Abstract
Sodium chloride (NaCl), as a eustressor, can trigger relevant pathways to cause plants to produce a series of metabolites, thus improving the quality of crops to a certain extent. However, there are few reports on the improvement of nutrient quality and flavor of hydroponic Chinese chives (Allium tuberosum Rottler) by sodium chloride. In this study, five NaCl concentrations were used to investigate the dose-dependent effects on growth, nutritional quality and flavor in Chinese chives. The results show that 10 mM NaCl had no significant effect on the growth of Chinese chives, but significantly decreased the nitrate content by 40% compared with 0 mM NaCl treatment, and the content of soluble protein and vitamin C was increased by 3.6% and 2.1%, respectively. In addition, a total of 75 volatile compounds were identified among five treatments using headspace solid-phase microextraction gas chromatography/mass spectrometry (HS-SPME/GC-MS). Compared with the 0 mM NaCl treatment, 10 mM NaCl had the greatest effect on the quantity and content of volatile compounds, with the total content increased by 27.8%. Furthermore, according to the odor activity values (OAVs) and odor description, there were 14 major aroma-active compounds (OAVs > 1) in Chinese chives. The “garlic and onion” odor was the strongest among the eight categories of aromas, and its highest value was observed in the 10 mM NaCl treatment (OAVs = 794).Taken together, adding 10 mM NaCl to the nutrient solution could improve the nutritional quality and flavor of Chinese chives without affecting their normal growth.
1. Introduction
Vegetables are one of the essential foods in people’s daily diets, providing a variety of nutrients for people, including sugar, minerals, vitamins, dietary fiber and so on [1,2]. In addition, the bioactive substances contained in different vegetables have special health care effects on the human body, such as lycopene in tomatoes, glucosinolates in cruciferous vegetables and prostaglandins in onions [3,4,5]. Therefore, the improvement of vegetable quality can enrich its nutritional and medicinal value, improve its market competitiveness and increase farmers’ income. There are many determinants of vegetable quality, including crop varieties, cultivation methods, management measures, environmental conditions and so forth [6,7]. Hence, we can take corresponding measures according to these influencing factors to obtain high-quality vegetables.
Chinese chive (A. tuberosum Rottl. ex. Spreng.) is a popular vegetable in Asia. It originated in China and is planted all over the country [8]. In addition to being a food, Chinese chive also has a certain medical value, because it contains rich active pharmaceutical ingredients, such as linalool, sulfides and flavonoid glycosides [9,10]. Moreover, the distinctive odor of Chinese chive is also one of the reasons for its popularity [11]. The distinctive odor of Chinese chive is mainly attributed to sulfur-containing compounds, which are produced by the degradation of S-alk(en)yl cysteine sulphoxide (CSO) [12]. In the field, the Bradysiaodoriphaga are attracted by this odor. Therefore, Bradysiaodoriphaga has become the main pest of Chinese chive, causing substantial reduction in Chinese chive production [13]. To solve this problem, farmers have applied large quantities of various pesticides to the roots of Chinese chives, which can reduce insect damage, but causes a high content of pesticide residues in Chinese chive leaves. It affects the safety of consumption and the sustainable development of the Chinese chive industry. The root systems of hydroponic vegetables are immersed in nutrient solution, and the anaerobic environment can prevent the occurrence of pests. Hence, hydroponic Chinese chive cultivation has become a new cultivation method of Chinese chives. According to the actual production effect, there is no Bradysiaodoriphaga in the hydroponic Chinese chive; however, it was also found that its quality is significantly lower than that of soil culture products in the same crop period [12]. For this reason, we need to take certain measures in the cultivation process to solve the problem of quality degradation of hydroponic Chinese chives.
During the growth and development of vegetable crops, appropriate environmental conditions are crucial for quality formation, including soil conditions, light, temperature, humidity, moisture, carbon dioxide (CO2) and nutrients [14,15]. For hydroponic vegetables, the precise management of the nutrient solution provides appropriate environmental conditions. Moderate salinity of nutrient solution as eustress (positive stress) can trigger relevant signal pathways to cause plants to produce a series of metabolites, thus improving the quality of vegetable crops, including nutritional quality, flavor, bioactive compounds and physical characteristics [16,17,18]. Research showed that melon firmness increased more with the application of saline water (6.1 dSm−1) than with control water (1.3 dSm−1) [19]. Adding 0 to 80 mM NaCl in the nutrient solution led to an increase in the fruit firmness of soilless melon [20]. Marín et al. [21] found that 30 mM NaCl increased the vitamin C content of red fruit in pepper. Cardeñosa et al. [22] reported that salinity stress promoted the accumulation of total phenols and antioxidant activity in strawberry fruits. In addition, the glucosinolate contents of Brassicaceae family vegetables can also be increased by salinity [23,24,25]. The flavor of a product is determined by its own quality attributes and the human olfactory system. The quality attributes include sourness, sweetness, bitterness, astringency and volatile compounds, whereas volatile compounds include aldehydes, ethers, alcohols, ketones, esters and so forth [26]. Previous research has shown that increasing salinity can improve the aroma and taste of many vegetables. Titratable acidity (TA) and total soluble solids (TSS) content were increased by adding inorganic salt to nutrient solution in cucumber fruits [27,28]. Similar results were found in cauliflower head [24], melon [20], strawberry [29,30] and watermelon [31]. However, there are few reports on the improvement of the nutritional quality and flavor quality of Chinese chives by moderate salinity. Our rationale is that moderate salinity in nutrient solution can trigger related metabolic pathways of Chinese chive quality formation and improve the content of metabolites, so as to improve its nutritional quality and flavor quality. We then further explain the reason for the improvement of flavor by using the odor activity value. Our findings could provide a theoretical basis for realizing high-quality production of hydroponic Chinese chives.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The seeds of the Chinese chive cultivar “Jiuxing 18” were used as the experimental material for this study. On 10 December 2020, the seeds were sterilized with 1% copper sulfate solution and then sown in a 32-cell tray filled with a mixture of perlite and vermiculite (1:1, v:v). The seedings were irrigated with 1/4 strength Japan Chiba farming vegetable nutrient solution once a day, as described previously [12]. On 8 February 2021, the seedlings were transplanted into the hydroponic cultivation system of a modern glass greenhouse located in the Gansu Agricultural University of Lanzhou (36°30′ N, 103°40′ E). The temperature and relative humidity of the greenhouse were 20 ± 3 °C/15 ± 3 °C (day/night) and 60–70%, respectively. The pH of the solution was adjusted to 6.2 with H3PO4. The concentration of the solution was the total nutrient solution and was renewed every 5 days.
