Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Essential Oils
2.3. Disk Diffusion Method
2.4. Broth Microdilution Method
2.5. Biofilm Formation
2.6. Biofilm Reduction
2.7. Scanning Electron Microscopy (SEM)
2.8. Minced Pork Meat Preparation
2.9. Microbiological Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Essential Oils
3.2. Antilisterial Activity of Essential Oils
3.3. MICs and MBCs Determination
3.4. Biofilm Formation
3.5. Biofilm Reduction
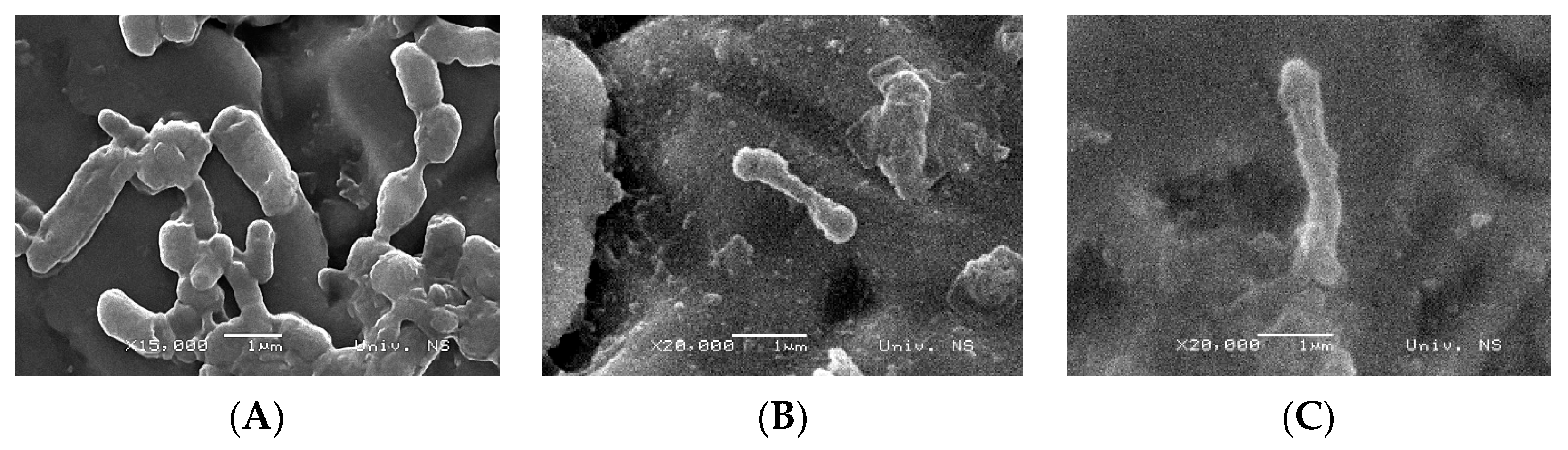
3.6. SEM Observation
3.7. Microbiological Analysis of Minced Pork Meat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- List of Prokaryotic Names with Standing in Nomenclature. Available online: https://lpsn.dsmz.de/ (accessed on 13 May 2020).
