Carbohydrate Sources Influence the Microbiota and Flavour Profile of a Lupine-Based Moromi Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Koji and Moromi Fermentation
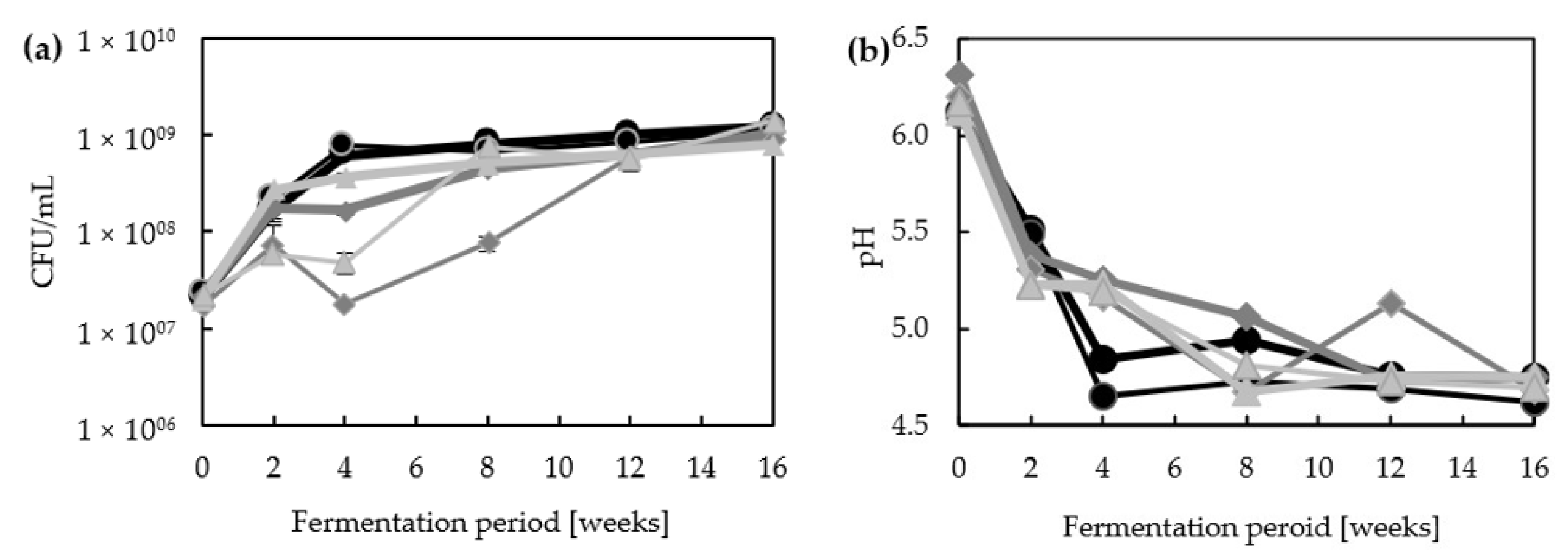
2.2. pH Measurement, Cell Counts and Chromatography
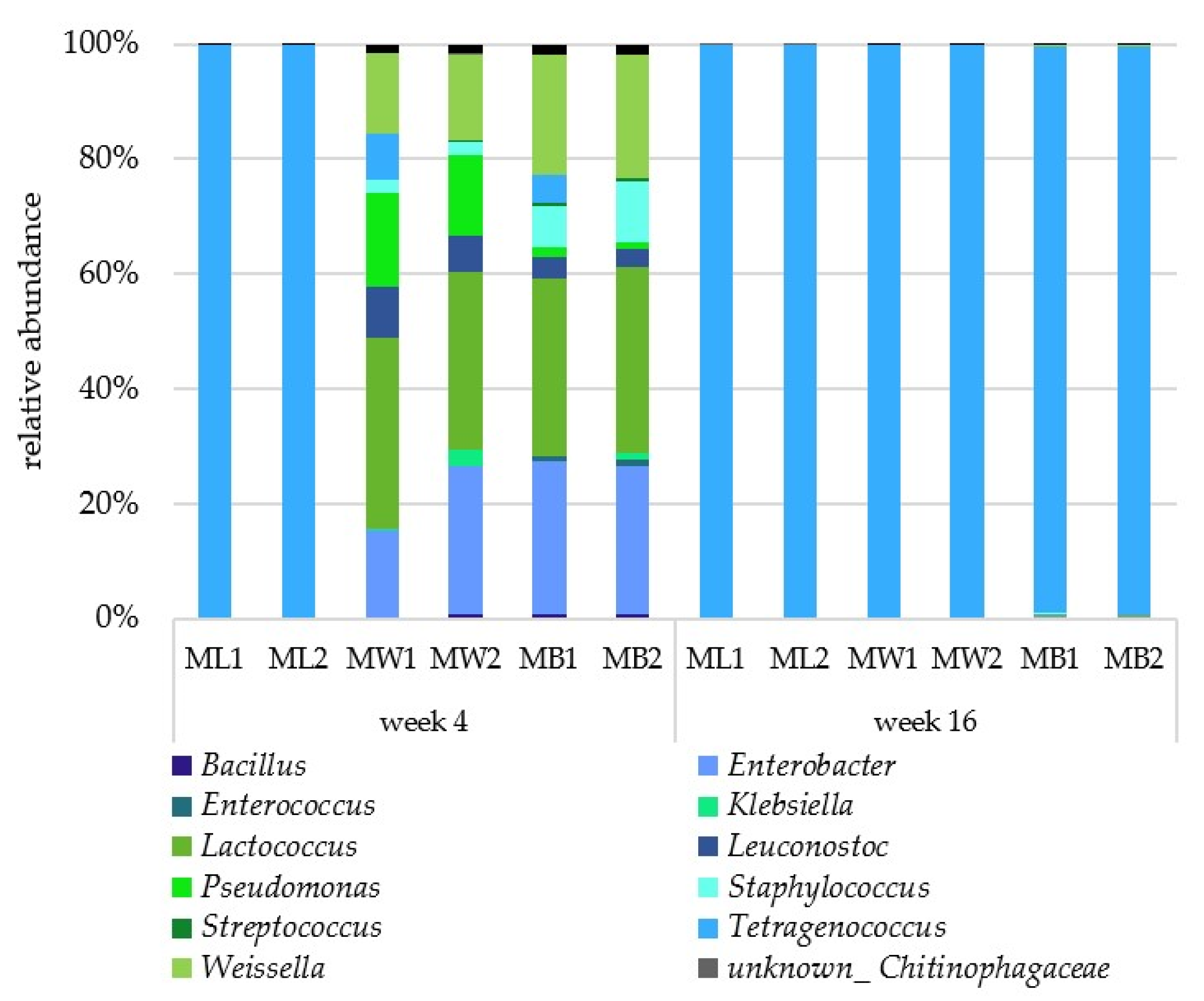
2.3. Identification of Organisms
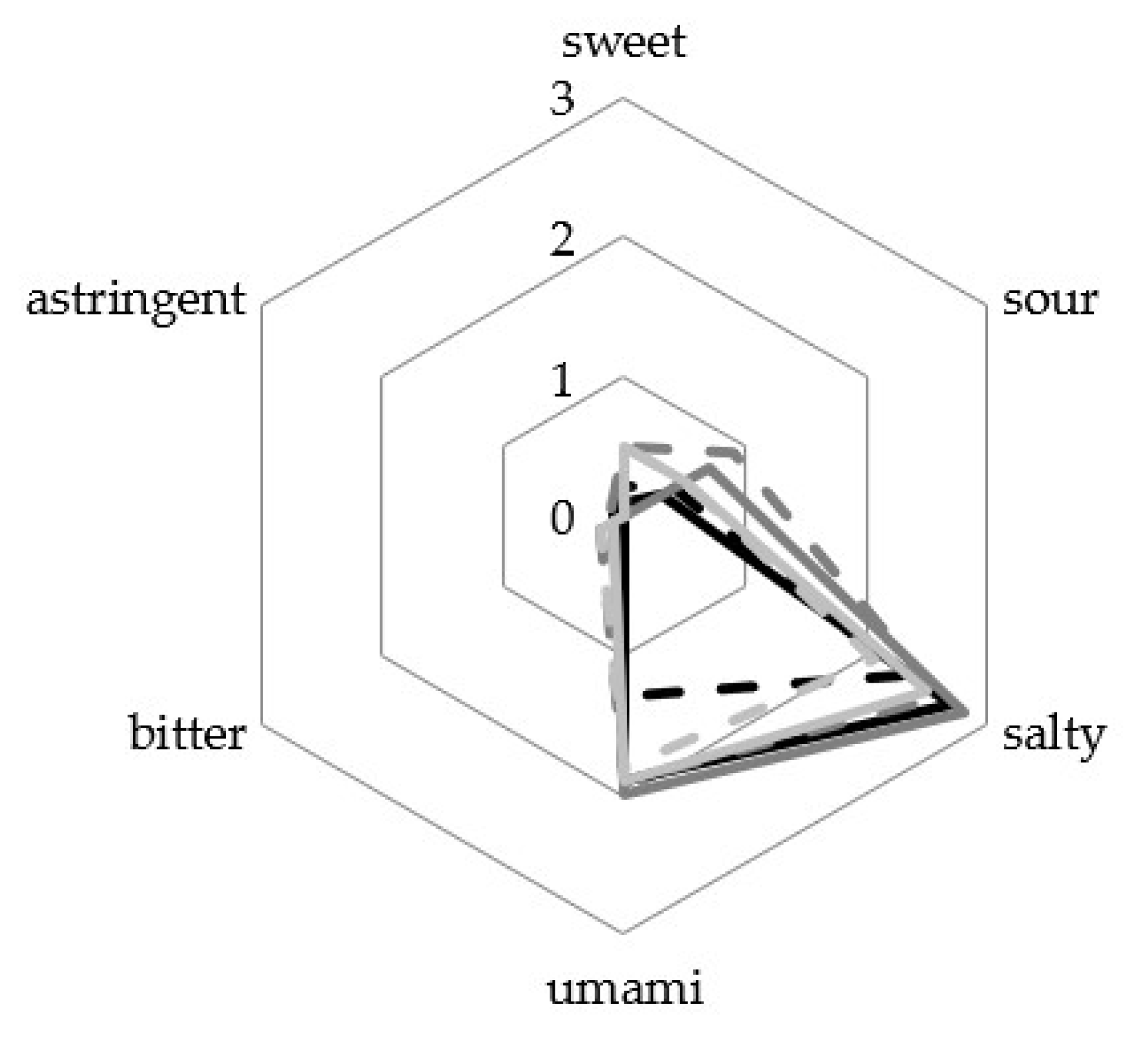
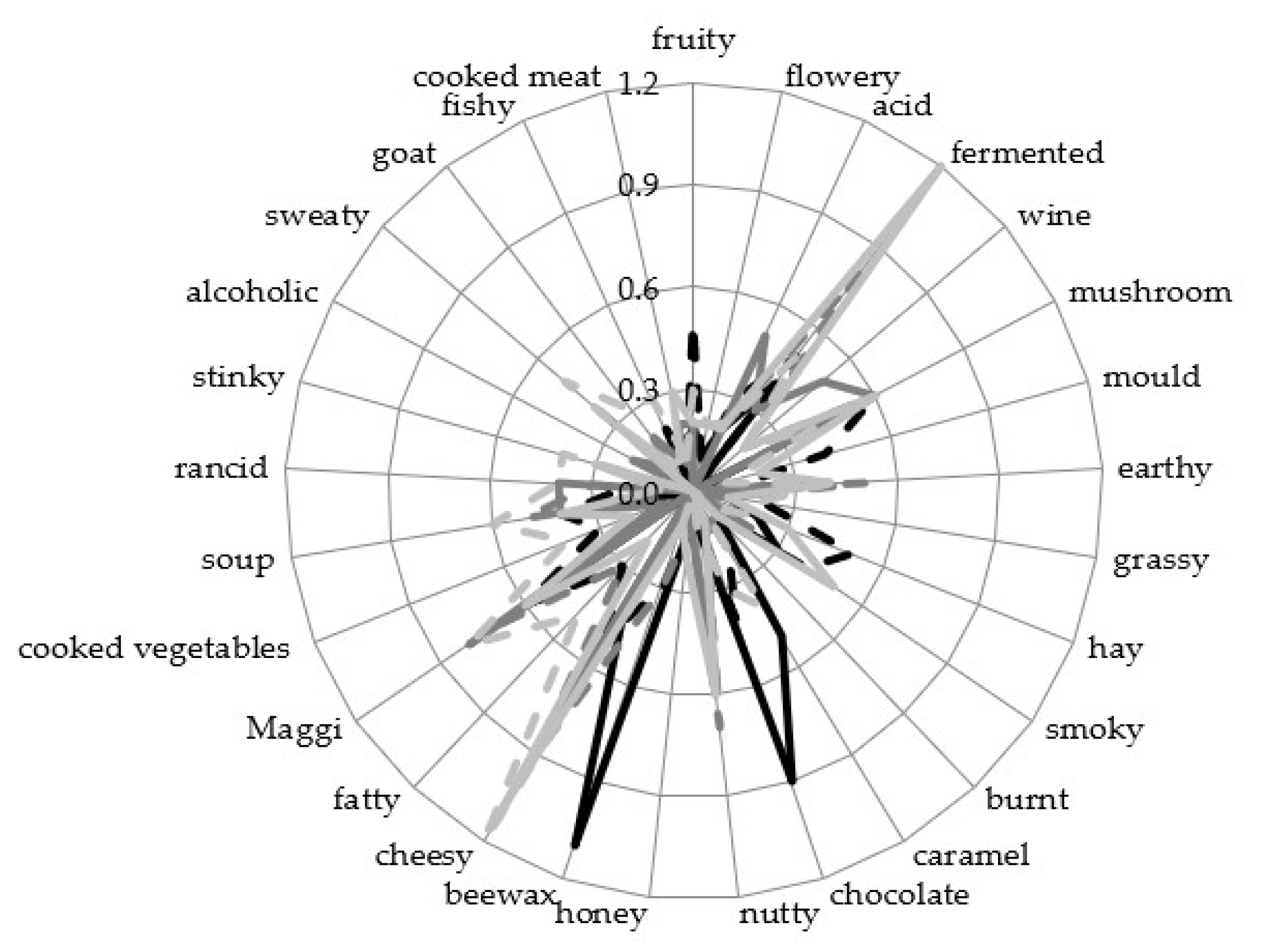
2.4. Sensory Analysis
3. Results
3.1. Major Characteristics of Lupine-Based Moromi Fermentation
3.2. Microbial Composition
3.3. Aromatic Compounds
3.4. Sensory Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouritsen, O.G.; Duelund, L.; Calleja, G.; Frøst, M.B. Flavour of Fermented Fish, Insect, Game, and Pea Sauces: Garum Revisited. Int. J. Gastron. Food Sci. 2017, 9, 16–28. [Google Scholar] [CrossRef]
- Lülf, R.H.; Vogel, R.F.; Ehrmann, M.A. Microbiota Dynamics and Volatile Compounds in Lupine Based Moromi Fermented at Different Salt Concentrations. Int. J. Food Microbiol. 2021, 354, 109316. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jeong, D.Y.; Baik, S.H. Effects of Indigenous Yeasts on Physicochemical and Microbial Properties of Korean Soy Sauce Prepared by Low-Salt Fermentation. Food Microbiol. 2015, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Lee, H.; Jeong, K.; Kim, C.T.; Shim, S.T.; Lee, J.H. Effects of Starter Candidates and NaCl on the Production of Volatile Compounds during Soybean Fermentation. J. Microbiol. Biotechnol. 