A Method to Directly Identify Cronobacter sakazakii in Liquid Medium by MALDI-TOF MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Cultivation Conditions
2.3. Direct Identification of C. sakazakii from Liquid Culture Media Pretreatment Method Process by MALDI-TOF MS
2.4. Single-Factor Test
2.5. Response Surface Methodology
2.6. Evaluation of the Accuracy of Identification of the MALDI-TOF MS Liquid Spotting Pretreatment Method
2.7. Comparison of Solid and Liquid Spotting Methods
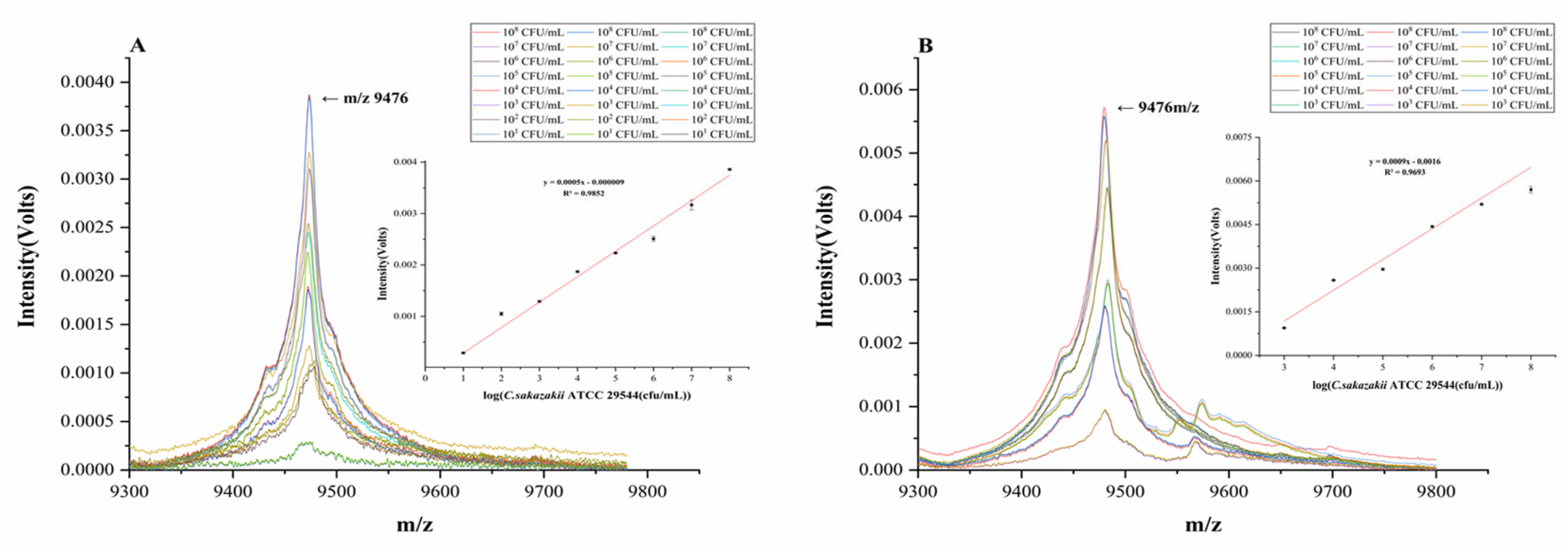
2.8. Direct Detection and Analysis of the Linear Relationship between the Cell Number of C. sakazakii in Environmental Samples
2.9. Direct Detection and Analysis of the Linear Relationship between the Cell Number of C. sakazakii in PIF Samples
3. Results and Discussion
3.1. Effects of Single Factors on the Identification Score of C. sakazakii from Liquid Culture Media by MALDI-TOF MS
3.2. Optimization of Direct Identification of C. sakazakii from Liquid Culture Media by MALDI-TOF MS by RSM
3.3. Statistical Analysis and Modeling of Direct Identification of C. sakazakii from Liquid Culture Media by MALDI-TOF MS
3.4. Analysis of Response Surface and Two-Dimensional Contour Plots
3.5. Optimization of Pretreatment Parameters and Validation of the Optimized Conditions
3.6. Evaluation of the Accuracy of Identification Using the Liquid Spotting Pretreatment Method
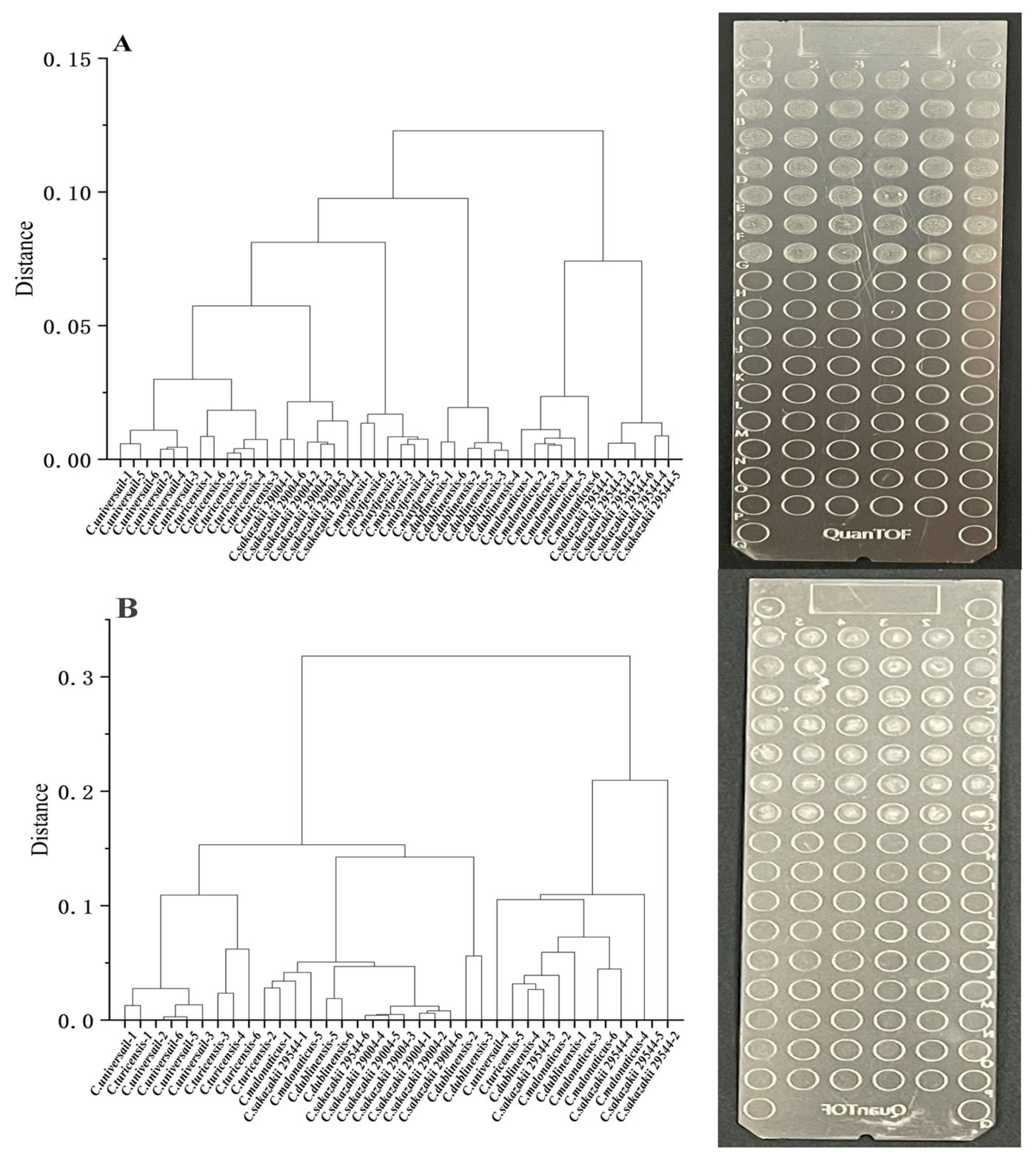
3.7. Comparison of Solid Spotting/Liquid Spotting Methods
3.8. Identification of the Analysis Relationship between the Cell Number of C. sakazakii and Peak Intensities Using Environmental and PIF Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Liu, G.; Wu, Y.; Pang, X.; Wu, Y.; Niu, J.; Chen, Q.; Zhang, X. Transposon sequencing: A powerful tool for the functional genomic study of food-borne pathogens. Trends Food Sci. Technol. 2021, 118, 679–687. [Google Scholar] [CrossRef]
- Hong, H.; Sun, C.; Wei, S.; Sun, X.; Mutukumira, A.; Wu, X. Development of a real-time recombinase polymerase amplification assay for rapid detection of Salmonella in powdered infant formula. Int. Dairy J. 2020, 102, 104579. [Google Scholar] [CrossRef]