- Kaszoni-Rückerl, I.; Mustedanagic, A.; Muri-Klinger, S.; Brugger, K.; Wagner, K.H.; Wagner, M.; Stessl, B. Predominance of distinct Listeria innocua and Listeria monocytogenes in recurrent contamination events at dairy processing facilities. Microorganisms 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Identification, subtyping and virulence determination of Listeria monocytogenes, an important foodborne pathogen. J. Med. Microbiol. 2006, 55, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.; Dernburg, A.; Vernozy-Rozand, C. An updated review of Listeria monocytogenes in the pork meat industry and its products. J. Appl. Microbiol. 2006, 101, 7–17. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- WHO; FAO. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Foods: Interpretative Summary; Microbiological Risk Assessment Series 4; World Health Organization: Geneva, Switzerland; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review controlling Listeria monocytogenes in ready-to-eat meat and poultry products: An overview of outbreaks, current legislations, challenges, and future prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- Jemmi, T.; Stephan, R. Listeria monocytogenes: Food-borne pathogen and hygiene indicator. Rev. Sci. Tech. OIE 2006, 25, 571–580. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ali, S.; Andrade, D.C.; Rezende, V.T.; Aoyanagi, M.M.; Franco, B.B.; Kamimura, E.S.; Oliveira, C.A. Essential Oils as Potential Tools to Control Listeria Monocytogenes in Foods. Food Sci. Eng. 2022, 3, 184–193. [Google Scholar] [CrossRef]
- Faleiro, M.L. The mode of antibacterial action of essential oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Basking Ridge, NJ, USA, 2011; Volume 2, pp. 1143–1156. [Google Scholar]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, antiviral and antifungal activity of essential oils: Mechanisms