2019, 29, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jeong, D.Y.; Baik, S.H. Monitoring of Yeast Communities and Volatile Flavor Changes during Traditional Korean Soy Sauce Fermentation. J. Food Sci. 2015, 80, 2005–2014. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Timotius, K.H.; Budhi Prasetyo, A.; Stouthamer, A.H.; van Verseveld, H.W. Changes in Microflora and Biochemical Composition during the Baceman Stage of Traditional Indonesian Kecap (Soy Sauce) Production. J. Ferment Bioeng. 1994, 77, 62–70. [Google Scholar] [CrossRef]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An Extremophilic Yeast with Biotechnological Potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Gori, K.; Sørensen, L.M.; Petersen, M.A.; Jespersen, L.; Arneborg, N. Debaryomyces hansenii Strains Differ in Their Production of Flavor Compounds in a Cheese-Surface Model. MicrobiologyOpen 2012, 1, 161–168. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy Sauce Fermentation: Microorganisms, Aroma Formation, and Process Modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Van Der Sluis, C.; Tramper, J.; Wijffels, R.H. Enhancing and Accelerating Flavour Formation by Salt-Tolerant Yeasts in Japanese Soy-Sauce Processes. Trends Food Sci. Technol. 2001, 12, 322–327. [Google Scholar] [CrossRef]
- Jansen, M.; Veurink, J.H.; Euverink, G.J.W.; Dijkhuizen, L. Growth of the Salt-Tolerant Yeast Zygosaccharomyces rouxii in Microtiter Plates: Effects of NaCl, PH and Temperature on Growth and Fusel Alcohol Production from Branched-Chain Amino Acids. FEMS Yeast Res. 2003, 3, 313–318. [Google