Mechanism of Reduced Glutathione Induced Lysozyme Defolding and Molecular Self-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Partially Unfolded Lys Nanofilm
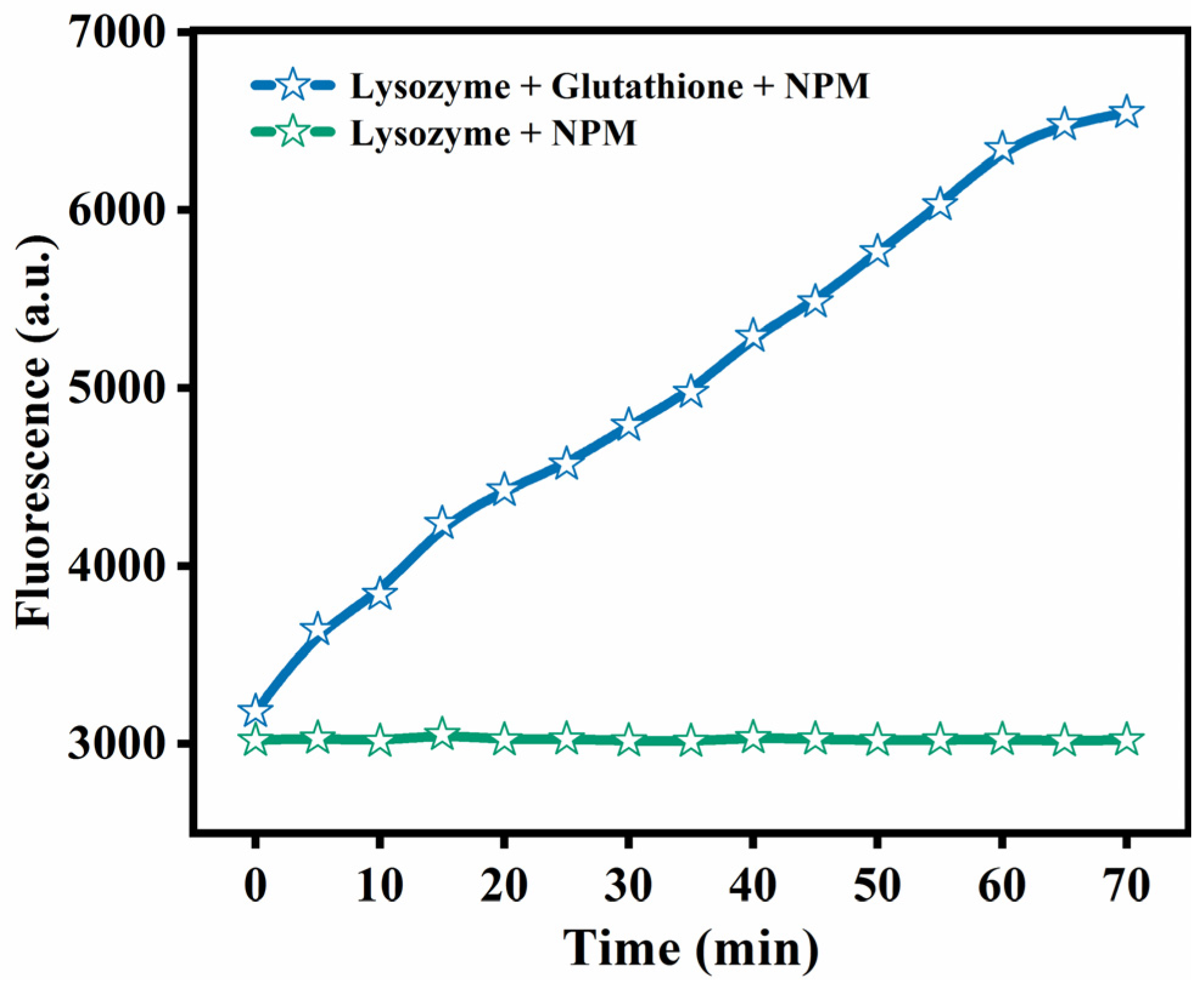
2.3. NPM Fluorescence Analysis
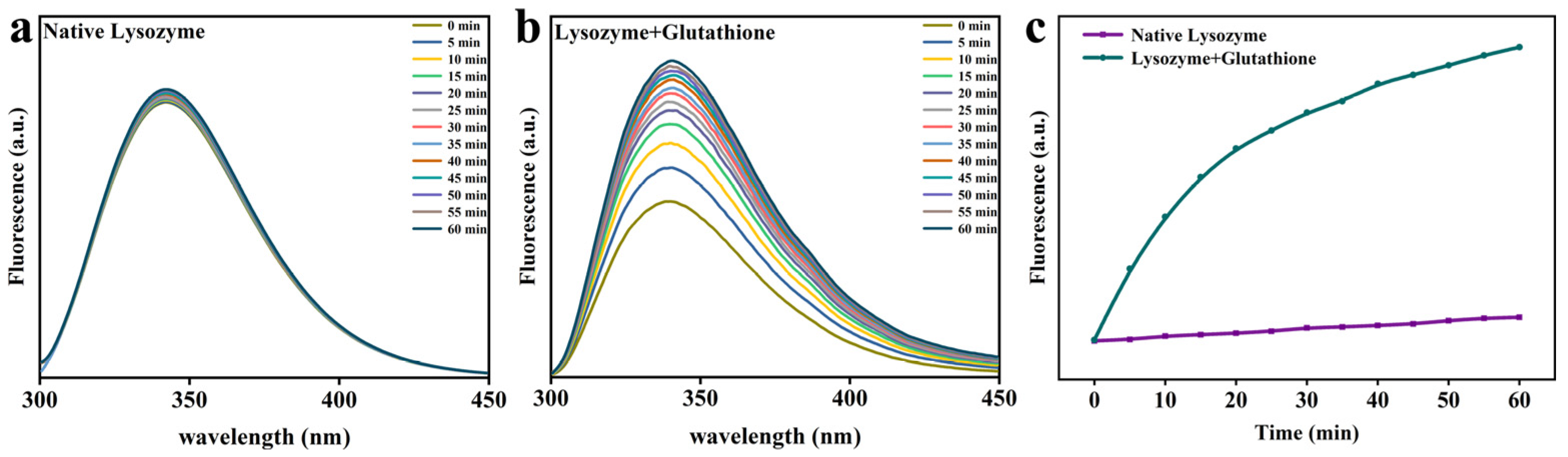
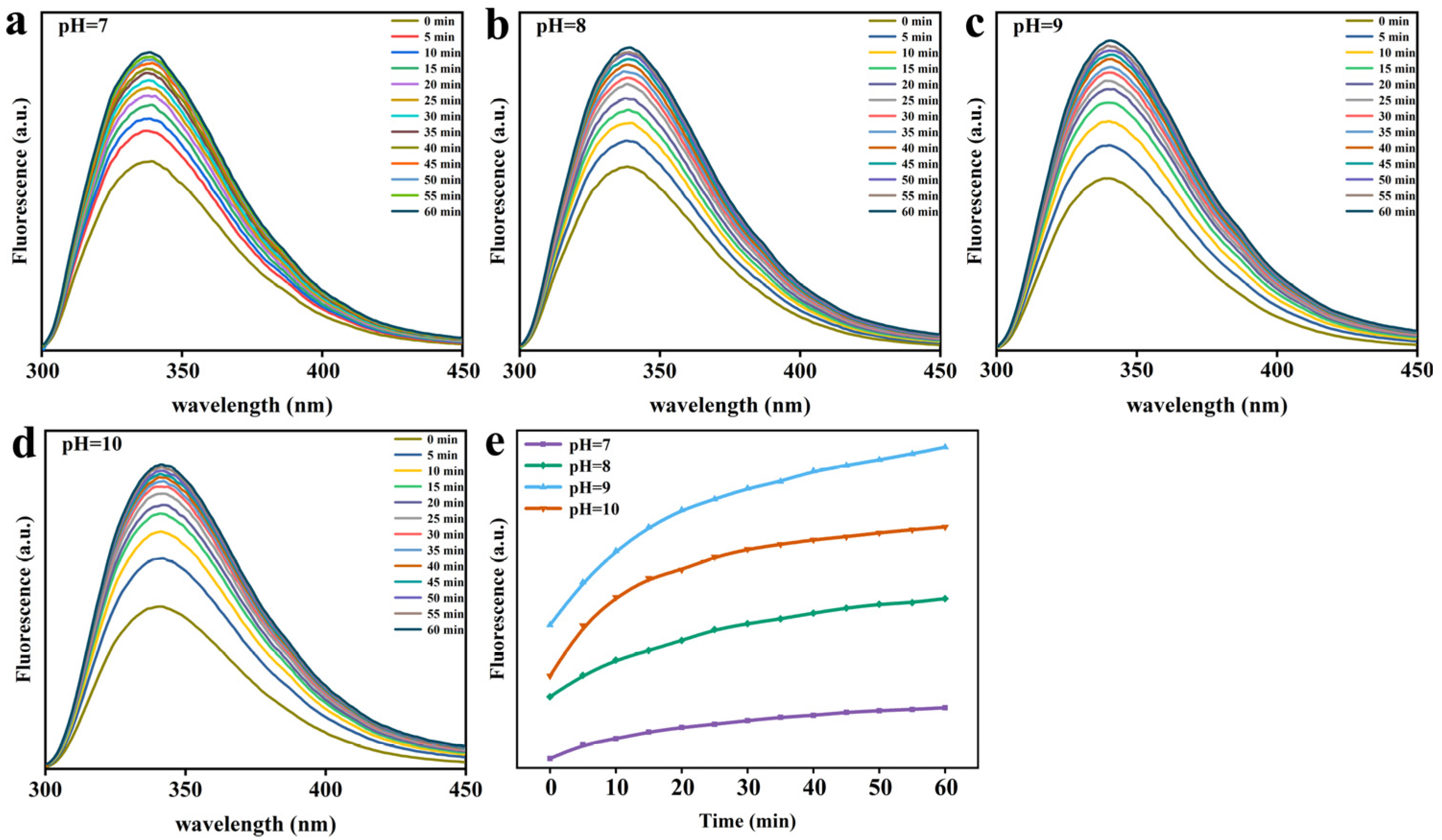
2.4. Trp Fluorescence Analysis
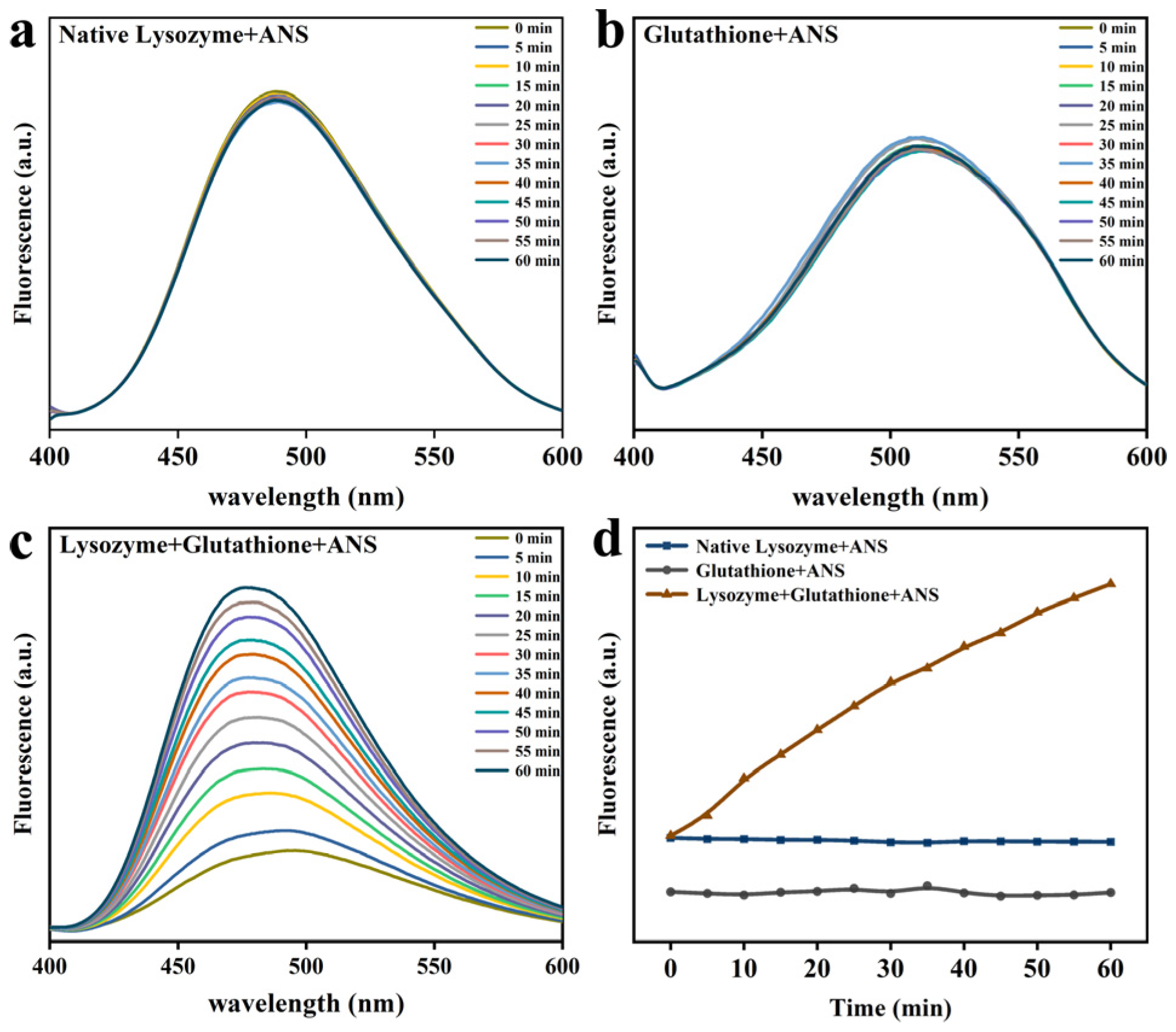
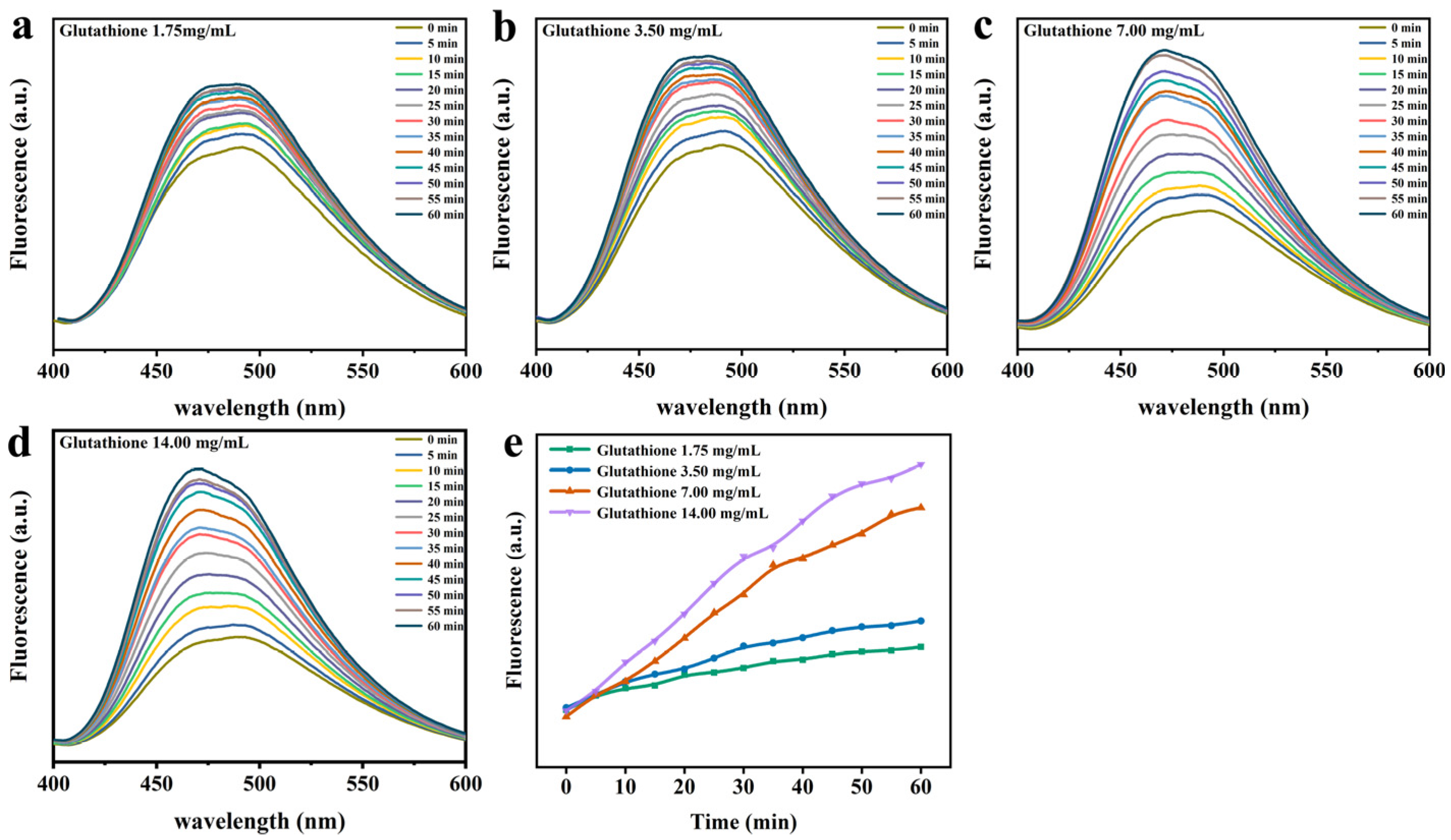
2.5. ANS Fluorescence Probe Analysis
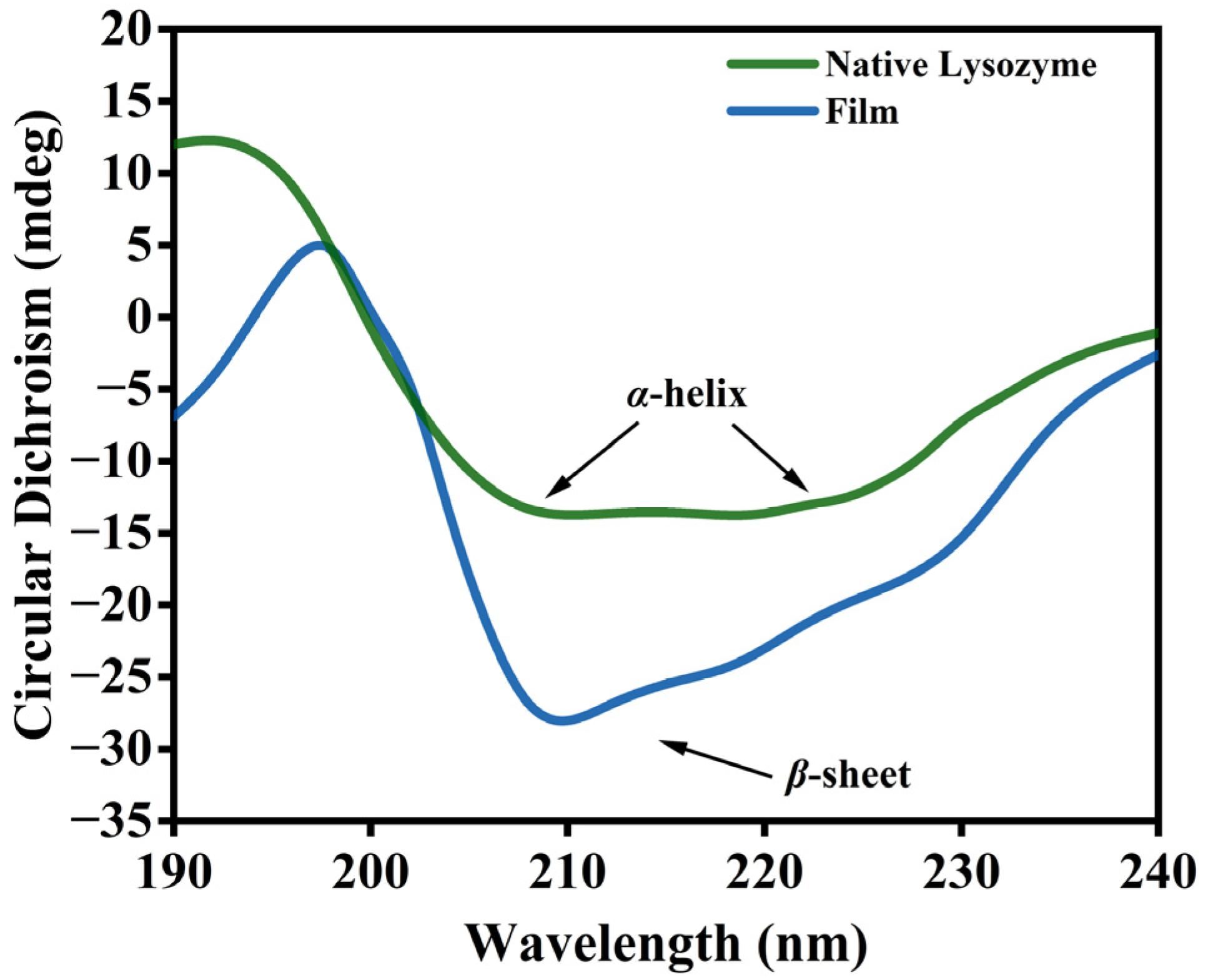
2.6. Circular Dichroism (CD) Spectrum
2.7. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR)
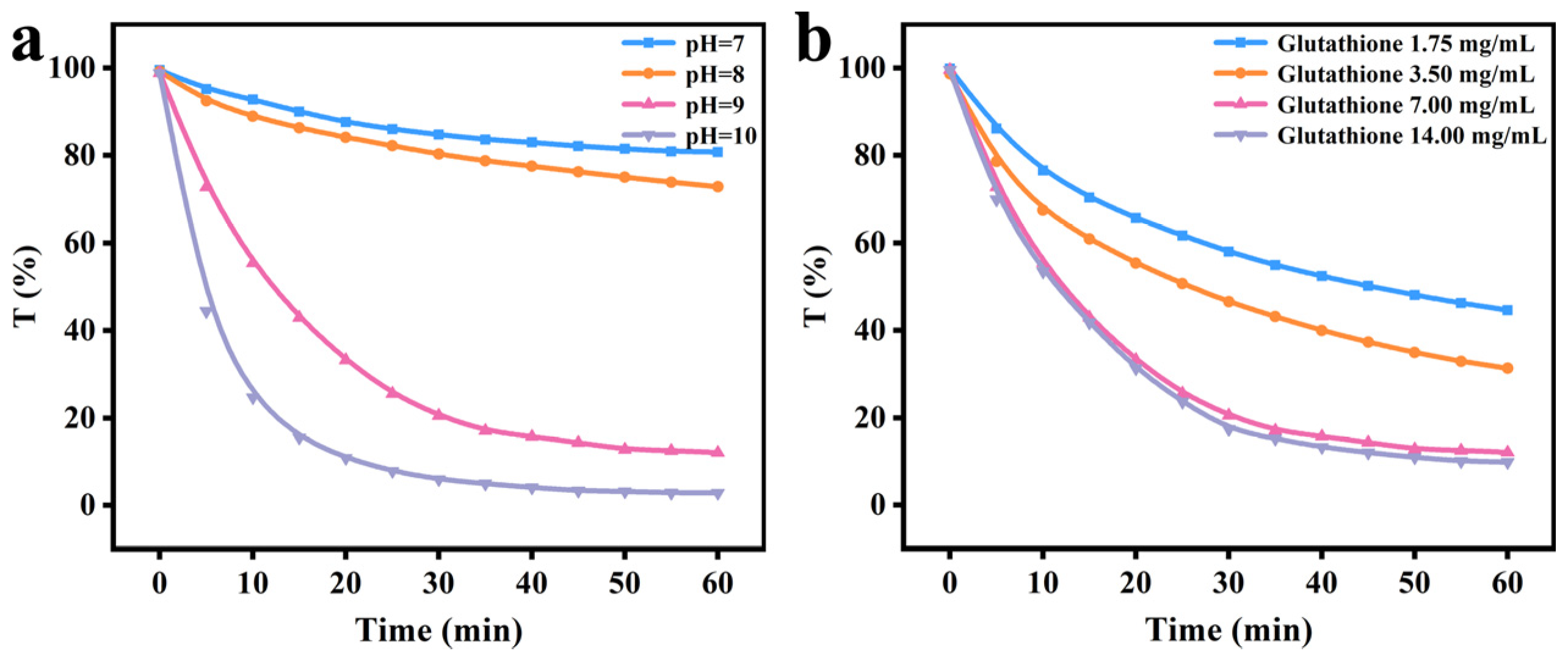
2.8. Turbidity Measurement
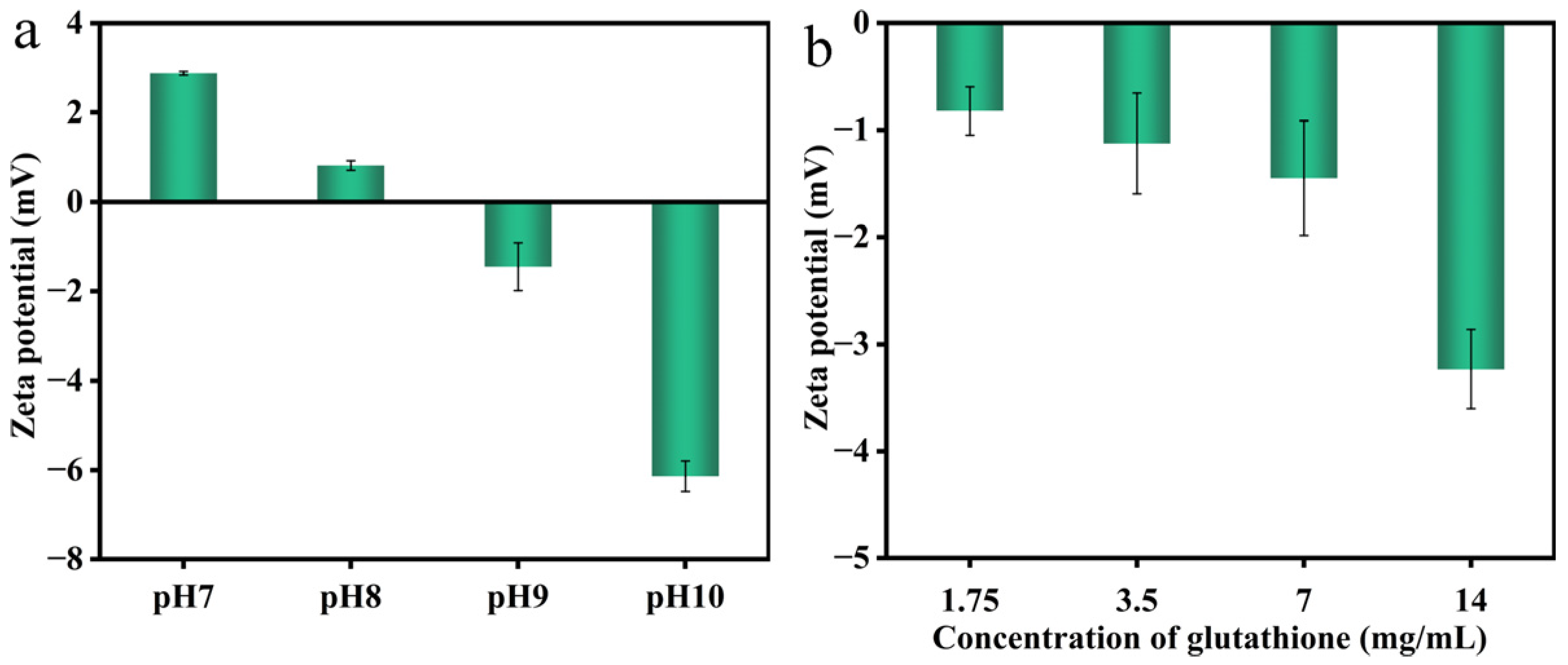
2.9. Zeta-Potential and Particle Size Analysis
2.10. Determination of Surface Tension
2.11. Atomic Force Microscopy (AFM)
2.12. Statistical Analysis
3. Results
3.1. Reduction of Disulfide Bonds in Defolded Lys
3.2. Changes in the Molecular Structure of Lys
3.3. Lys Molecular Interaction
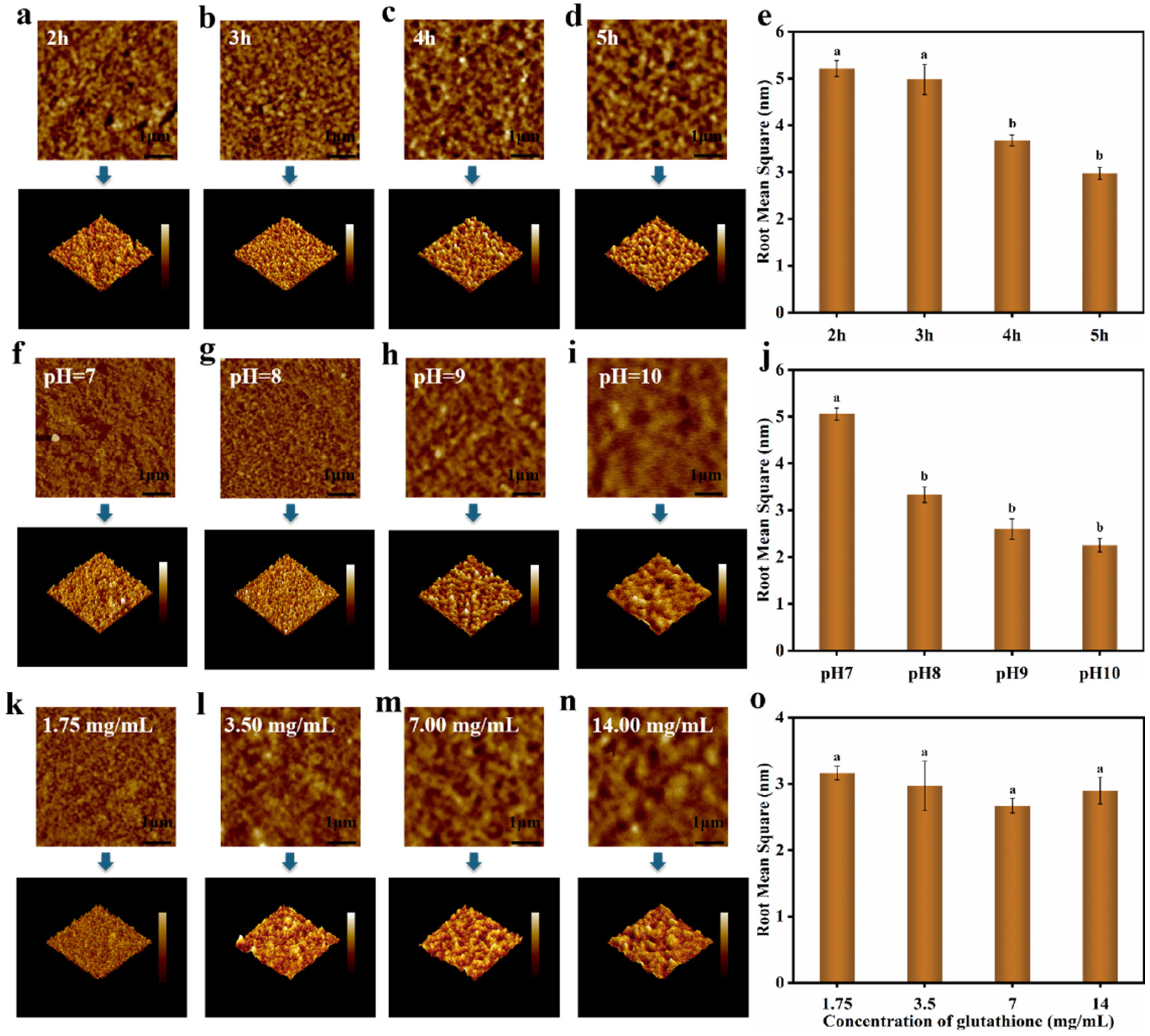