and applications. In Antimicrobial Compounds; Villa, T., Veiga-Crespo, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–81. [Google Scholar]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Vidaković Knežević, S.; Kocić-Tanackov, S.; Kravić, S.; Knežević, S.; Vranešević, J.; Savić Radovanović, R.; Karabasil, N. In vitro antibacterial activity of some essential oils against Salmonella Enteritidis and Salmonella Typhimurium isolated from meat. J. Food Saf. Food Qual. 2021, 72, 4–11. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Approved Standard Document M02-A11; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2012. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2012. [Google Scholar]
- Kocić-Tanackov, S.; Blagojev, N.; Suturović, I.; Dimić, G.; Pejin, J.; Tomović, V.; Šojić, B.; Savanović, J.; Kravić, S.; Karabasil, N. Antibacterial activity essential oils against Escherichia coli, Salmonella enterica and Listeria monocytogenes. J. Food Saf. Food Qual. 2017, 68, 88–95. [Google Scholar]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS—J. Pathol. Microbiol. Immunol. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Stefano, V.D. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- ISO 11290-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Nivinskienë, O.; Butkienë, R.; Mockutë, D. Chemical composition of seed (fruit) essential oils of Angelica archangelica L. growing wild in Lithuania. Chemija 2005, 16, 51–54. [Google Scholar]
- Telci, I.; Bayram, E.; Yılmaz, G.; Avcı, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem. Syst. Ecol. 2006, 34, 489–497. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical composition and cytotoxic and antioxidant activities of Satureja montana L. essential oil and its antibacterial potential against Salmonella spp. strains. J. Chem. 2013, 2013, 275698. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS J. 