Scholar] [CrossRef][Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and Sensory Characteristics of Soy Sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A Transparent and Modular R Pipeline for Microbial Profiling Based on 16S rRNA Gene Amplicons. PeerJ 2017, 2017, e2836. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A Comprehensive Open Resource of Processed 16S rRNA Microbial Profiles for Ecology and Diversity Studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef]
- Tanaka, Y.; Watanabe, J.; Mogi, Y. Monitoring of the Microbial Communities Involved in the Soy Sauce Manufacturing Process by PCR-Denaturing Gradient Gel Electrophoresis. Food Microbiol. 2012, 31, 100–106. [Google Scholar] [CrossRef]
- Sulaiman, J.; Gan, H.M.; Yin, W.F.; Chan, K.G. Microbial Succession and the Functional Potential during the Fermentation of Chinese Soy Sauce Brine. Front. Microbiol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Guo, H.; Qiu, Y.; Wei, J.; Niu, C.; Zhang, Y.; Yuan, Y.; Yue, T. Genomic Insights Into Sugar Adaptation in an Extremophile Yeast Zygosaccharomyces rouxii. Front. Microbiol. 2020, 10, 3157. [Google Scholar] [CrossRef]
- Bubnová, M.; Zemančíková, J.; Sychrová, H. Osmotolerant Yeast Species Differ in Basic Physiological Parameters and in Tolerance of Non-Osmotic Stresses. Yeast 2014, 31, 309–321. [Google Scholar] [CrossRef]
- Lee, K.E.; Lee, S.M.; Choi, Y.H.; Hurh, B.S.; Kim, Y.S. Comparative Volatile Profiles in Soy Sauce According to Inoculated Microorganisms. Biosci. Biotechnol. Biochem. 2013, 77, 2192–2200. [Google Scholar] [CrossRef]