When the height of the seedings reached 30 cm (8 June 2021), we cut off the old leaves of the plant to grow new leaves. At the same time, the hydroponic Chinese chives were randomly divided into 5 groups, each group having 5 rectangular hydroponic boxes. The cover of hydroponic box had 11 holes, and 4 seedings were fixed in every hole. The salinity of the nutrient solution was regulated by adding NaCl to the nutrient solution to the following concentration: 0 mM, 5 mM, 10 mM, 20 mM and 30 mM. Other management of the nutrient solution was the same as the above description. On 8 August 2021, healthy Chinese chive leaves of the same size were selected for sampling from each treatment, and each treatment was repeated three times. All samples were ground into powder in liquid nitrogen and stored at −80 °C until analysis. The overall flowchart of the experiment and the hydroponic system are shown in Figure 1.
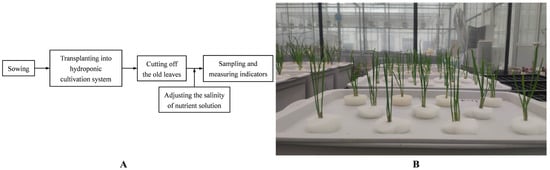
Figure 1.
The overall flowchart of the experiment (A) and the hydroponic system (B). Each hydroponic box cover had 11 holes, and 4 seedings were fixed in every hole.
2.2. Determination of Growth Index and Photosynthetic Pigment Content of Chinese Chive
During harvest, 30 seedings were selected for each treatment to measure the plant height, leaf length and pseudo-stem diameter, and leaf width with a tape measure and vernier caliper, respectively. After harvest, the shoots and roots of the Chinese chives were dried at 105 °C for 30 min, and then at 75 °C to a constant weight for recording fresh weight and dry weight [32]. Root activity was determined using the triphenyl tetrazolium chloride (TTC) method [33] with slight modifications. Briefly, 0.5 g of fresh root tips were cut into 1 cm segments and placed in test tubes containing 5 mL of 1% TTC and 5 mL of 100 mM phosphate buffer (pH 7.5). After holding at 37 °C for 1h, the reaction was terminated by adding 2 mL of 1 M H2SO4. Subsequently, the roots were transferred to a mortar containing 3–5 mL of ethyl acetate (compared with methanol, ethyl acetate has low toxicity, so ethyl acetate was used instead) and a small amount of quartz sand. The grinding homogenate was filtered to the graduated test tube, and the residue was washed 2–3 times with a small amount of ethyl acetate. Finally, the volume of ethyl acetate was fixed at 10 mL, and then the absorbance values were performed with a UV-1780 spectrophotometer at 485 nm.
The photosynthetic pigment content was measured according to a previous study with some modifications [34]. Briefly, 0.1 g of fresh leaves were accurately weighed into the test tube, and then 10 mL of 80% acetone was added. The mixture was extracted in the dark for 48h until the leaves turned white(to ensure complete extraction) and shaken many times during this period (to ensure adequate contact between the leaves and acetone). After extraction, the optical density (OD) was measured with a UV-1780 spectrophotometer at 663 nm and 645 nm. The photosynthetic pigment content was calculated with the following formulas:
where V and FW are the total volume of acetone and the fresh weight of the sample, respectively.
Chl. a (mg∙g−1 FW) = (12.71 × OD663 − 2.59 × OD645) × V/FW
Chl. b (mg∙g−1 FW) = (22.88 × OD645 − 4.67 × OD663) × V/FW
Chl. T (mg∙g−1 FW) = (20.29 × OD645 + 8.04 × OD663) × V/FW
2.3. Determination of Nutritional Quality of Chinese Chive
The soluble sugar content was determined using the anthrone colorimetric method [35]. The soluble protein content was determined using the Coomassie brilliant blue method [36]. The vitamin C content was determined using the 2,6-dichloroindophenol stain method [37]. The nitrate content was determined according to the salicylic acid methods [38].
2.4. Determination of Volatile Flavor Compounds of Chinese Chive
The volatile flavor compounds of Chinese chive were determined using headspace solid-phase microextraction-gas chromatography/mass spectrometry (HS-SPME/GC-MS) [39]. Fresh Chinese chive leaves (1.5 g) were quickly ground into a homogenate and placed in a headspace vial fitted with the PTFE/silicone septa, and then ultrapure water (2 mL), Na2SO4 (0.75 g), 4 µL of difurfuryl sulfide (21.4 mg/L) and a magnetic stirring rotor were added into the vial. In order to extract more compounds, the headspace vial was heated at 70 °C for 15 min for equilibrating. After equilibration, the 85 µm CAR/PDMS fiber (Sigma-Aldrich, St. Louis, MO, USA) was inserted into the headspace vial for extracting with heating and agitation (50 min). At last, the SPME fiber was desorbed for 5 min in GC-MS (Agilent 7890B-7000D, Agilent, Santa Clara, CA, USA) using splitless mode.
The conditions of gas chromatography (GC) and mass spectrometry (MS) were as follows: DB-WAX capillary column (30 m × 0.25 mm, 0.25 µm); carrier gas and flow rate: He (>99.999% purity) at 1 mL/min; temperature program: initially 40 °C held for 1 min, increased to 80 °C at a rate of 8 °C/min, then increased to 130 °C at 2 °C/min and finally increased to 220 °C at 6 °C/min maintained for 3 min; MS ionization, electron ionization (EI), ionization energy: 70 eV; source temperature: 230 °C; scan area: 30–660 amu.
Matching score and retention index (RI) were used for the qualitative analysis of volatile compounds. For matching score, after comparison with the mass spectrometry library (NIST 2014), only compounds with a matching score of more than 70 were maintained. The RI was calculated on a DB-WAX chromatographic column with a C7–C40 n-alkanes series as external references under the same chromatographic conditions. The quantitative analysis of volatile compounds was performed according to the internal standard method, and the formula is as follows [40]:
where S1 and S2 represent the peak area of detected composition and the internal standard, respectively; M1 and M2 represent the amounts of the internal standard (µg) and the sample (g), respectively.
2.5. Calculation of Odor Activity Values
Odor activity values (OAVs) reflect the contribution of volatile compounds to the overall flavor. The calculation formula is as follows: OAVs = Ci/OTi(5) (Ci: the actual concentration of a certain volatile in Chinese chive; OTi: the odor threshold of the corresponding volatile) [41].
2.6. Statistical Analysis
The experimental data were analyzed using single factor variance (ANOVA) and Duncan’s multiple range tests of variance (p < 0.05) with SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). All data were expressed as means ± standard error (SE). A Principal Components Analysis (PCA) was performed to determine differences between treatment groups. All figures were generated using OriginPro 2021 (OriginLab, Northampton, MA, USA).