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microb. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Abu Ghoush, M.H.; Al-Nabulsi, A.A.; Osaili, T.M.; Holley, R.A. Inhibitory effects of cinnamon and thyme essential oils against Salmonella spp. in hummus (chickpea dip). J. Food Process. Pres. 2019, 43, e13925. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef]
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnone, D.; Serio, A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control 2015, 50, 794–803. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT-Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.; Mendes, A.; Martins da Costa, P.; Belo, A.D. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus Labill. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; McAllister, T.A. Biofilm formation by shiga toxin-producing Escherichia coli on stainless steel coupons as affected by temperature and incubation time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Lundén, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Protect. 2000, 63, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Fernandes Júnior, A.; Araújo Júnior, J.P.; Tsunemi, M.H.; Rall, V.L.M. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Lemon, K.P.; Freitag, N.E.; Kolter, R. The virulence regulator PrfA promotes biofilm formation by Listeria monocytogenes. J. Bacteriol. 2010, 192, 3969–3976. [Google Scholar] [CrossRef]
- Way, S.S.; Thompson, L.J.; Lopes, J.E.; Hajjar, A.M.; Kollmann, T.R.; Freitag, N.E.; Wilson, C.B. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 2004, 6, 235–242. [Google Scholar] [CrossRef]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Camilo, R.; Magalhães, R.; Ferreira, V.; Santos, I.; Silva, J.; Almeida, G.; Teixeira, P. Biofilm formation among clinical and food isolates of Listeria monocytogenes. Int. J. Microbiol. 