- Yang, S.; Pei, X.; Wang, G.; Yan, L.; Hu, J.; Li, Y.; Li, N.; Yang, D. Prevalence of food-borne pathogens in ready-to-eat meat products in seven different Chinese regions. Food Control 2016, 65, 92–98. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naitali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Chen, D.; Peng, P.; Zhou, N.; Cheng, Y.; Min, M.; Ma, Y.; Mao, Q.; Chen, P.; Chen, C.; Ruan, R. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 2019, 290, 270–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Tang, J.; Wang, S.; Wang, L.; Zhu, G.; Li, X.; Liu, Y. Thermal inactivation of Cronobacter sakazakii ATCC 29544 in powdered infant formula milk using thermostatic radio frequency. Food Control 2020, 114, 107270. [Google Scholar] [CrossRef]
- Wang, L.; Forsythe, S.J.; Yang, X.; Fu, S.; Man, C.; Jiang, Y. Invited review: Stress resistance of Cronobacter spp. affecting control of its growth during food production. J. Dairy Sci. 2021, 104, 11348–11367. [Google Scholar] [CrossRef] [PubMed]
- Badawy, B.; Gwida, M.; Sadat, A.; El-Toukhy, M.; Sayed-Ahmed, M.; Alam, N.; Ahmad, S.; Ali, M.D.S.; Elafify, M. Prevalence and Antimicrobial Resistance of Virulent Listeria monocytogenes and Cronobacter sakazakii in Dairy Cattle, the Environment, and Dried Milk with the In Vitro Application of Natural Alternative Control. Antibiotics 2022, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yi, L.; Chen, J.; Liu, B.; Lü, X. Antibacterial mechanisms of bacteriocin BM1157 against Escherichia coli and Cronobacter sakazakii. Food Control 2021, 123, 107730. [Google Scholar] [CrossRef]
- Priego, R.; Medina, L.M.; Jordano, R. Bactometer system versus traditional methods for monitoring bacteria populations in salchichon during its ripening process. J. Food Prot. 2011, 74, 145–148. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Z. Simultaneous enumeration of Cronobacter sakazakii and Staphylococcus aureus in powdered infant foods through duplex TaqMan real-time PCR. Int. Dairy J. 2021, 117, 105019. [Google Scholar] [CrossRef]
- Duan, M.; Xiao, X.; Huang, Y.; Li, G.; Shan, S.; Lv, X.; Zhou, H.; Peng, S.; Liu, C.; Liu, D.; et al. Immuno-HCR based on contact quenching and fluorescence resonance energy transfer for sensitive and low background detection of Escherichia coli O157:H7. Food Chem. 2021, 334, 127568. [Google Scholar] [CrossRef] [PubMed]
- Amrane, S.; Lagier, J.-C. Metagenomic and clinical microbiology. Hum. Microbiome J. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Elbir, H.; Robert, C.; Nguyen, T.T.; Gimenez, G.; El Sanousi, S.M.; Flock, J.-I.; Raoult, D.; Drancourt, M. Staphylococcus aureus subsp. anaerobius strain ST1464 genome sequence. Stand. Genom. Sci. 2013, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, J.; Yang, H.; Yu, J.; Wei, H. Accurate Detection of Methicillin-Resistant Staphylococcus aureus in Mixtures by Use of Single-Bacterium Duplex Droplet Digital PCR. J. Clin. Microbiol. 