3. Results
3.1. Effects of Adding Sodium Chloride in Nutrient Solution on Growth and Photosynthetic Pigment Content of Hydroponic Chinese Chive
As shown in Figure 2, the sodium chloride level had a significant effect on the growth and photosynthetic pigment content of the hydroponic Chinese chive. Compared with 0 mM treatment, 5–20 mM NaCl treatments had no significant effect on plant height and leaf length; however, 30 mM NaCl significantly decreased plant height and leaf length (Figure 2A). The pseudo-stem diameter treated with NaCl was significantly lower than the control (0 mM), especially 30 mM NaCl, which decreased by 38%. As the concentration of NaCl increased, the leaf width and leaf number increased at first and then decreased, reaching the maximum at 10 mM. When treated with 5–20 mM NaCl, the fresh weight of the root had no significant effect compared with the control (0 mM); however, the dry weight of the root treated with NaCl was significantly lower than the control (0 mM) (Figure 2B). The fresh and dry weights of the shoot had no significant effects when treated with 5 and 10 mM NaCl. Similarly, compared with 0 mM treatment, 5 and 10 mM NaCl had no significant effect on the content of Chl.a andChl.T, whereas the content of Chl.a treated with 20 and 30 mM NaCl significantly decreased by 21 and 25%, respectively (Figure 2C). In addition, 5–30 mM NaCl treatments had no significant effect on the Chl.b content. As the concentration of NaCl increased, the root activity significantly increased by 40, 42, 77 and 79%, respectively, compared with 0 mM treatment (Figure 2D).
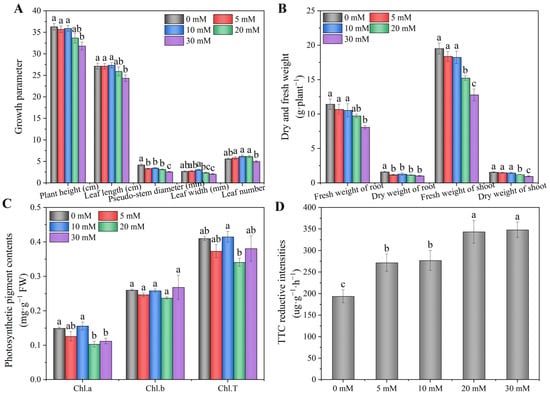
Figure 2.
Effects of adding sodium chloride in nutrient solution on growth (A,B), photosynthetic pigment content (C) and root activity (D) of hydroponic Chinese chive. Values are represented as mean ± SE (n = 3). Different lowercase letters denote significant differences by Duncan’s multiple range test (p < 0.05).
3.2. Effects of Adding Sodium Chloride in Nutrient Solution on Soluble Sugar, Soluble Protein, Vitamin C and Nitrate Content of Hydroponic Chinese Chive
Compared with 0 mM treatment, 5 and 10 mM NaCl treatments had no significant effect on the soluble sugar content; however, 20 and 30 mM NaCl significantly decreased the soluble sugar content by 50 and 40%, respectively (Figure 3A). With the increase in NaCl concentration, the content of soluble protein and vitamin C presented an uptrend as a whole, and increased by 9.6 and 3.2% from 0 to 30 mM NaCl, respectively (Figure 3B,C). Moreover, when treated with 5–30 mM NaCl, the nitrate content significantly decreased by 21, 40, 39 and 44%, respectively (Figure 3D).
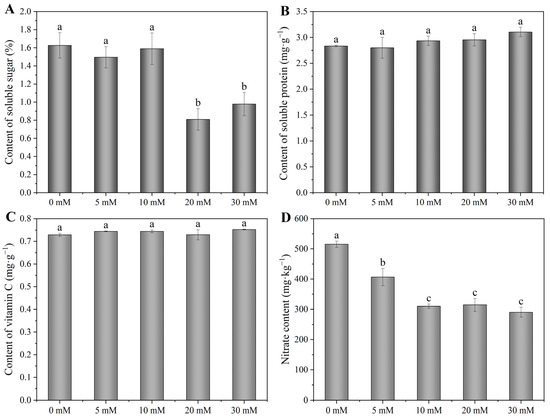
Figure 3.
Effects of adding sodium chloride in nutrient solution on soluble sugar (A), soluble protein (B), vitamin C (C) and nitrate content (D) of hydroponic Chinese chive. Values are represented as mean ± SE (n = 3). Different lowercase letters denote significant differences by Duncan’s multiple range test (p < 0.05).
3.3. Effects of Adding Sodium Chloride in Nutrient Solution on Volatile Flavor Compounds of Hydroponic Chinese Chive
As shown in Table 1, a total of 75 volatile compounds were detected in hydroponic Chinese chive by using HS-SPME/GC-MS technology. These volatile compounds can be classified into nine categories according to their chemical structures, including 14 aldehydes, 19 ethers, 11 alcohols, 7 ketones, 6 hydrocarbons, 9 esters, 2 phenols, 2 furans, and 5 others. In terms of the amounts of volatile compounds, 65 compounds were detected in the 10 mM NaCl treatment, which was the highest among all treatments, followed by the 20 mM treatment with 59 compounds. Additionally, 58 compounds were found in both the 5 mM treatment and the 20 mM treatment. The control group contained the least volatile compounds, with only 48 compounds. From the content point of view, the total content of volatile compounds in the 10 mM NaCl treatment was the highest, reaching 18,733.52 μg/kg and increasing by 27.8% compared with the 0 mM treatment (14,659.02 μg/kg), followed by the 5 mM treatment with 16,408.35 μg/kg. However, 20 and 30 mM NaCl decreased the content of volatile compounds by 22.3 and 14.5%, respectively, compared with the 0 mM treatment.

Table 1.
Effects of adding sodium chloride in nutrient solution on volatile compounds of hydroponic Chinese chive.
There were 14 aldehydes detected in all treatment groups, of which 11 were detected in each treatment (Table 1). Compared with the 0 mM treatment, more aldehydes were detected in the NaCl treatment, such as (E)-2-octenal, 2-undecenal and (E)-2-tridecenal. Treatment with 20 and 30 mM NaCl induced production of all 14 aldehydes. The amounts of ethers were the largest among the volatile compounds of Chinese chive, followed by aldehydes. Treatment with 10 mM NaCl promoted the production of four ethers compared with 0 mM treatment, namely 2,4-dimethylthiophene, methyl propyl disulfide, methyl propyl trisulfide and 1-allyl-3-propyltrisulfane. Although the amounts of ethers treated with 5, 20 and 30 mM NaCl were the same, the constituent components were different; for example, methyl propyl trisulfide was detected in the 20 and 30 mM NaCl treatments, while 3-ethenyl-3,6-dihydrodithiine was only detected in the 5 mM treatment. Additionally, most kinds of ketones and esters in Chinese chive were obtained under 5 mM NaCl treatment, while the alcohols were lower than other NaCl treatments. For other compounds, 30 mM NaCl promoted the production of more compounds, such as 2-methyl-2-phenyl oxirane and dodecanoic acid.
