2.3 ab | 96.8 ± 5.6 ab | 99.8 ± 4.0 ab | 93.6 ± 1.4 ab |
| 48 | Cyclohexanone (D) | C108941 | 428.0 ± 18.5 cd | 459.4 ± 9.7 bc | 471.5 ± 6.6 b | 513.0 ± 3.2 a | 513.2 ± 6.9 a | 485.1 ± 17.9 ab | 463.5 ± 16.2 b | 518.0 ± 18.2 a | 418.5 ± 10.6 d | 454.5 ± 24.7 bc |
| 49 | 6-Methyl-5-hepten-2-one | C110930 | 580.6 ± 18.8 d | 603.9 ± 5.9 cd | 536.6 ± 21.0 e | 652.3 ± 4.1 a | 639.9 ± 5.0 ab | 608.1 ± 11.5 cd | 596.8 ± 3.5 cd | 615.2 ± 17.0 bc | 541.4 ± 1.5 e | 600.9 ± 17.0 cd |
| 50 | Acetophenone | C98862 | 341.5 ± 10.1 ab | 333.9 ± 10.6 ab | 305.9 ± 9.4 cde | 344.0 ± 9.5 a | 328.7 ± 7.9 abc | 300.1 ± 19.7 de | 335.2 ± 11.5 ab | 334.4 ± 7.3 ab | 284.8 ± 13.7 e | 313.4 ± 12.0 bcd |
| Others (5) | ||||||||||||
| 51 | Triethylamine | C121448 | 490.5 ± 22.9 a | 425.3 ± 15.3 b | 416.0 ± 7.8 bcd | 387.4 ± 7.4 de | 404.9 ± 11.6 bcd | 368.1 ± 6.1 e | 422.9 ± 1.6 bc | 420.6 ± 22.7 bc | 392.1 ± 1.9 cde | 406.2 ± 4.9 bcd |
| 52 | 2-Methylpropanoic acid | C79312 | 171.1 ± 21.9 abcd | 166.3 ± 8.3 bcd | 181.9 ± 11.3 abc | 199.4 ± 11. 6 a | 191.7 ± 11.3 ab | 183.9 ± 4.2 abc | 184.4 ± 11.7 abc | 166.5 ± 13.8 bcd | 156.7 ± 1.0 cd | 146.1 ± 7.7 d |
| 53 | Methylpyrazine | C109080 | 696.5 ± 23.0 a | 542.5 ± 46.1 c | 470.7 ± 10.6 d | 646.6 ± 2.7 b | 456.2 ± 22.4 d | 437.9 ± 5.8 d | 652.2 ± 24.2 ab | 644.5 ± 17.3 b | 443.8 ± 6.0 d | 556.5 ± 2.2 c |
| 54 | 2-Pentylfuran | C3777693 | 186.5 ± 24.9 bcd | 178.6 ± 3.0 cd | 192.2 ± 16.9 bc | 194.9 ± 6.4 bc | 215.6 ± 4.0 ab | 187.4 ± 7.9 bcd | 225.6 ± 2.1 a | 214.8 ± 6.0 ab | 183.8 ± 18.0 cd | 161.0 ± 13.1 d |
| 55 | Decalin | C91178 | 271.9 ± 109.0 bc | 331.3 ± 6.0 b | 319.1 ± 30.4 b | 321.1 ± 8.8 b | 280.4 ± 12.2 bc | 309.8 ± 26.1 b | 213.5 ± 17.0 c | 189.8 ± 1.3 c | 418.7 ± 14.7 a | 256.4 ± 26.6 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Wu, J.; Liu, Z.; Zhang, X.; Lu, X.; Yin, L.; Lu, G.; Pang, L. Identifying Early-Stage Changes in Volatile Organic Compounds of Ceratocystis fimbriata Ellis & Halsted-Infected Sweet Potatoes (Ipomoea batatas L. Lam) Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Foods 2023, 12, 2224. https://doi.org/10.3390/foods12112224
Cheng J, Wu J, Liu Z, Zhang X, Lu X, Yin L, Lu G, Pang L. Identifying Early-Stage Changes in Volatile Organic Compounds of Ceratocystis fimbriata Ellis & Halsted-Infected Sweet Potatoes (Ipomoea batatas L. Lam) Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Foods. 2023; 12(11):2224. https://doi.org/10.3390/foods12112224
Chicago/Turabian StyleCheng, Jiyu, Jiawen Wu, Zhihao Liu, Xiaoqiong Zhang, Xinghua Lu, Liqing Yin, Guoquan Lu, and Linjiang Pang. 2023. "Identifying Early-Stage Changes in Volatile Organic Compounds of Ceratocystis fimbriata Ellis & Halsted-Infected Sweet Potatoes (Ipomoea batatas L. Lam) Using Headspace Gas Chromatography-Ion Mobility Spectrometry" Foods 12, no. 11: 2224. https://doi.org/10.3390/foods12112224
APA StyleCheng, J., Wu, J., Liu, Z., Zhang, X., Lu, X., Yin, L., Lu, G., & Pang, L. (2023). Identifying Early-Stage Changes in Volatile Organic Compounds of Ceratocystis fimbriata Ellis & Halsted-Infected Sweet Potatoes (Ipomoea batatas L. Lam) Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Foods, 12(11), 2224. https://doi.org/10.3390/foods12112224