2017, 55, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Bachli, P.; Baars, S.; Simmler, A.; Zbinden, R.; Schulthess, B. Impact of MALDI-TOF MS identification on anaerobic species and genus diversity in routine diagnostics. Anaerobe 2022, 75, 102554. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef]
- Oviaño, M.; Rodríguez-Sánchez, B. MALDI-TOF mass spectrometry in the 21st century clinical microbiology laboratory. Enferm. Infecc. Y Microbiol. Clin. 2021, 39, 192–200. [Google Scholar] [CrossRef]
- Moreno, E.; Miller, E.; Miller, E.; Totty, H.; Deol, P. A novel liquid media mycobacteria extraction method for MALDI-TOF MS identification using VITEK(R) MS. J. Microbiol. Methods 2018, 144, 128–133. [Google Scholar] [CrossRef]
- Sun, J.; Shi, H.; Xue, Y.; Cheng, W.; Yu, M.; Ding, C.; Xu, F.; Yu, S. Releasing bacteria from functional magnetic beads is beneficial to MALDI-TOF MS based identification. Talanta 2021, 225, 121968. [Google Scholar] [CrossRef]
- Lau, A.F.; Walchak, R.C.; Miller, H.B.; Slechta, E.S.; Kamboj, K.; Riebe, K.; Robertson, A.E.; Gilbreath, J.J.; Mitchell, K.F.; Wallace, M.A.; et al. Multicenter Study Demonstrates Standardization Requirements for Mold Identification by MALDI-TOF MS. Front. Microbiol. 2019, 10, 2098. [Google Scholar] [CrossRef] [PubMed]
- Petry, S.; Py, J.S.; Wilhelm, A.; Duquesne, F.; Bayon-Auboyer, M.H.; Morvan, H.; Gassilloud, B. Evaluation of MALDI-TOF MS and an expanded custom reference spectra database for the identification and differentiation of Taylorella equigenitalis and Taylorella asinigenitalis. Diagn. Microbiol. Infect. Dis. 2019, 94, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, L.; Rocca, F.; Armitano, R.I.; Martinez, C.; Almuzara, M.; Faccone, D.; Vay, C.; Prieto, M. Development and evaluation of an in-house database for quick identification of Burkholderia contaminans by MALDI-TOF MS. Rev. Argent Microbiol. 2019, 51, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Mailler, S.; Welker, M.; Arsac, M.; Celliere, B.; Cotte-Pattat, P.J.; Chatellier, S.; Durand, G.; Beni, A.M.; Schrenzel, J.; et al. Identification of mycobacterium spp. and nocardia spp. from solid and liquid cultures by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Diagn. Microbiol. Infect. Dis. 2016, 86, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Oviano, M.; Rodriguez-Sanchez, B.; Gomara, M.; Alcala, L.; Zvezdanova, E.; Ruiz, A.; Velasco, D.; Gude, M.J.; Bouza, E.; Bou, G. Direct identification of clinical pathogens from liquid culture media by MALDI-TOF MS analysis. Clin. Microbiol. Infect. 2018, 24, 624–629. [Google Scholar] [CrossRef]