2013, 2013, 524975. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Biofilms and meat safety: A mini-review. J. Food Protect. 2019, 82, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Akbari, A.M.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hulankova, R.; Borilova, G. Modeling dependence of growth inhibition of Salmonella Typhimurium and Listeria monocytogenes by oregano or thyme essential oils on the chemical composition of minced pork. J. Food Saf. 2020, 40, e12818. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage microorganism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef]
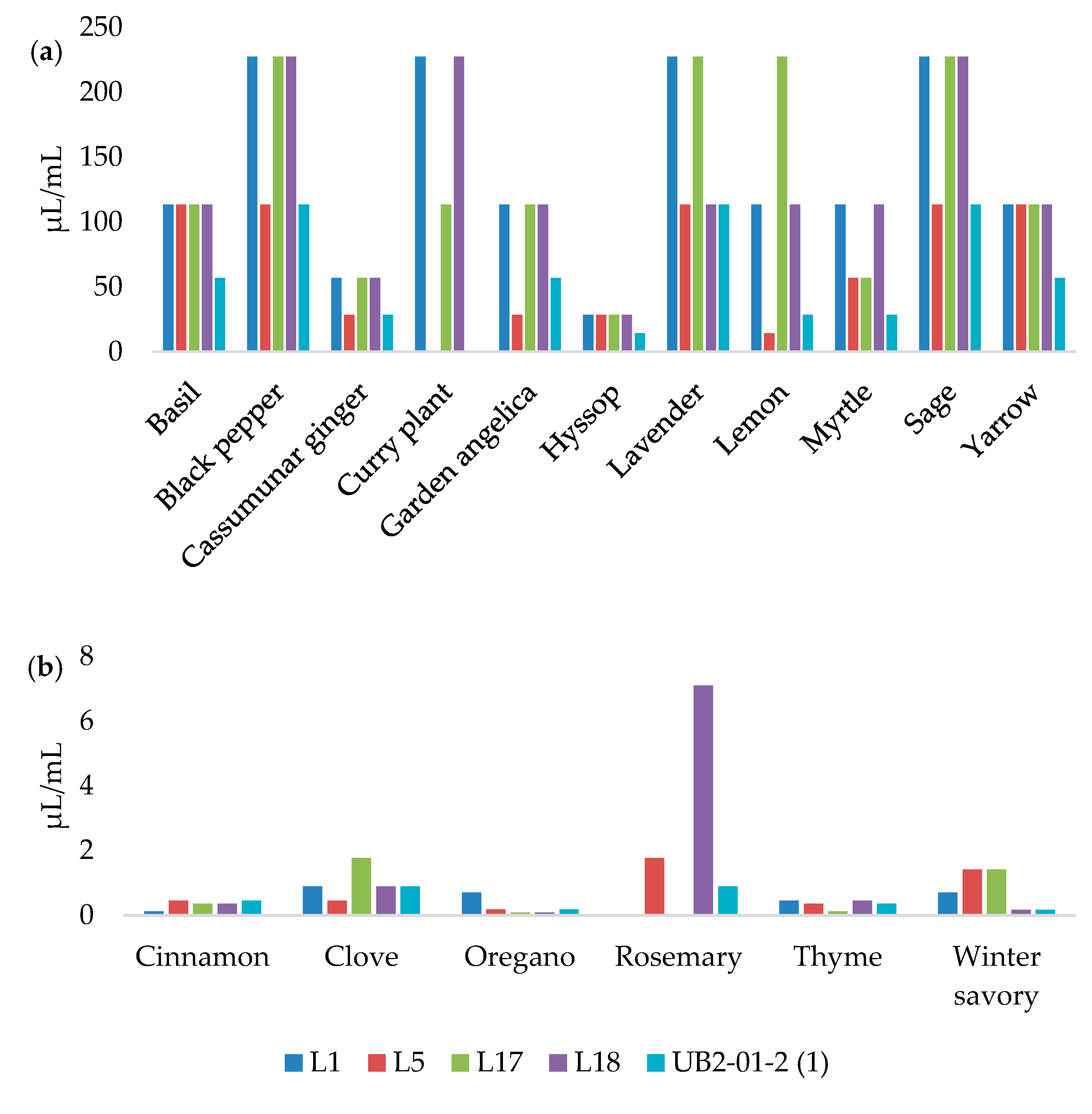
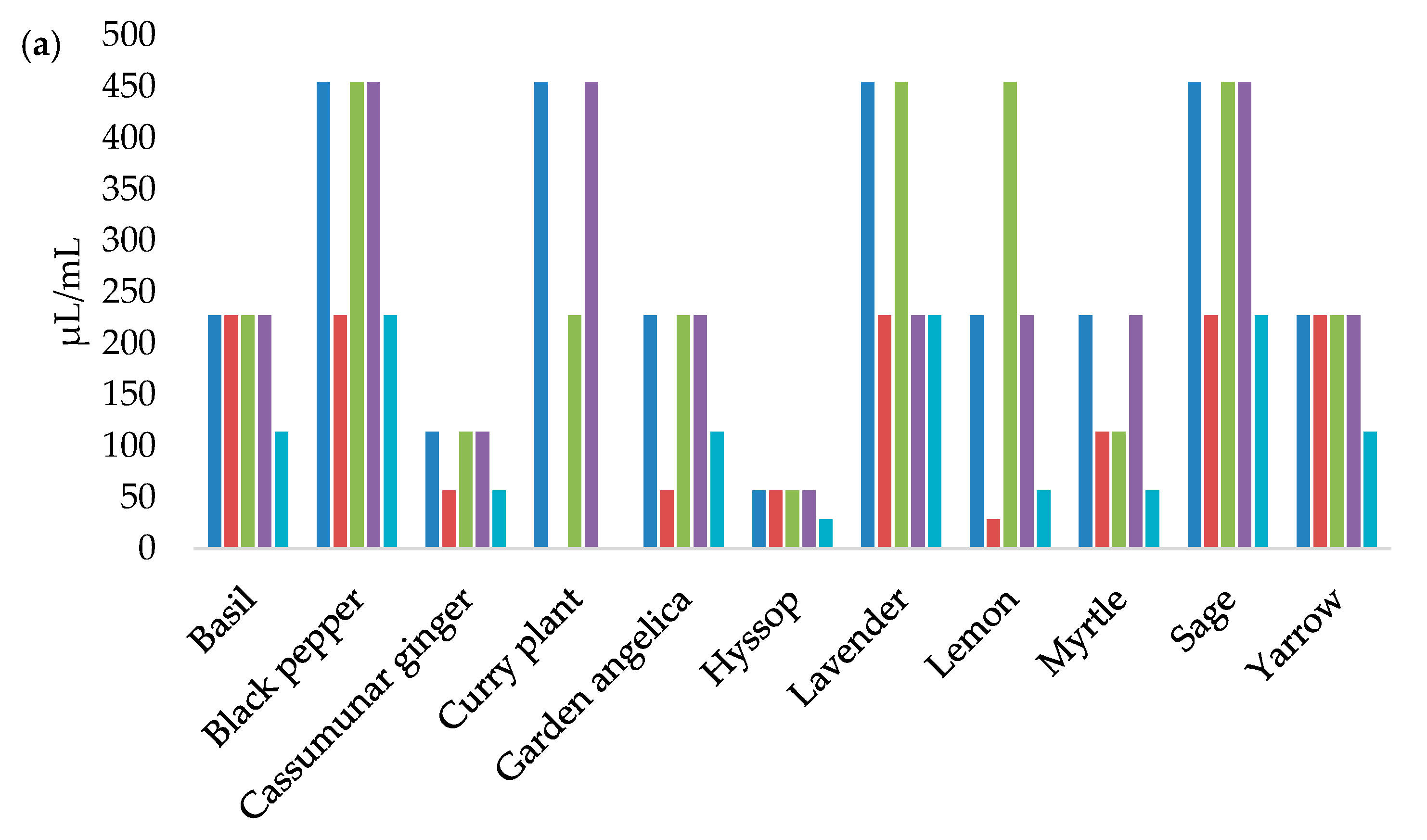
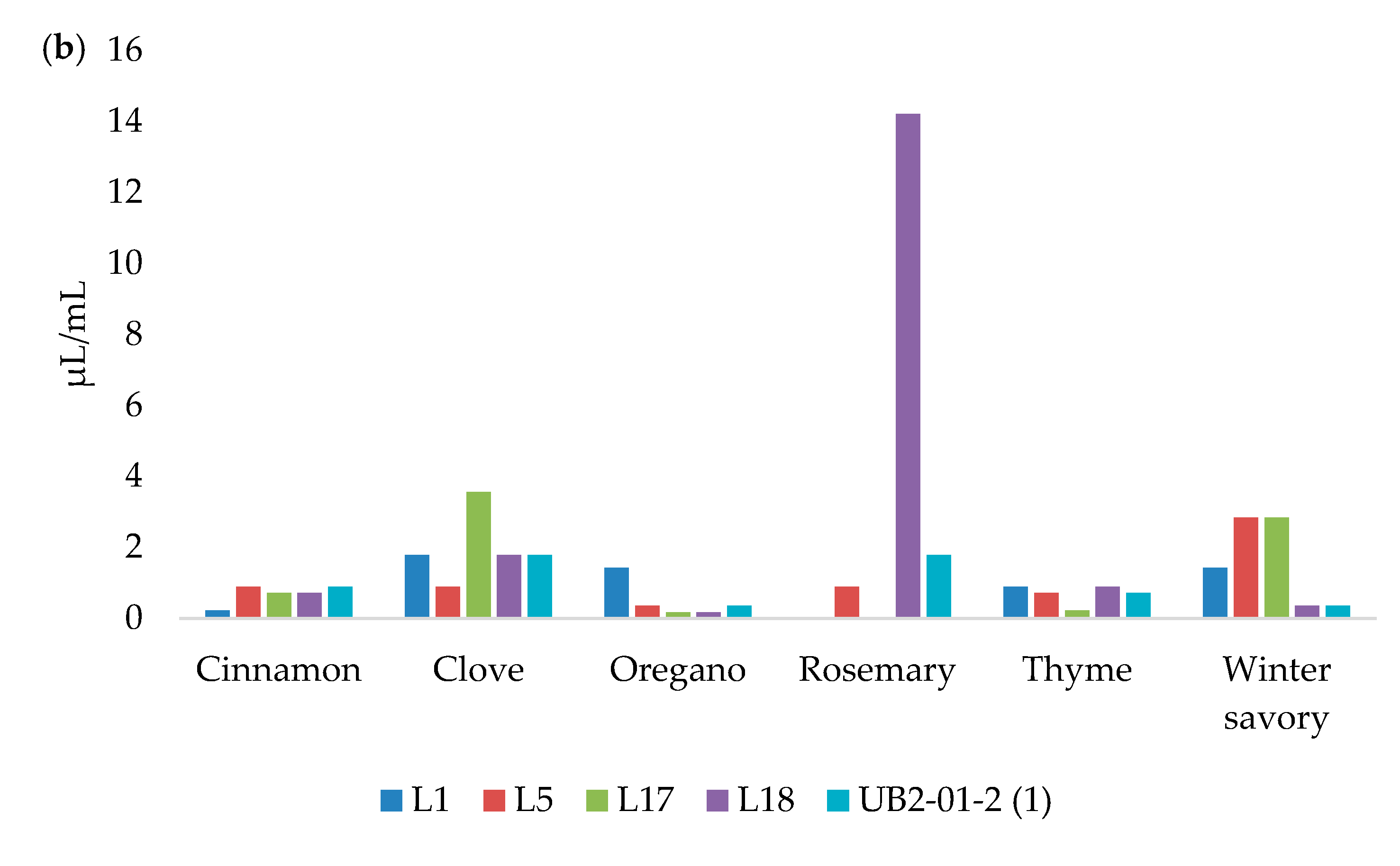
| Essential Oils | L1 | L5 | L17 | L18 | UBL2–01-2(1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 µL | 10 µL | 5 µL | 10 µL | 5 µL | 10 µL | 5 µL | 10 µL | 5 µL | 10 µL | |
| Basil | - 1 | 8.68 ± 1.15 jk | 7.33 ± 0.58 gh | 10.33 ± 0.58 kl | 9.67 ± 0.58 de | 11.00 ± 3.61 ef | 9.67 ± 0.58 de | 13.00 ± 2.00 fg | - | 8.00 ± 0.00 i |
| Black pepper | 10.00 ± 0.00 de | 14.33 ± 1.15 gh | 7.00 ± 0.00 h | 16.33 ± 0.58 fg | 12.00 ± 1.00 cd | 14.33 ± 3.21 def | 12.00 ± 0.00 d | 15.00 ± 2.65 fg | 10.68 ± 0.58 ef | 15.00 ± 0.00 efg |
| Cassumunar ginger | 10.33 ± 1.15 de | 18.00 ± 0.00 e | 11.33 ± 1.15 e | 18.00 ± 2.00 f | 11.33 ± 0.58 d | 18.00 ± 2.00 d | 10.33 ± 0.58 de | 21.00 ± 1.00 d | 7.00 ± 0.00 g | 16.00 ± 1.73 ef |
| Cinnamon | 34.67 ± 1.15 b | 35.67 ± 1.15 c | 31.00 ± 1.00 c | 33.33 ± 1.15 d | 30.33 ± 0.58 b | 33.67 ± 1.53 c | 38.33 ± 1.53 a | 41.00 ± 1.73 c | 34.67 ± 0.58 b | 36.33 ± 2.31 c |
| Clove | 11.33 ± 1.15 d | 17.00 ± 1.00 ef | 15.67 ± 0.58 d | 16.67 ± 0.58 fg | 14.67 ± 1.53 c | 15.33 ± 2.52 def | 15.00 ± 0.00 c | 17.33 ± 1.15 def | 15.33 ± 1.15 d | 16.67 ± 1.15 e |