3.4. Characterization of Nanofilms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lesnierowski, G.; Yang, T. Lysozyme and its modified forms: A critical appraisal of selected properties and potential. Trends Food Sci. Technol. 2020, 107, 333–342. [Google Scholar] [CrossRef]
- Jansens, K.J.A.; Rombouts, I.; Grootaert, C.; Brijs, K.; Van Camp, J.; Van der Meeren, P.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Rational Design of Amyloid-like Fibrillary Structures for Tailoring Food Protein Techno-Functionality and Their Potential Health Implications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hwang, H.J.; Bhak, G.; Jang, Y.; Paik, S.R.; Char, K. In Situ Fibril Formation of κ-Casein by External Stimuli within Multilayer Thin Films. ACS Macro Lett. 2013, 2, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, 1908291. [Google Scholar] [CrossRef]
- Gao, A.; Wu, Q.; Wang, D.; Ha, Y.; Chen, Z.; Yang, P. Protein Self-Assembly: A Superhydrophobic Surface Templated by Protein Self-Assembly and Emerging Application toward Protein Crystallization. Adv. Mater. 2016, 28, 592. [Google Scholar] [CrossRef]
- Gu, J.; Su, Y.; Liu, P.; Li, P.; Yang, P. An Environmentally Benign Antimicrobial Coating Based on a Protein Supramolecular Assembly. ACS Appl. Mater. Interfaces 2017, 9, 198–210. [Google Scholar] [CrossRef]