- Stevenson, L.G.; Drake, S.K.; Murray, P.R. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2010, 48, 444–447. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Gao, M.; Zhang, X. High throughput detection of tetracycline residues in milk using graphene or graphene oxide as MALDI-TOF MS matrix. J. Am. Soc. Mass. Spectrom. 2012, 23, 1424–1427. [Google Scholar] [CrossRef]
- Cunsolo, V.; Muccilli, V.; Saletti, R.; Foti, S. MALDI-TOF mass spectrometry for the monitoring of she-donkey’s milk contamination or adulteration. J. Mass. Spectrom. 2013, 48, 148–153. [Google Scholar] [CrossRef]
- Aseev, L.V.; Boni, I.V. Extraribosomal functions of bacterial ribosomal proteins. Mol. Biol. 2011, 45, 739–750. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Li, H.; Miao, S.; Zheng, B. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.). Innov. Food Sci. Emerg. Technol. 2019, 54, 51–63. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing cultivation of Cordyceps militaris for fast growth and cordycepin overproduction using rational design of synthetic media. Comput. Struct. Biotechnol. J. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deepika, D.; Saigal, K.; Ghosh, A.; Saikia, D.; Balaji, V.; John, J.; Kang, G. Spatial cluster analysis of invasive typhoidal Salmonella infections from paediatric population in North India. Int. J. Infect. Dis. 2020, 101, 125. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.J.; Wang, Z.W.; Liu, L.; Wei, Y.X.; Han, X.; Zeng, J.; Liao, W.J. Identification of Cronobacter species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with an optimized analysis method. J. Microbiol. Methods 2017, 139, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Dubois, D.; Leyssene, D.; Chacornac, J.P.; Kostrzewa, M.; Schmit, P.O.; Talon, R.; Bonnet, R.; Delmas, J. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2010, 48, 941–945. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Tseng, C.L.; Lee, Y.S.; Kuo, A.J.; Sun, C.F.; Lin, Y.H.; Chen, J.K. Highly efficient classification and identification of human pathogenic bacteria by MALDI-TOF MS. Mol. Cell Proteom. 2008, 7, 448–456. [Google Scholar] [CrossRef]
- Javůrková, B.; Blažková, M.; Fukal, L.; Rauch, P. Rapid detection of genus Cronobacter in powdered infant formula milk. Eur. Food Res. Technol. 2012, 234, 1099–1104. [Google Scholar] [CrossRef]