As shown in Figure 4 and Table 1, the contents of various volatile compounds in different treatments of Chinese chive had great differences. Ethers (7465.47–13,585.64 μg/kg) were the most abundant of all compounds, followed by aldehydes (1065.56–2324.82 μg/kg). The phenols content (56.04–75.57 μg/kg) was the lowest, and furans (14.59–140.52 μg/kg) were only detected in the 5 and 10 mM treatment. Compared with 0 mM NaCl treatment, 5–30 mM NaCl significantly promoted the content of aldehydes, especially the 10 mM NaCl treatment, which increased by 118%. However, when the concentration of NaCl exceeded 10 mM, the content of ethers significantly decreased. The 10 mM NaCl treatment significantly promoted the content of alcohols and ketones, and the 5–20 mM NaCl treatment significantly promoted the content of hydrocarbons.
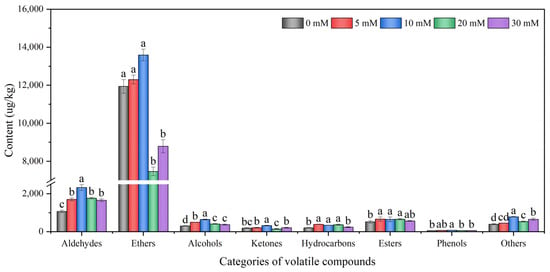
Figure 4.
Effects of adding sodium chloride in nutrient solution on the content of volatile compounds of hydroponic Chinese chive. Values are represented as mean ± SE (n = 3). Different lowercase letters denote significant differences by Duncan’s multiple range test (p < 0.05).
The Venn diagram shows common and specific volatile compounds among different treatments of hydroponic Chinese chive (Figure 5). A total of 41 volatile compounds were common substances among the five treatments. The 41 common compounds comprised 11 aldehydes, 13 ethers, 4 alcohols, 2 ketones, 1 hydrocarbon, 6 esters, 2 phenols and 2 other compounds. Treatment with 5, 10, 20 and 30 mM NaCl produced one, four, one and three specific compounds, respectively. The one specific compound produced by 5 and 20 mM NaCl treatment was 2,3-dihydrofuran and 7-tetradecyne, respectively. The four special compounds produced by 10 mM NaCl treatment were methyl propyl disulfide, (Z)-2-penten-1-ol, 1-hexadecanol and 2-pentylfuran, of which the content of 1-hexadecanol was the highest, reaching 45.54 μg/kg. Treatment with 30 mM NaCl produced three specific compounds: cis-4-Isopropenyl-1-methylcyclohexanol, 2,3,4-trimethyl-2-cyclopenten-1-one and 2-methyl-2-phenyl oxirane. Of these, cis-4-Isopropenyl-1-methylcyclohexanol was the most abundant, reaching 56.41 μg/kg.
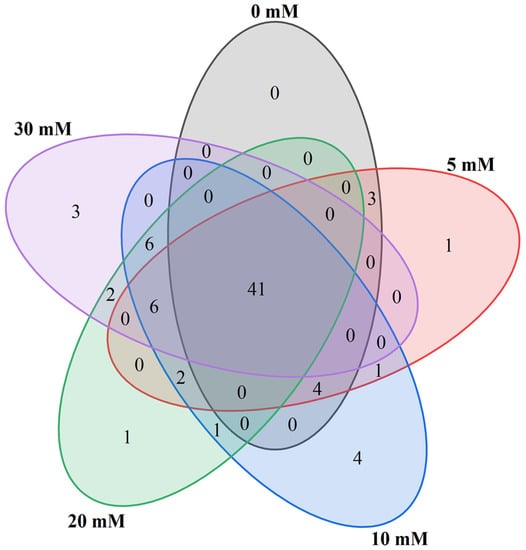
Figure 5.
Effects of adding sodium chloride in nutrient solution on common and specific volatile compounds of hydroponic Chinese chive.
3.4. Odor Activity Values Analysis and Radar Fingerprint Chart of Volatile Compounds in Chinese Chive
In general, volatile compounds with OAVs > 1 have an actual contribution to the overall flavor, that is, major aroma-active compounds. As shown in Table 2, there were only 14 volatile compounds with OAVs > 1, mainly aldehydes and ethers, but also ketones, phenols and furan. Moreover, the compounds with OAVs < 1 also have a certain effect on the overall flavor. According to the odor description, the aroma of volatile compounds in Chinese chive can be divided into eight categories, including “green and grassy”, “floral”, “fatty”, “sweet”, “garlic and onion”, “fresh”, “spicy” and “fruity” (Figure 6). The “garlic and onion” odor was the strongest scent, which mainly derived from ethers. Dimethyl disulfide, dimethyl trisulfide and diallyl disulfide are three major aroma-active compounds in ethers. Due to the lower threshold (6 μg/kg) and high concentration of dimethyl trisulfide, it contributed greatly to the “garlic and onion” odor. Among these treatments, the 10 mM NaCl treatment had a stronger “garlic and onion” odor than other treatments because it promoted the production of more ethers.

Table 2.
Odor activity values (OAVs) and odor description of volatile compounds in Chinese chive at different sodium chloride concentrations of nutrient solution.
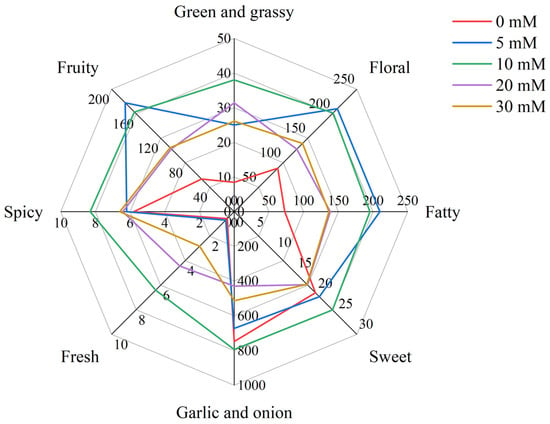
Figure 6.
Radar fingerprint chart of volatile compounds in Chinese chive at different sodium chloride concentrations of nutrient solution.
As shown in the radar map, the outline gradually changed after treatment with different concentrations of NaCl. The “fruity”, “floral” and “fatty” odors of treatment with 5 mM NaCl were stronger than in other treatments. The “fruity” odor mainly came from aldehydes, especially nonanal, whose threshold was only 1 μg/kg, so we could easily smell its aroma. Besides the “fruity” odor, nonanal also contained a “floral” and “fatty” odor. The other two major contributors to “floral” were decanal and β-ionone, both of which were major aroma-active compounds. The “fatty” odor was composed of aldehydes, containing (E)-2-octenal in addition to the five aldehydes that were common in the 10, 20 and 30 mM NaCl treatments. Compared with 0 mM treatment, NaCl treatments strengthened the “green and grassy” and “fresh” and “spicy” odor of Chinese chive, but the “sweet” odor was weakened when the NaCl concentration reached 20 mM. The “sweet” odor mainly came from the decanal mentioned above and two phenols of 2-methoxy-4-vinylphenol and eugenol, which also contributed to the “spicy” odor of Chinese chives. The “fresh” odor came from just two compounds, but only one of them was a major aroma-active compound, that is, (E)-2-octenal, whose content determined the intensity of “fresh” odor in Chinese chives. There were as many as 11 compounds in Chinese chive that could contribute to the “green and grassy” odor, and the two most important compounds were trans, trans-2,4-heptadienal and (2E,4E)-2,4-octadienal, which were the major aroma-active compounds.