| Curry plant | 9.67 ± 1.53 de | 14.67 ± 0.58 gh | 10.33 ± 0.58 ef | 11.00 ± 1.00 jkl | - | 12.33 ± 1.53 ef | 9.00 ± 2.00 e | 15.33 ± 1.15 efg | - | - |
| Fennel | - | - | - | - | - | - | - | - | - | - |
| Garden angelica | - | 10.00 ± 0.00 ij | - | 9.00 ± 0.00 l | - | 11.33 ± 1.15 ef | - | 11.00 ± 0.00 g | - | - |
| Hyssop | - | 11.33 ± 1.15 i | 9.67 ± 0.58 ef | 21.33 ± 1.15 e | 10.00 ± 0.00 de | 13.33 ± 1.15 ef | - | 12.67 ± 0.58 fg | 8.67 ± 1.15 fg | 24.00 ± 2.00 d |
| Lavender | 9.00 ± 1.00 ef | 14.00 ± 1.73 gh | 10.00 ± 0.00 ef | 15.00 ± 0.00 gh | 9.00 ± 0.00 de | 13.33 ± 0.58 ef | 10.00 ± 0.00 de | 13.33 ± 1.15 fg | 8.00 ± 0.00 g | 13.33 ± 1.15 fg |
| Lemon | 7.67 ± 1.15 fg | 15.67 ± 0.58 fg | 11.00 ± 2.65 e | 16.33 ± 1.15 fg | 9.33 ± 1.53 de | 12.67 ± 3.06 ef | 10.00 ± 0.00 de | 14.33 ± 2.08 fg | 8.33 ± 1.53 fg | 15.67 ± 1.15 ef |
| Myrtle | 8.00 ± 0.00 fg | 11.67 ± 0.58 i | 9.00 ± 1.00 fg | 13.33 ± 0.58 hij | 11.67 ± 0.58 cd | 15.67 ± 1.15 de | 11.33 ± 0.58 de | 13.67 ± 0.58 fg | 11.67 ± 1.15 e | 13.67 ± 1.15 efg |
| Oregano | 40.00 ± 0.00 a | 43.67 ± 1.53 b | 40.33 ± 0.58 a | 42.67 ± 3.21 b | 37.33 ± 2.89 a | 40.67 ± 1.15 b | 37.67 ± 1.15 a | 46.00 ± 5.57 b | 34.00 ± 1.73 b | 35.33 ± 0.58 c |
| Rosemary | 7.00 ± 0.00 g | 8.00 ± 0.00 k | 7.67 ± 0.58 gh | 13.33 ± 1.15 hij | - | - | 10.67 ± 1.15 de | 20.00 ± 0.00 de | 8.00 ± 0.00 g | 10.33 ± 0.58 hi |
| Sage | - | 13.67 ± 1.53 h | 10.33 ± 1.15 ef | 14.67 ± 0.58 ghi | 11.33 ± 1.53 d | 12.33 ± 1.15 ef | 10.00 ± 0.00 de | 14.67 ± 0.58 fg | 8.33 ± 0.58 fg | 12.33 ± 0.58 gh |
| Thyme | 32.67 ± 1.53 c | 69.00 ± 1.00 a | 34.33 ± 0.58 b | 54.00 ± 2.65 a | 30.33 ± 4.93 b | 65.00 ± 5.57 a | 31.67 ± 4.62 b | 64.67 ± 8.08 a | 45.00 ± 3.61 a | 67.00 ± 2.65 a |
| Winter savory | 32.00 ± 0.00 c | 33.33 ± 1.53 d | 31.33 ± 0.58 c | 36.67 ± 2.08 c | 28.33 ± 0.58 b | 43.00 ± 2.65 b | 33.33 ± 0.58 b | 42.67 ± 2.31 bc | 30.33 ± 2.31 c | 39.67 ± 0.58 b |