- Verhagen, L.C. Beer Flavor. Compr. Nat. Prod. II Chem. Biol. 2010, 3, 967–997. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Su, G.; Zhao, H.; Wang, C.; Zhao, M. Evaluation of Aroma Differences between High-Salt Liquid-State Fermentation and Low-Salt Solid-State Fermentation Soy Sauces from China. Food. Chem. 2014, 145, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, Formation and Biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.; Landmann, C.; Brühlmann, F.; Schwab, W. Formation of 5-Methyl-4-Hydroxy-3[2H]-Furanone in Cytosolic Extracts Obtained from Zygosaccharomyces rouxii. J. Agric. Food Chem. 2003, 51, 1410–1414. [Google Scholar] [CrossRef]

| Moromi with Lupine Seeds | Moromi with Wheat | Moromi with Buckwheat | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | 0 | 12 | 16 | 0 | 12 | 16 | 0 | 12 | 16 |
| 2-Methylpropanoic acid | X | X | X | X | X | X | |||
| 3-Methylbutanoic acid | X | X | > | > | X | X | |||
| Acetic acid | X | X | X | > | X | X | |||
| Benzene acetic acid | < | X | X | X | X | X | |||
| n-Decanoic acid | X | > | |||||||
| Nonanoic acid | < | X | < | > | |||||
| Octanoic acid | X | X | > | X | < | < | > | ||
| 2-Methylbutanoic acid ethyl ester | X | X | X | X | |||||
| 2-Methylpropanoic acid ethyl ester | X | X | X | X | |||||
| 2-Phenylethyl acetate | X | X | X | < | |||||
| 3-Methyl-1-butyl acetate | X | < | |||||||
| 3-Methylbutanoic acid ethyl ester | > | X | X | X | |||||
| 3-Methylbutyl 2-methylbutanoate | < | > | X | ||||||