3.5. Principal Component Analysis
The principal component analysis (PCA) of growth and quality parameters, as well as the 75 volatile compounds in this study, are shown in Figure 7. The sum of the first two principal components of growth and quality parameters reached 77.3%, of which PC1 and PC2 accounted for 53.6% and 23.7% of the total variance, respectively (Figure 7A). The first principal component sorted the treatments into two groups: 0, 5, and 10 mM were close to one group, while 20 and 30 mM were close to one group. It could be observed that the soluble sugar and Chl.b showed strong loadings with the first and second principal component, respectively. The root activity was highly negative to PC1, and nitrate was highly negative to PC2. The sum of the first two principal components of the 75 volatile compounds reached 57.3%, of which PC1 and PC2 accounted for 32.1% and 25.2% of the total variance, respectively (Figure 7B). The second principal component sorted the treatments into two groups: 0, 5, and 10 mM were close to one group, while 20 and 30 mM were close to one group, which was consistent with the results of growth and quality parameters.
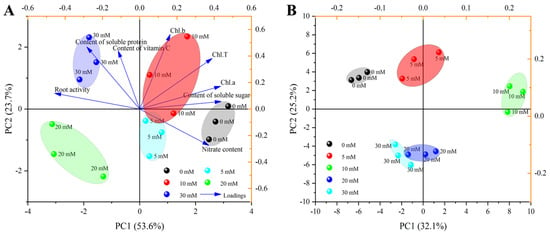
Figure 7.
Principal component analysis (PCA) of growth and quality parameters (A) and 75 volatile compounds (B).
4. Discussion
Adding different concentrations of sodium chloride in nutrient solution can induce different biochemical, morphological and physiological responses in plants. In the present study, we found that except for pseudo-stem diameter, low concentrations of NaCl treatment (5 and 10 mM) did not significantly inhibit the growth of Chinese chive and even had a certain extent of promotion (Figure 2A), which is consistent with the findings of Wang et al. [45] and Cheng et al. [46]. Wang et al. reported that the shoot growth of canola was promoted by 50 mM NaCl. Cheng et al. found that 10 mM NaCl promoted the growth of cassava by increasing and changing the morphological characteristics and number of root cells. To sum up, a low sodium chloride level of nutrient solution doesn’t inhibit plant growth and even has a certain growth-promoting effect on some plants. Dry and fresh weight is an important index reflecting the growth status of plants and the content of nutrients and structural substances. Our results show that low concentrations of NaCl (5 and 10 mM) had no significant effect on the fresh weight of the root or the fresh and dry weight of the shoot, while high concentrations of NaCl inhibited the fresh and dry weight of the root and shoot (Figure 2B). High concentration of NaCl caused salt stress on Chinese chives, resulting in reduced uptake of nutrients and water by roots, thus affecting the growth and development of plants [47,48]. Photosynthesis is the basis of plant growth and development, and chlorophyll is the key substance of photosynthesis. Therefore, chlorophyll content is an important indicator of plant growth. This experimental study showed that salinity mainly affected the content of Chl.a and Chl.T. When the concentration of NaCl reached 30 mM, the decrease in Chl.T was less than that in Chl.a due to no significant effects of salinity on Chl.b, but overall, the content of Chl.a and Chl.T decreased when the NaCl concentration reached 20 mM (Figure 2C), which was similar to the results of previous studies on alfalfa [49]. This may be due to a large amount of reactive oxygen species produced in Chinese chives under high concentrations of NaCl, which destroyed the structure of chloroplasts and slowed the synthesis of chlorophyll, resulting in a decrease in chlorophyll content [50]. The root is an important organ for plants to absorb water and nutrients, and its growth, development and root activity directly affects the life activities of individual plants. In this study, we found that with the increase in NaCl concentration, the root activity of Chinese chive was continuously enhanced, reaching the highest value at 30 mM (Figure 2D). The results were consistent with the findings of Wang et al. [51], who reported that 200 mM NaCl treatment significantly increased the root activity of Limonium bicolor, compared with 0 mM treatment. However, Zhang et al. [52] reported that 150 mM NaCl significantly decreased the root activity of wheat seedlings. This indicates that different plants at different growth stages respond differently to NaCl. Therefore, it is necessary to determine the optimum concentration of NaCl through corresponding pre-experiments in actual production.
With the improvement of people’s living standards, safe, healthy and high-quality agricultural products have become a mainstream demand in the agricultural market [16]. Therefore, how to improve the quality of agricultural products through corresponding agronomic measures has become one of the hotspots of current research. Compared with conventional breeding and genetic transformation, improving crop quality through environmental and agronomic factors has the advantages of safety, speed, high success rate and wide adaptability, and can also reduce consumers’ worries about genetically transformed or modified products [16,18]. At present, it has become a practicable method to improve the quality of crops by adding NaCl in nutrient solution [17]. This study found that the content of soluble sugar significantly decreased when the NaCl concentration reached 20 mM (Figure 3A), which may be due to the high concentration of NaCl-caused abiotic stress to Chinese chives, resulting in the decrease in chlorophyll content, then the weakening of photosynthesis, and finally, the reduction in soluble sugar content. However, compared with the 0 mM treatment, the content of soluble protein and vitamin C presented an uptrend with the increase in NaCl concentration (Figure 3B,C), which was similar to the results of Abdullah et al. [53] and Li et al. [54]. Soluble protein is an important osmotic regulator, so increasing it under salt stress helps to improve the stress resistance of plants and also improves the nutritional quality to a certain extent. In addition, we observed that NaCl treatments significantly decreased the nitrate content compared with the control (0 mM) (Figure 3D), which may be because related genes encoding nitrate reductase enzyme were reduced by NaCl [55]. In general, this study demonstrated that adding NaCl in a nutrient solution improved the nutritional quality and safety quality of Chinese chives.