| Yarrow | 10.33 ± 0.58 de | 16.67 ± 1.15 ef | 8.00 ± 0.00 gh | 12.33 ± 0.58 ijk | 7.00 ± 0.00 e | 10.67 ± 1.15 f | - | 11.00 ± 0.00 g | 8.00 ± 0.00 g | 15.00 ± 3.61 efg |
| Strains | TSB | MB | LB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 15 °C | 37 °C | 5 °C | 15 °C | 37 °C | 5 °C | 15 °C | 37 °C | |
| L1 | 0.111 ± 0.010 º | 0.221 ± 0.031 * | 0.349 ± 0.046 ** | 0.121 ± 0.007 º | 0.243 ± 0.051 * | 0.290 ± 0.072 ** | 0.076 ± 0.007 º | 0.124 ± 0.021 º | 0.129 ± 0.022 * |
| L5 | 0.103 ± 0.006 º | 0.584 ± 0.081 ** | 0.341 ± 0.047 ** | 0.138 ± 0.009 º | 0.221 ± 0.038 * | 0.324 ± 0,086 ** | 0.081 ± 0.009 º | 0.442 ± 0.100 ** | 0.149 ± 0.010 * |
| L17 | 0.110 ± 0.008 º | 0.144 ± 0.034 º | 0.285 ± 0.037 ** | 0.116 ± 0.015 º | 0.211 ± 0.075 * | 0.279 ± 0.033 ** | 0.076 ± 0.010 º | 0.139 ± 0.011 º | 0.105 ± 0.012 º |
| L18 | 0.095 ± 0.007 º | 0.124 ± 0.011 º | 0.316 ± 0.070 ** | 0.095 ± 0.010 º | 0.143 ± 0.025 º | 0.232 ± 0.075 * | 0.081 ± 0.008 º | 0.122 ± 0.013 º | 0.109 ± 0.015 º |
| UB2-01-2 (1) | 0.111 ± 0.016 º | 0.259 ± 0.030 * | 0.519 ± 0.047 *** | 0.122 ± 0.011 º | 0.178 ± 0.024 * | 0.242 ± 0.026 * | 0.080 ± 0.011 º | 0.382 ± 0.058 * | 0.142 ± 0.018 * |
| Strains | Growth Medium and Temperature | Essential Oils | ||||
|---|---|---|---|---|---|---|
| Oregano | Thyme | Cinnamon | Winter Savory | Clove | ||
| L1 | TSB 37 °C | 51.72 a | 69.25 d | 57.16 ab | 64.92 cd | 61.44 bc |
| L1 | MB 37 °C | 56.72 ab | 53.76 a | 59.20 bc | 62.64 c | 55.57 ab |
| L5 | TSB 37 °C | 62.41 ab | 58.06 a | 59.70 a | 66.01 b | 64.76 b |
| L5 | TSB 15 °C | 69.72 c | 66.32 b | 59.63 a | 66.05 b | 67.54 bc |
| L5 | MB 37 °C | 46.58 b | 32.61 a | 42.90 b | 42.03 b | 41.02 b |
| L5 | LB 15 °C | 78.62 b | 66.82 a | 77.85 b | 78.62 b | 76.75 b |
| L17 | TSB 37 °C | 42.87 ab | 38.98 a | 44.01 ab | 51.64 b | 41.35 a |
| L17 | MB 37 °C | 43.88 a | 46.86 ab | 51.28 b | 44.44 ab | 48.57 ab |