| Level | 70% FA Added Amounts (μL) | Ultrasonic Power (W) | Ultrasonic Time (min) | ACN Added Amounts (μL) |
|---|---|---|---|---|
| 1 | 10 | 300 | 1 | 40 |
| 2 | 20 | 350 | 2 | 60 |
| 3 | 30 | 400 | 3 | 80 |
| No. | 70% FA Added Amounts (μL) | Ultrasonic Power (W) | Ultrasonic Time (min) | ACN Added Amounts (μL) | Identification Score |
|---|---|---|---|---|---|
| 1 | 10 | 300 | 2 | 60 | 1770 |
| 2 | 10 | 350 | 1 | 60 | 1648 |
| 3 | 10 | 350 | 2 | 80 | 1695 |
| 4 | 10 | 350 | 3 | 60 | 1464 |
| 5 | 10 | 350 | 2 | 40 | 1530 |
| 6 | 10 | 400 | 2 | 60 | 1626 |
| 7 | 20 | 300 | 1 | 60 | 1721 |
| 8 | 20 | 300 | 3 | 60 | 1747 |
| 9 | 20 | 300 | 2 | 80 | 1891 |
| 10 | 20 | 300 | 2 | 40 | 1534 |
| 11 | 20 | 350 | 1 | 40 | 1812 |
| 12 | 20 | 350 | 1 | 80 | 1505 |
| 13 | 20 | 350 | 3 | 40 | 1553 |
| 14 | 20 | 350 | 3 | 80 | 1777 |
| 15 | 20 | 400 | 1 | 60 | 1636 |
| 16 | 20 | 400 | 2 | 40 | 1696 |
| 17 | 20 | 400 | 2 | 80 | 1728 |
| 18 | 20 | 400 | 3 | 60 | 1867 |
| 19 | 30 | 300 | 2 | 60 | 1601 |
| 20 | 30 | 350 | 1 | 60 | 1543 |
| 21 | 30 | 350 | 2 | 40 | 1399 |
| 22 | 30 | 350 | 2 | 80 | 1877 |
| 23 | 30 | 350 | 3 | 60 | 1650 |
| 24 | 30 | 400 | 2 | 60 | 1723 |
| 25 | 20 | 350 | 2 | 60 | 1879 |
| 26 | 20 | 350 | 2 | 60 | 1809 |
| 27 | 20 | 350 | 2 | 60 | 1725 |
| 28 | 20 | 350 | 2 | 60 | 1901 |
| 29 | 20 | 350 | 2 | 60 | 1924 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | R2 | Significance Level |
|---|---|---|---|---|---|---|
| Model | 4.50 × 105 | 14 | 32,170.79 | 3.78 | 0.0091 | Significant |
| A-70% FA added amounts | 300 | 1 | 300 | 0.035 | 0.8538 | |
| B-ultrasonic power | 12 | 1 | 12 | 1.41 × 10−3 | 0.9706 | |
| C-ultrasonic time | 3104.08 | 1 | 3104.08 | 0.36 | 0.5557 | |
| D-ACN added amounts | 75,050.08 | 1 | 75,050.08 | 8.81 | 0.0102 | Significant |
| AB | 17,689 | 1 | 17,689 | 2.08 | 0.1716 | |
| AC | 21,170.25 | 1 | 21,170.25 | 2.49 | 0.1372 | |
| AD | 24,492.25 | 1 | 24,492.25 | 2.88 | 0.1121 | |
| BC | 10,506.25 | 1 | 10,506.25 | 1.23 | 0.2855 | |
| BD | 26,406.25 | 1 | 26,406.25 | 3.1 | 0.1001 | |
| CD | 70,490.25 | 1 | 70,490.25 | 8.28 | 0.0122 | Significant |
| A2 | 1.45 × 105 | 1 | 1.45 × 105 | 17 | 0.001 | Significant |
| B2 | 3335.06 | 1 | 3335.06 | 0.39 | 0.5416 | |
| C2 | 64,605.66 | 1 | 64,605.66 | 7.58 | 0.0155 | Significant |
| D2 | 53,184.66 | 1 | 53,184.66 | 6.24 | 0.0255 | Significant |
| Residual | 1.19 × 105 | 14 | 8518.41 | |||