| 3-Methylbutyl 3-methylbutanoate | < | X | X | X | |||||
| 3-Methylbutyl butanoate | > | < | X | ||||||
| Ethyl 9-octadecenoate | > | X | X | X | X | ||||
| Ethyl Acetate | X | X | |||||||
| Ethyl benzene acetate | X | > | < | X | |||||
| Ethyl hexadecanoate | X | > | X | X | X | X | |||
| Isopentyl 2-methylpropanoate | > | > | < | X | |||||
| Linoleic acid ethyl ester | X | X | X | X | X | X | |||
| Octanoic acid ethyl ester | < | X | |||||||
| Phenylethyl 3-methyl-butanoate | > | > | < | X | |||||
| β-Phenylethyl butyrate | X | X | X | X | |||||
| Dimethyl ether | X | X | X | < | |||||
| 2,3,5-Trimethyl-6-ethylpyrazine | X | X | X | X | |||||
| 2,3-Dimethyl-5-ethylpyrazine | > | < | > | ||||||
| 2,3-Dimetyhlpyrazine | X | X | X | X | > | X | |||
| 2,5-Diethylpyrazine | X | X | < | ||||||
| 2,5-Dimethylpyrazine | X | X | X | X | X | X | X | X | X |
| 2,6-Diethylpyrazine | > | X | X | ||||||
| 2,6-Dimethylpyrazine | X | X | X | X | > | ||||
| 2-Ethyl-3-methylpyrazine | X | X | X | X | > | > | X | ||
| 2-Ethyl-5-methylpyrazine | X | X | X | X | > | > | X | ||
| 2-Ethyl-6-methylpyrazine | X | X | X | < | X | X | X | X | |
| 2-Methyl-5-((E)-1-propenyl)-pyrazine | > | < | < | ||||||
| 2-Methyl-5-((Z)-1-propenyl)-pyrazine | X | < | |||||||
| 3,5-Diethyl-2-methylpyrazine | X | X | X | ||||||
| 3-Ethyl-2,5-dimethylpyrazine | X | X | X | X | |||||
| 5H-5-Methyl-6,7-dihydrocyclopentapyrazine | X | X | X | X | > | > | X | < | |
| 6,7-Dihydro-2,5-dimethyl-5H-cyclopentapyrazine | X | < | > | ||||||
| Ethyl pyrazine | X | X | X | X | > | X | |||