| L18 | TSB 37 °C | 44.38 a | 41.24 a | 38.63 a | 40.77 a | 42.46 a |
| UB2-01-2 (1) | TSB 37 °C | 55.19 a | 54.32 a | 58.77 ab | 58.04 a | 63.31 b |
| Treatments | Days | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Control | 4.18 ± 0.08 aD | 4.18 ± 0.06 aD | 4.56 ± 0.17 aC | 4.85 ± 0.07 aB | 5.28 ± 0.11 aA |
| Oregano EO concentration 0.18 µL/g | 4.20 ± 0.05 aC | 4.20 ± 0.14 aC | 4.54 ± 0.15 aB | 4.65 ± 0.17 bB | 5.03 ± 0.24 bA |
| Oregano EO concentration 0.36 µL/g | 4.23 ± 0.10 aC | 4.16 ± 0.10 aC | 4.30 ± 0.07 bC | 4.60 ± 0.13 bcB | 4.77 ± 0.15 cA |
| Thyme EO concentration 0.36 µL/g | 4.21 ± 0.04 aB | 4.10 ± 0.06 aC | 4.29 ± 0.03 bB | 4.42 ± 0.23 cdB | 5.06 ± 0.12 bA |
| Thyme EO concentration 0.72 µL/g | 4.19 ± 0.09 aC | 4.13 ± 0.08 aC | 4.14 ± 0.05 cC | 4.30 ± 0.10 dB | 4.99 ± 0.13 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidaković Knežević, S.; Knežević, S.; Vranešević, J.; Kravić, S.Ž.; Lakićević, B.; Kocić-Tanackov, S.; Karabasil, N. Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System. Foods 2023, 12, 1930. https://doi.org/10.3390/foods12101930
Vidaković Knežević S, Knežević S, Vranešević J, Kravić SŽ, Lakićević B, Kocić-Tanackov S, Karabasil N. Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System. Foods. 2023; 12(10):1930. https://doi.org/10.3390/foods12101930
Chicago/Turabian StyleVidaković Knežević, Suzana, Slobodan Knežević, Jelena Vranešević, Sneẑana Ž. Kravić, Brankica Lakićević, Sunčica Kocić-Tanackov, and Nedjeljko Karabasil. 2023. "Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System" Foods 12, no. 10: 1930. https://doi.org/10.3390/foods12101930
APA StyleVidaković Knežević, S., Knežević, S., Vranešević, J., Kravić, S. Ž., Lakićević, B., Kocić-Tanackov, S., & Karabasil, N. (2023). Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System. Foods, 12(10), 1930. https://doi.org/10.3390/foods12101930