Compared with the nutritional value of Chinese chives, it seems that people are more familiar with their unique flavor. The main source of the unique aroma of Chinese chive is secondary metabolites, such as sulfur-containing compounds [56,57]. Salinity can induce plants to produce related secondary metabolites, so it can be inferred that moderate salinity can improve the flavor quality of Chinese chives. In this study, we detected a total of 75 volatile compounds among the five treatments, which mainly comprised aldehydes, ethers, alcohols, ketones, hydrocarbons, esters, phenols, furans and other compounds (Table 1). Compared with 0 mM treatment, 5 and 10 mM NaCl treatments improved the quantity and content of volatile compounds, suggesting that moderate NaCl concentration stimulated the metabolic pathway related to the production of volatile compounds in Chinese chive, thereby improving its flavor quality. Similar results were found by Neffati [58], who reported that the essential oil yield of coriander leaves increased significantly under moderate salinity (25 and 50 mM NaCl) and decreased significantly under 75 mM NaCl. Chatterjee et al. [59] also found that 25 and 50 mM NaCl increased the green leaf volatiles of rice. Ethers are the main volatile compounds of Allium vegetables, which are called thioethers. Among the volatile compounds of Chinese chive, we detected 19 thioethers in five treatments with the largest quantity and content of all the compounds, which is consistent with previous research results [56,57]. Compared with 0 mM treatment, 5 and 10 mM NaCl increased the thioethers content by 3 and 13.8%, respectively (Figure 4), indicating that moderate NaCl concentration may stimulate the expression of key genes involved in the metabolic pathway of thioethers generation [12]. The content of aldehydes in the volatile compounds of Chinese chive was second only to that of ethers, but unlike ethers, the total content was increased in all NaCl treatment groups, indicating that the effects of nutrient solution salt on various substances were different (Figure 4). The content of trans-2-hexenal was the highest in aldehydes, which are released when plant cells are damaged under external stress [60]. Therefore, the growth status of plants can be judged by measuring the content of trans-2-hexenal in the air in the field, so as to take corresponding protective measures [61]. More alcohols were detected when the concentration of NaCl reached 10 mM, such as linalool, isophytol, 3-hexen-1-ol, etc. Linalool is a volatile compound that mainly exists in flowers. It not only endows plants with floral fragrance, but also participates in multiple biological processes of plant growth and development, such as pollinator attraction and plant defense [62]. In this experiment, only two phenols were detected in Chinese chive, namely eugenol and 2-methoxy-4-vinylphenol (Table 1), among which eugenol is not only the aromatic compound of many fruits [63,64], but also has antioxidant and antimicrobial properties [65]. Therefore, phenols and sulfur-containing compounds contribute to both the antioxidant and antimicrobial properties of Chinese chives.
When volatile compounds enter the human olfactory system, they react with olfactory receptors and eventually produce the corresponding olfaction in the cerebral cortex. The intensity of odor depends on two factors, the actual concentration and its odor threshold, of which the odor threshold refers to the lowest concentration that can be smelled by the human body [66,67]. The ratio between actual concentration and odor threshold is odor activity values (OAVs), which reflect the contribution of volatile compounds to the overall flavor [68]. In this study, we detected 14 major aroma-active compounds according to OAVs > 1 which are essential for the aroma quality of Chinese chive (Table 2). In addition, the volatile compounds with OAVs < 1 influence the flavor of the Chinese chive through internal synergistic effects [69]. Therefore, according to the odor description and OAVs of each volatile compound, we have drawn the radar fingerprints of volatile compounds in Chinese chives under different NaCl concentrations of nutrient solution (Figure 6). As shown in the radar map, the OAVs of the “garlic and onion” odor are much higher than others; that is why the aroma of Chinese chives is similar to garlic and onion. At the same time, the “garlic and onion” odor comes from thioethers, so it is crucial to improve the flavor quality of Chinese chive by increasing the content of thioethers, especially dimethyl trisulfide, which widely exists in Allium [70]. The “floral” odor was mainly comprised of nonanal and β-ionone, wherein β- Ionone is widely found in vegetables and fruits and is the major aroma-active compound in some plants [71,72]. The OAVs of the “fresh” and “spicy” odors were the lowest of the eight categories because there were few kinds and low concentrations of odor contributing compounds. Among the five treatments, the effects of NaCl treatments on the eight categories of odor were different, indicating that NaCl, as a eustressor, had different stimulating effects on the generation pathways of various volatile compounds in Chinese chive, which needs further research.
Principal component analysis (PCA) is a commonly used multivariate analysis method which can clearly show the differences between samples [73]. As shown in Figure 7, with the increase in NaCl concentration, each treatment was divided into different groups according to growth and quality parameters and 75 volatile compounds. For growth and quality parameters, 0, 5 and 10 mM were close to one group, indicating that low salinity would not inhibit the growth of Chinese chives, which was similar to the research results of Zhao et al. [74]. For volatile compounds, grouping was similar to growth and quality parameters, indicating that high salinity weakened the flavor of Chinese chives. Similar results were found in Lycopersicum esculentum leaves [75] and rosemary [76]. On the whole, adding 10 mM NaCl to the nutrient solution can improve the nutritional quality and flavor of Chinese chive without affecting their normal growth, which provides a theoretical basis for the high-quality cultivation of Chinese chive in hydroponics.
5. Conclusions
In this study, nutrient solution treatment with 10 mM NaCl had no significant effect on growth parameters, dry and fresh weight, photosynthetic pigment content and soluble sugar content of Chinese chive. Differently, treatment with 10 mM NaCl significantly decreased the nitrate content and increased the root activity. Moreover, soluble protein and vitamin C content were increased under all NaCl treatments. A total of 75 volatile compounds were detected in Chinese chive, including 14 aldehydes, 19 ethers, 11 alcohols, 7 ketones, 6 hydrocarbons, 9 esters, 2 phenols, 2 furans and 5 others. Treatment with 10 mM NaCl had the greatest effect on the quantity and content of volatile compounds, especially aldehydes and ethers. According to the odor activity values (OAVs), there are 14 major aroma-active compounds (OAVs > 1) in Chinese chives. The “garlic and onion” odor was the strongest among the eight categories of aroma in Chinese chives. The 10 mM NaCl treatment had the strongest “garlic and onion” odor among the different treatments. In conclusion, nutrient solution treatment with 10 mM NaCl could improve the nutritional quality and flavor of Chinese chives without affecting their normal growth. The research results provide a feasible application basis for improving the nutritional quality and flavor of the hydroponic Chinese chives. In addition, vegetables with stronger flavor or special medical value can be produced by using similar methods, which can not only meet people’s daily health care needs, but also increase farmers’ income and improve the local economic level.