- Yang, F.; Tao, F.; Li, C.; Gao, L.; Yang, P. Self-assembled membrane composed of amyloid-like proteins for efficient size-selective molecular separation and dialysis. Nat. Commun. 2018, 9, 5443. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Hu, X.; Qin, R.; Su, H.; Li, J.; Yang, P. The Synthesis of a 2D Ultra-Large Protein Supramolecular Nanofilm by Chemoselective Thiol–Disulfide Exchange and its Emergent Functions. Angew. Chem. Int. Ed. 2020, 59, 2850–2859. [Google Scholar] [CrossRef]
- Wang, D.; Ha, Y.; Gu, J.; Li, Q.; Zhang, L.; Yang, P. 2D Protein Supramolecular Nanofilm with Exceptionally Large Area and Emergent Functions. Adv. Mater. 2016, 28, 7414–7423. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Smith, K.M.; Darmanin, C.; Ryan, T.M.; Drummond, C.J.; Greaves, T.L. Lysozyme conformational changes with ionic liquids: Spectroscopic, small angle X-ray scattering and crystallographic study. J. Colloid Interface Sci. 2021, 585, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Özyiğit, İ.; Karakuş, E.; Pekcan, Ö. The modifier effects of chymotrypsin and trypsin enzymes on fluorescence lifetime distribution of “N-(1-pyrenyl)maleimide-bovine serum albumin” complex. Spectrochim. Acta Part A 2015, 154, 8–12. [Google Scholar] [CrossRef]