| Lack of fit | 93,062.58 | 10 | 9306.26 | 1.42 | 0.3928 | Not significant |
| Pure error | 26,195.2 | 4 | 6548.8 | |||
| Cor total | 5.70 × 105 | 28 |
| Culture Medium | Identification Score | Average Value | Standard Deviation |
|---|---|---|---|
| LB-1 | 2000 | ||
| LB-2 | 1916 | 1972.000 | 48.497 |
| LB-3 | 2000 | ||
| NB-1 | 1943 | ||
| NB-2 | 1988 | 1938.666 | 51.636 |
| NB-3 | 1885 | ||
| TSB-1 | 2000 | ||
| TSB-2 | 1930 | 1976.666 | 40.414 |
| TSB-3 | 2000 |
| Strains of Label | Territory | Source | MALDI-TOF MS Identification Result | 16S rDNA Identification Result |
|---|---|---|---|---|
| CE1 | Commercially available PIF | C. sakazakii | C. sakazakii | |
| CE7 | C. sakazakii | C. sakazakii | ||
| CE9 | C. sakazakii | C. sakazakii | ||
| CE10 | C. sakazakii | C. sakazakii | ||
| CE21 | C. sakazakii | C. sakazakii | ||
| CE24 | C. sakazakii | C. sakazakii | ||
| CE25 | C. sakazakii | C. sakazakii | ||
| CE26 | C. sakazakii | C. sakazakii | ||
| CE27 | C. sakazakii | C. sakazakii | ||
| CE28 | C. sakazakii | C. sakazakii | ||
| CE29 | C. sakazakii | C. sakazakii | ||
| CE34 | C. sakazakii | C. sakazakii | ||
| CE38 | C. sakazakii | C. sakazakii | ||
| CE41 | C. sakazakii | C. sakazakii | ||
| CE43 | Northeastern China | C. sakazakii | C. sakazakii | |
| CE44 | C. sakazakii | C. sakazakii | ||
| CE47 | C. sakazakii | C. sakazakii | ||
| CE48 | C. sakazakii | C. sakazakii | ||
| CE49 | C. sakazakii | C. sakazakii | ||
| CE50 | C. sakazakii | C. sakazakii | ||
| CE54 | C. sakazakii | C. sakazakii | ||
| CE55 | C. sakazakii | C. sakazakii | ||
| CE56 | C. sakazakii | C. sakazakii | ||
| CE69 | C. sakazakii | C. sakazakii | ||
| CE30 | Plant product | C. sakazakii | C. sakazakii | |
| CE31 | C. sakazakii | C. sakazakii | ||
| CE32 | C. sakazakii | C. sakazakii | ||
| CE61 | C. sakazakii | C. sakazakii | ||
| CE64 | C. sakazakii | C. sakazakii | ||
| CE67 | C. sakazakii | C. sakazakii | ||
| CE70 | C. sakazakii | C. sakazakii | ||
| CE71 | C. sakazakii | C. sakazakii | ||
| CE72 | C. sakazakii | C. sakazakii | ||
| CE73 | C. sakazakii | C. sakazakii | ||
| CE75 | C. sakazakii | C. sakazakii | ||
| CE76 | C. sakazakii | C. sakazakii | ||
| CE77 | C. sakazakii | C. sakazakii | ||
| CE78 | C. sakazakii | C. sakazakii | ||
| CE33 | Raw material | C. sakazakii | C. sakazakii | |
| CE58 | C. sakazakii | C. sakazakii | ||
| CE59 | C. sakazakii | C. sakazakii | ||
| CE36 | Fluidized bed powder | C. sakazakii | C. sakazakii | |
| CE62 | C. sakazakii | C. sakazakii | ||
| CE68 | C. sakazakii | C. sakazakii | ||