Author Contributions
Conceptualization, X.X. and J.Y.; methodology, B.X.; software, S.W.; validation, B.X., S.W. and H.L.; formal analysis, J.L.; investigation, B.X. and Y.G.; resources, X.X. and J.Y.; data curation, J.Y.; writing—original draft preparation, B.X.; writing—review and editing, B.X., X.X. and J.Y.; visualization, X.X.; supervision, J.Y.; project administration, X.X. and J.Y.; funding acquisition, X.X. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32160705), Top Leading Talent Plan of Gansu Province (GSBJLJ-2021-14), Youth Tutor Support Fund Project of Gansu Agricultural University (GAU-QDFC-2022-03), Modern Silk Road Cold and Dry Agri-cultural Science and Technology Support Project (GSLK-2021-6).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, L.; Hao, N.; Wu, T.; Cao, J. Advances in Understanding and Harnessing the Molecular Regulatory Mechanisms of Vegetable Quality. Front. Plant Sci. 2022, 13, 836515. [Google Scholar] [CrossRef]
- Drewnowski, A.; Maillot, M.; Vieux, F. Multiple Metrics of Carbohydrate Quality Place Starchy Vegetables Alongside Non-starchy Vegetables, Legumes, and Whole Fruit. Front. Nutr. 2022, 9, 867378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Li, J.; Dawuda, M.M.; Ali, B.; Wu, Y.; Yu, J.; Tang, Z.; Lyu, J.; Xiao, X.; et al. Exogenous Application of 5-Aminolevulinic Acid Promotes Coloration and Improves the Quality of Tomato Fruit by Regulating Carotenoid Metabolism. Front. Plant Sci. 2021, 12, 683868. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Jin, L.; Wei, S.; Wang, S.; Jin, N.; Wang, J.; Xie, J.; Feng, Z.; Zhang, G.; et al. Combined Straw and Plastic Film Mulching Can Increase the Yield and Quality of Open Field Loose-Curd Cauliflower. Front. Nutr. 2022, 9, 888728. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pena, D.; Checa, A.; de Ancos, B.; Wheelock, C.E.; Sanchez-Moreno, C. New insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biol. 2017, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Barth, M.M. Preharvest Factors Affecting Postharvest Quality of Vegetables. HortScience Publ. Am. Soc. Hortic. Sci. 1997, 32, 812–816. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Mau, J.L.; Chen, C.P.; Hsieh, P.C. Antimicrobial Effect of Extracts from Chinese Chive, Cinnamon, and Corni Fructus. J. Agric. Food Chem. 2001, 49, 183. [Google Scholar] [CrossRef]
- Han, S.H.; Suh, W.S.; Park, K.J.; Kim, K.H.; Lee, K.R. Two new phenylpropane glycosides from Allium tuberosum Rottler. Arch. Pharm. Res. 2015, 38, 1312–1316. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhang, H.L.; Lu, C.Q.; Luo, J.P.; Zha, X.Q. A new kinetic model of ultrasound-assisted extraction of polysaccharides from Chinese chive. Food Chem. 2016, 212, 274–281. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tong, J.; Hu, M.; Ji, Y.; Wang, B.; Liang, H.; Liu, M.; Wu, Z. Transcriptome landscapes of multiple tissues highlight the genes involved in the flavor metabolic pathway in Chinese chive (Allium tuberosum). Genomics 2021, 113, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, C.; Liu, Y.; Shan, T.; Shi, X.; Gao, X. Integration analysis of PacBio SMRT- and Illumina RNA-seq reveals P450 genes involved in thiamethoxam detoxification in Bradysia odoriphaga. Pestic. Biochem. Physiol. 2022, 186, 105176. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water Deficit and Salinity Stress Reveal Many Specific QTL for Plant Growth and Fruit Quality Traits in Tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Botía, P.; Navarro, J.M.; Cerdá, A.; Martínez, V. Yield and fruit quality of two melon cultivars irrigated with saline water at different stages of development. Eur. J. Agron. 2005, 23, 243–253. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Marín, A.; Rubio, J.S.; Martínez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Cardenosa, V.; Medrano, E.; Lorenzo, P.; Sanchez-Guerrero, M.C.; Cuevas, F.; Pradas, I.; Moreno-Rojas, J.M. Effects of salinity and nitrogen supply on the quality and health-related compounds of strawberry fruits (Fragaria × ananassa cv. Primoris). J. Sci. Food Agric. 2015, 95, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta Mdel, C.; Muries, B.; Moreno, D.A.; Dominguez-Perles, R.; Garcia-Viguera, C.; Carvajal, M. Involvement of a glucosinolate (sinigrin) in the regulation of water transport in Brassica oleracea grown under salt stress. Physiol. Plant. 2014, 150, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Cassaniti, C.; Malvuccio, A.; Leonardi, C. Effects of salt stress imposed during two growth phases on cauliflower production and quality. J. Sci. Food Agric. 2017, 97, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-H.; Cao, W.; Peng, H.-H.; Wang, F.; Tatsumi, E.; Kohyama, K.; Li, L.-T. Effect of fermentation metabolites on rheological and sensory properties of fermented rice noodles. J. Sci. Food Agric. 2008, 88, 2134–2141. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Gil, M.I.; Izumi, H.; Colelli, G.; Watkins, C.B.; Zude, M. Quality and safety of fresh horticultural commodities: Recent advances and future perspectives. Food Packag. Shelf Life 2017, 14, 2–11. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardarelli, M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Jawad, R.; Kumar, P.; Rea, E.; Cardarelli, M. The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 2013, 164, 380–391. [Google Scholar] [CrossRef]
- Galli, V.; da Silva Messias, R.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. LWT 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Keutgen, A.; Pawelzik, E. Modifications of taste-relevant compounds in strawberry fruit under NaCl salinity. Food Chem. 2007, 105, 1487–1494. [Google Scholar] [CrossRef]
- Lei, T.; Xiao, J.; Li, G.; Mao, J.; Wang, J.; Liu, Z.; Zhang, J. Effect of Drip Irrigation with Saline Water on Water Use Efficiency and Quality of Watermelons. Water Resour. Manag. 2003, 17, 395–408. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Xie, J.; Yu, J.; Li, J.; Lv, J.; Gao, Y.; Niu, T.; Patience, B.E. Effects of Preharvest Methyl Jasmonate and Salicylic Acid Treatments on Growth, Quality, Volatile Components, and Antioxidant Systems of Chinese Chives. Front. Plant Sci. 2021, 12, 767335. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, J.; Sun, L.; Gao, Y.; Cao, M.; Luo, J. Impacts of water deficit and post-drought irrigation on transpiration rate, root activity, and biomass yield of Festuca arundinacea during phytoextraction. Chemosphere 2022, 294, 133842. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Wen, C.F.; Dong, A.W.; Li, G.Z.; Shu, L.; Yong, L. Determination of Total Sugar and Reducing Sugar in Viola Philippicassp Munda, W. Becker by Anthrone Colorimetry. Guangzhou Food Sci. Technol. 2005, 21, 122–124. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Arya, S.P.; Mahajan, M.; Jain, P. Non-spectrophotometric methods for the determination of Vitamin C. Anal. Chim. Acta 2000, 417, 1–14. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant-Tissue by Nitration of Salicylic-Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Xie, B.; Wu, Q.; Wei, S.; Li, H.; Wei, J.; Hanif, M.; Li, J.; Liu, Z.; Xiao, X.; Yu, J. Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler). Molecules 2022, 27, 2425. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC-MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2003. [Google Scholar]