- Lacy, E.R.; Baker, M.; Brigham-Burke, M. Free sulfhydryl measurement as an indicator of antibody stability. Anal. Biochem. 2008, 382, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Dang, L.; Zhang, B.; Wang, Z. The activity and molecular interaction of lysozyme in adding four ionic liquids aqueous solutions. J. Mol. Liq. 2022, 353, 118788. [Google Scholar] [CrossRef]
- Seo, J.-H.; Matsuno, R.; Lee, Y.; Konno, T.; Takai, M.; Ishihara, K. Effect of hydrophilic polymer conjugation on heat-induced conformational changes in a protein. Acta Biochim. 2010, 7, 1477–1484. [Google Scholar] [CrossRef]
- Yang, P.; Yang, W. Hydroxylation of Organic Polymer Surface: Method and Application. ACS Appl. Mater. Interfaces 2014, 6, 3759–3770. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Goel, M.; Kotamarthi, H.; Ainavarapu, S.R.K.; Swaminathan, R. Preventing Disulfide Bond Formation Weakens Non-Covalent Forces among Lysozyme Aggregates. PLoS ONE 2014, 9, e87012. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, H.-X. Protein Allostery and Conformational Dynamics. Chem. Rev. 2016, 116, 6503–6515. [Google Scholar] [CrossRef]