| CE74 | C. sakazakii | C. sakazakii | ||
| CE79 | C. sakazakii | C. sakazakii | ||
| CE65 | Fixed bed | C. sakazakii | C. sakazakii | |
| CE66 | C. sakazakii | C. sakazakii | ||
| CE60 | U-shaped valve | C. sakazakii | C. sakazakii | |
| C. sakazakii | C. sakazakii | |||
| CE63 | Spray dried powder | C. sakazakii | C. sakazakii | |
| C. sakazakii | C. sakazakii | |||
| CE51 | Northwestern China | Commercially available PIF | C. sakazakii | C. sakazakii |
| CE52 | C. sakazakii | C. sakazakii | ||
| CE53 | C. sakazakii | C. sakazakii | ||
| CE13 | Northern China | Commercially available PIF | C. sakazakii | C. sakazakii |
| CE14 | C. sakazakii | C. sakazakii | ||
| CE15 | C. sakazakii | C. sakazakii | ||
| CE16 | C. sakazakii | C. sakazakii | ||
| CE17 | C. sakazakii | C. sakazakii | ||
| CE18 | C. sakazakii | C. sakazakii | ||
| CE19 | C. sakazakii | C. sakazakii | ||
| CE20 | C. sakazakii | C. sakazakii | ||
| CE22 | C. sakazakii | C. sakazakii | ||
| CE23 | C. sakazakii | C. sakazakii | ||
| CE11 | Eastern China | Commercially available PIF | C. sakazakii | C. sakazakii |
| CE12 | C. sakazakii | C. sakazakii | ||
| CE8 | North China | Commercially available PIF | C. sakazakii | C. sakazakii |
| ATCC BAA-894 | America | PIF Manufacturing environment | C. sakazakii | C. sakazakii |
| ATCC 29004 | Commercially available PIF | C. sakazakii | C. sakazakii | |
| ATCC 29544 | C. sakazakii | C. sakazakii | ||
| ATCC 12868 | C. sakazakii | C. sakazakii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Su, Q.; Jia, A.; Fu, S.; Ma, X.; Li, T.; Man, C.; Yang, X.; Jiang, Y. A Method to Directly Identify Cronobacter sakazakii in Liquid Medium by MALDI-TOF MS. Foods 2023, 12, 1981. https://doi.org/10.3390/foods12101981
Song D, Su Q, Jia A, Fu S, Ma X, Li T, Man C, Yang X, Jiang Y. A Method to Directly Identify Cronobacter sakazakii in Liquid Medium by MALDI-TOF MS. Foods. 2023; 12(10):1981. https://doi.org/10.3390/foods12101981
Chicago/Turabian StyleSong, Danliangmin, Qunchao Su, Ai Jia, Shiqian Fu, Xiaoming Ma, Tiantian Li, Chaoxin Man, Xinyan Yang, and Yujun Jiang. 2023. "A Method to Directly Identify Cronobacter sakazakii in Liquid Medium by MALDI-TOF MS" Foods 12, no. 10: 1981. https://doi.org/10.3390/foods12101981
APA StyleSong, D., Su, Q., Jia, A., Fu, S., Ma, X., Li, T., Man, C., Yang, X., & Jiang, Y. (2023). A Method to Directly Identify Cronobacter sakazakii in Liquid Medium by MALDI-TOF MS. Foods, 12(10), 1981. https://doi.org/10.3390/foods12101981