- Alenyorege, E.A.; Ma, H.; Aheto, J.H.; Agyekum, A.A.; Zhou, C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. LWT 2020, 117, 108666. [Google Scholar] [CrossRef]
- Wei, L.; Wei, S.; Hu, D.; Feng, L.; Liu, Y.; Liu, H.; Liao, W. Comprehensive Flavor Analysis of Volatile Components during the Vase Period of Cut Lily (Lilium spp. ‘Manissa’) Flowers by HS-SPME/GC-MS Combined with E-Nose Technology. Front. Plant Sci. 2022, 13, 822956. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Sun, L.; Yang, L.; Yang, Y.; Wang, Y.; Siddique, K.H.M.; Pang, J. Alkaline Salt Inhibits Seed Germination and Seedling Growth of Canola more than Neutral Salt. Front. Plant. Sci. 2022, 13, 814755. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-E.; Dong, M.-Y.; Fan, X.-W.; Nong, L.-L.; Li, Y.-Z. A study on cassava tolerance to and growth responses under salt stress. Environ. Exp. Bot. 2018, 155, 429–440. [Google Scholar] [CrossRef]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary Metabolites from Halotolerant Plant Growth Promoting Rhizobacteria for Ameliorating Salinity Stress in Plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Guo, R.; Shi, D.; Liu, B.; Lin, X.; Yang, C. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Diao, P.; Kong, L.; Yu, R.; Wuriyanghan, H. Ethylene Enhances Seed Germination and Seedling Growth under Salinity by Reducing Oxidative Stress and Promoting Chlorophyll Content via ETR2 Pathway. Front. Plant Sci. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Agastian, P.; Kingsley, S.J.; Vivekanandan, M. Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Wang, L.S.; Li, W.L.; Qi, X.W.; Ma, L.; Wu, W.L. Physiological and proteomic response of Limonium bicolor to salinity. Russ. J. Plant Physiol. 2017, 64, 349–360. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Abdullah; Mahmood, A.; Bibi, S.; Naqve, M.; Javaid, M.M.; Zia, M.A.; Jabbar, A.; Ud-Din, W.; Attia, K.A.; Khan, N.; et al. Physiological, Biochemical, and Yield Responses of Linseed (Linum usitatissimum L.) in alpha-Tocopherol-Mediated Alleviation of Salinity Stress. Front. Plant Sci. 2022, 13, 867172. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wan, S.; Li, X.; Kang, Y.; Han, X. Effect of water-salt regulation drip irrigation with saline water on tomato quality in an arid region. Agric. Water Manag. 2022, 261, 107347. [Google Scholar] [CrossRef]
- Sathee, L.; Jha, S.K.; Rajput, O.S.; Singh, D.; Kumar, S.; Kumar, A. Expression dynamics of genes encoding nitrate and ammonium assimilation enzymes in rice genotypes exposed to reproductive stage salinity stress. Plant Physiol. Biochem. 2021, 165, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Fuentes., V.; Correa., M.T. Volatile constituents of Chinese chive (Allium tuberosum Rottl. ex Sprengel) and rakkyo (Allium chinense G. Don). J. Agric. Food Chem. 2001, 49, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Mukaida, Y.; Saito, Y.; Oshima, K.; Takahashi, T.; Muroi, E.; Hashimoto, K.; Uda, Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem. 2010, 120, 343–348. [Google Scholar] [CrossRef]
- Neffati, M.; Marzouk, B. Changes in essential oil and fatty acid composition in coriander (Coriandrum sativum L.) leaves under saline conditions. Ind. Crops Prod. 2008, 28, 137–142. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kanagendran, A.; Samaddar, S.; Pazouki, L.; Sa, T.M.; Niinemets, U. Methylobacterium oryzae CBMB20 influences photosynthetic traits, volatile emission and ethylene metabolism in Oryza sativa genotypes grown in salt stress conditions. Planta 2019, 249, 1903–1919. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Wouter, V.D.G.; Van Kooij, A.J.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F.G. Development of a biocatalytic process for the production of C6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Zhang, W.; Xu, C.; Li, B.; Zhang, D.; Mu, W.; Liu, F. Nematicidal Activity of trans-2-Hexenal against Southern Root-Knot Nematode (Meloidogyne incognita) on Tomato Plants. J. Agric. Food Chem. 2017, 65, 544–550. [Google Scholar] [CrossRef]
- Raguso, R.A. More lessons from linalool: Insights gained from a ubiquitous floral volatile. Curr. Opin. Plant Biol. 2016, 32, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Baudino, S.; Caissard, J.C.; Florence, N.; Zhang, H.; Tang, K.; Li, S.; Lu, S. Functional characterization of the eugenol synthase gene (RcEGS1) in rose. Plant Physiol. Biochem. 2018, 129, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hidalgo, F.J.; Medina-Puche, L.; Canete-Gomez, C.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Caballero, J.L.; Rodriguez-Franco, A.; Blanco-Portales, R.; Munoz-Blanco, J.; et al. The fruit-specific transcription factor FaDOF2 regulates the production of eugenol in ripe fruit receptacles. J. Exp. Bot. 2017, 68, 4529–4543. [Google Scholar] [CrossRef]
- Scremin, F.R.; Veiga, R.S.; Silva-Buzanello, R.A.; Becker-Algeri, T.A.; Corso, M.P.; Torquato, A.S.; Bittencourt, P.R.S.; Flores, E.L.M.; Canan, C. Synthesis and characterization of protein microcapsules for eugenol storage. J. Therm. Anal. Calorim. 2017, 131, 653–660. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Chen, S.; Tang, J.; Fan, S.; Zhang, J.; Chen, S.; Liu, Y.; Yang, Q.; Xu, Y. Comparison of Potent Odorants in Traditional and Modern Types of Chinese Xiaoqu Liquor (Baijiu) Based on Odor Activity Values and Multivariate Analyses. Foods 2021, 10, 2392. [Google Scholar] [CrossRef]
- Anon, A.; Lopez, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, W.; Lao, F.; Mi, R.; Liao, X.; Luo, D.; Wu, J. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography-Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.G.; Chen, F.L.; Guan, H.N.; Liu, L.L.; Zhang, C.Y.; Zhu, P.Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Z.; Guo, Q.; Mao, S.; Li, S.; Sun, F.; Wang, H.; Yang, C. Transcriptomic Profiling and Physiological Responses of Halophyte Kochia sieversiana Provide Insights into Salt Tolerance. Front. Plant Sci. 2017, 8, 1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, L.; Chen, S.; Sun, H.; Ma, S. Transcription profile analysis of Lycopersicum esculentum leaves, unravels volatile emissions and gene expression under salinity stress. Plant Physiol. Biochem. 2018, 126, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of Salinity and Water Stress on the Essential Oil Components of Rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).