| Methylpyrazine | X | X | X | X | X | ||||
| Trimethyl pyrazine | X | X | X | X | X | X | X | X | |
| 2,3-Dihydrobenzofuran | > | X | X | ||||||
| 5-Isopropyl-3,3-dimethyl-2-methylene-2,3-dihydrofuran | > | ||||||||
| Dihydro-5-((Z)-2-octenyl)-2(3H)-furanone | X | > | X | X | < | > | X | ||
| HDMF (µg/g) | HMF (µg/g) | |
|---|---|---|
| ML1 | 1.47 | 1.74 |
| ML2 | 0.92 | 1.16 |
| MW1 | 0.76 | 2.67 |
| MW2 | 0.60 | 3.62 |
| MB1 | 0.83 | 2.68 |
| MB2 | 1.87 | 4.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lülf, R.H.; Selg-Mann, K.; Hoffmann, T.; Zheng, T.; Schirmer, M.; Ehrmann, M.A. Carbohydrate Sources Influence the Microbiota and Flavour Profile of a Lupine-Based Moromi Fermentation. Foods 2023, 12, 197. https://doi.org/10.3390/foods12010197
Lülf RH, Selg-Mann K, Hoffmann T, Zheng T, Schirmer M, Ehrmann MA. Carbohydrate Sources Influence the Microbiota and Flavour Profile of a Lupine-Based Moromi Fermentation. Foods. 2023; 12(1):197. https://doi.org/10.3390/foods12010197
Chicago/Turabian StyleLülf, Rebekka H., Karl Selg-Mann, Thomas Hoffmann, Tingting Zheng, Melanie Schirmer, and Matthias A. Ehrmann. 2023. "Carbohydrate Sources Influence the Microbiota and Flavour Profile of a Lupine-Based Moromi Fermentation" Foods 12, no. 1: 197. https://doi.org/10.3390/foods12010197
APA StyleLülf, R. H., Selg-Mann, K., Hoffmann, T., Zheng, T., Schirmer, M., & Ehrmann, M. A. (2023). Carbohydrate Sources Influence the Microbiota and Flavour Profile of a Lupine-Based Moromi Fermentation. Foods, 12(1), 197. https://doi.org/10.3390/foods12010197