- Ashrafi, N.; Shareghi, B.; Farhadian, S.; Hosseini-Koupaei, M. A comparative study of the interaction of naringenin with lysozyme by multi-spectroscopic methods, activity comparisons, and molecular modeling procedures. Spectrochim. Acta Part A 2022, 271, 120931. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Sakakibara, Y.; Kamada, Y.; Watanabe, K.-I.; Sugimoto, Y. Analysis of Core Region from Egg White Lysozyme Forming Amyloid Fibrils. Int. J. Biol. Sci. 2013, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Qin, R.; Liu, Y.; Tao, F.; Li, C.; Cao, W.; Yang, P. Protein-Bound Freestanding 2D Metal Film for Stealth Information Transmission. Adv. Mater. 2019, 31, 1803377. [Google Scholar] [CrossRef]
- Meister, K.; Bäumer, A.; Szilvay, G.R.; Paananen, A.; Bakker, H.J. Self-Assembly and Conformational Changes of Hydrophobin Classes at the Air–Water Interface. J. Phys. Chem. Lett. 2016, 7, 4067–4071. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Zhou, J.; Yao, Y.; Wu, X.; Bolisetty, S.; Diener, M.; Handschin, S.; Lu, C.; Mezzenga, R. Interfacial Electrostatic Self-Assembly of Amyloid Fibrils into Multifunctional Protein Films. Adv. Sci. 2023, 10, 2206867. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, H.; Li, Z.; Zhou, J.; Chen, X.; Bai, S.; Yang, H. Monodisperse phase transfer and surface bioengineering of metal nanoparticles via silk fibroin protein corona. Nanoscale 2017, 9, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, A.S.; Dexter, A.F.; Middelberg, A.P.J. Mechanical Properties of Interfacial Films Formed by Lysozyme Self-Assembly at the Air−Water Interface. Langmuir 2006, 22, 8897–8905. [Google Scholar] [CrossRef]
- Wosten, H.; Scholtmeijer, K. Applications of hydrophobins: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 1587–1597. [Google Scholar] [CrossRef]
- Hermann, S.; de las Heras, D.; Schmidt, M. Non-negative Interfacial Tension in Phase-Separated Active Brownian Particles. Phys. Rev. Lett. 2019, 123, 268002. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Z.; Xue, Y. Determination of Interfacial Tension of Nanomaterials and the Effect of Particle Size on Interfacial Tension. Langmuir 2021, 37, 14463–14471. [Google Scholar] [CrossRef]
- Vialetto, J.; Nussbaum, N.; Bergfreund, J.; Fischer, P.; Isa, L. Influence of the interfacial tension on the microstructural and mechanical properties of microgels at fluid interfaces. J. Colloid Interface Sci. 2022, 608, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Fainerman, V.; Aksenenko, E.; Makievski, A.; Trukhin, D.; Yeganehzad, S.; Gochev, G. Surface tension and dilational rheology of mixed β-casein–β-lactoglobulin aqueous solutions at the water/air interface. Food Hydrocoll. 2020, 106, 105883. [Google Scholar]
- Narayan, M. Revisiting the Formation of a Native Disulfide Bond: Consequences for Protein Regeneration and Beyond. Molecules 2020, 25, 5337. [Google Scholar] [CrossRef] [PubMed]
- Warenda, M.; Richter, A.; Schmidt, D.; Janke, A.; Müller, M.; Simon, F.; Zimmermann, R.; Eichhorn, K.; Voit, B.; Appelhans, D. Fabricating pH-Stable and Swellable Very Thin Hyperbranched Poly(ethylene imine)-Oligosaccharide Films Fabricated without Precoating: First View on Protein Adsorption. Macromol. Rapid Commun. 2012, 33, 1466–1473. [Google Scholar] [CrossRef]
- Jiang, Y.; Löbling, T.I.; Huang, C.; Sun, Z.; Müller, A.H.E.; Russell, T.P. Interfacial Assembly and Jamming Behavior of Polymeric Janus Particles at Liquid Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 33327–33332. [Google Scholar] [CrossRef] [PubMed]
- Khor, J.W.; Lee, U.N.; Berthier, J.; Berthier, E.; Theberge, A.B. Interfacial Tension Driven Open Droplet Microfluidics. Adv. Mater. Interfaces 2023, 10, 2202234. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Hou, Y.; Liang, H.; Han, L.; Li, B.; Zhou, B. Mechanism of Reduced Glutathione Induced Lysozyme Defolding and Molecular Self-Assembly. Foods 2023, 12, 1931. https://doi.org/10.3390/foods12101931
Guo D, Hou Y, Liang H, Han L, Li B, Zhou B. Mechanism of Reduced Glutathione Induced Lysozyme Defolding and Molecular Self-Assembly. Foods. 2023; 12(10):1931. https://doi.org/10.3390/foods12101931
Chicago/Turabian StyleGuo, Dashan, Yuwei Hou, Hongshan Liang, Lingyu Han, Bin Li, and Bin Zhou. 2023. "Mechanism of Reduced Glutathione Induced Lysozyme Defolding and Molecular Self-Assembly" Foods 12, no. 10: 1931. https://doi.org/10.3390/foods12101931
APA StyleGuo, D., Hou, Y., Liang, H., Han, L., Li, B., & Zhou, B. (2023). Mechanism of Reduced Glutathione Induced Lysozyme Defolding and Molecular Self-Assembly. Foods, 12(10), 1931. https://doi.org/10.3390/foods12101931





