In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Culture Conditions
2.2. Yeasts Identification
2.3. Testing the Influence of Temperature, pH, and Sodium Chloride (NaCl) on Yeast Growth
2.4. Auto-Aggregation and Hydrophobicity
2.5. Antioxidant Activity
2.6. Yeast Survival Rate to Gastrointestinal Barriers In Vitro
2.7. Antibacterial Activity
2.8. Antibiotic Susceptibility
2.9. Catalase and Hemolytic Activity
2.10. Conditioning of Yeast by Freeze-Drying Procedure
2.11. Statistical Analysis
3. Results
3.1. Molecular Identification of Yeast Strains
3.2. Influence of Temperature, pH, and Sodium Chloride (NaCl) on Yeast Growth
3.3. Auto-Aggregation and Hydrophobicity Ability
3.4. Antioxidant Properties
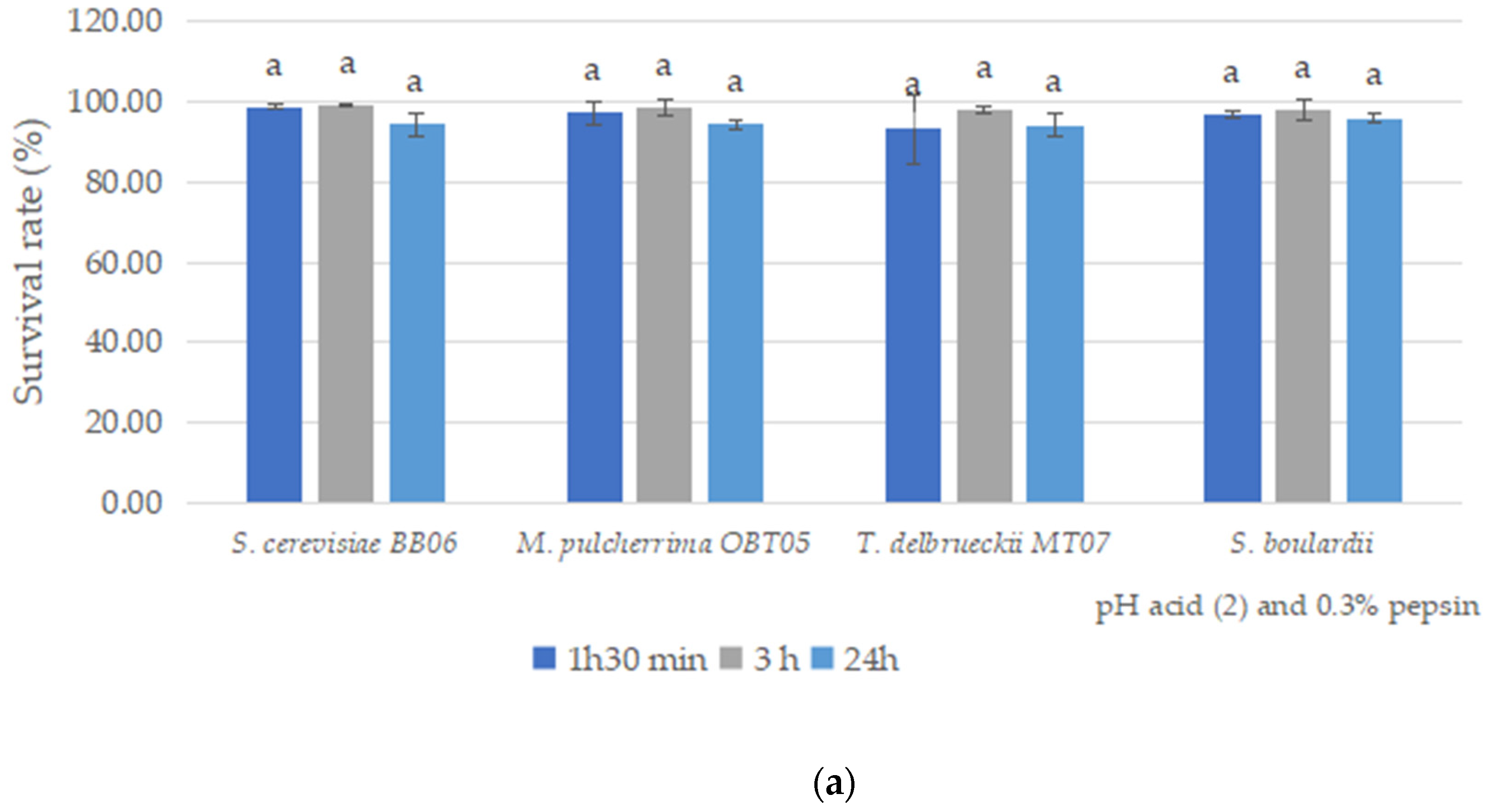
3.5. Resistance to Gastric Acidity and Bile Salts
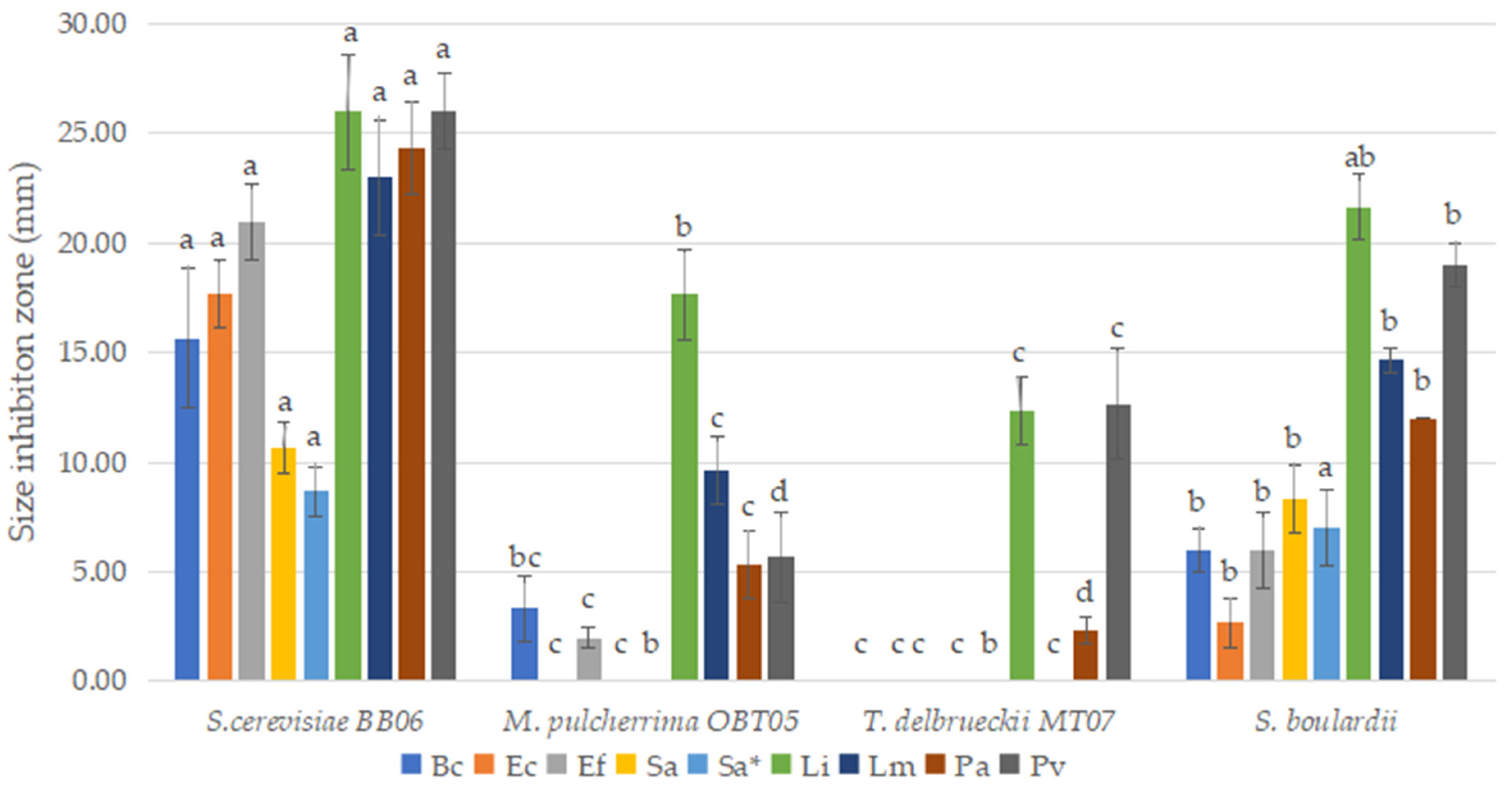
3.6. Antibacterial Properties
3.7. Antibiotic Susceptibility
3.8. Catalase and Hemolysis Assay
3.9. Conditioning by Lyophilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavelle, T.; Lester, S.E.; Gentry, R.; Froehlich, H.E. Interactions and management for the future of marine aquaculture and capture fisheries. Fish Fish. 2019, 20, 368–388. [Google Scholar] [CrossRef]
- Deepak, A.; Vasava, R.; Elchelwar, V.; Tandel, D.; Vadher, K.; Shrivastava, V.; Prabhakar, P. Aquamimicry: New an innovative apporoach for sustainable development of aquaculture. J. Entomol. Zool. Stud. 2020, 8, 1029–1031. [Google Scholar]
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Pyla, R.; Kim, T.J.; Silva, J.L.; Jung, Y.S. Antibiotic resistance in Listeria species isolated from catfish fillets and processing environment. Lett. Appl. Microbiol. 2010, 50, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.T.; Sumon, A.A.; Pugazhendi, A.; Al Harbi, M.; Hussain, A.; Haque, F. Use of probiotics in commercially important finfish aquaculture. Int. J. Probiot. Prebiot. 2020, 15, 7–21. [Google Scholar] [CrossRef]
- Budiati, T.; Rusul, G.; Wan-Abdullah, W.N.; Arip, Y.M.; Ahmad, R.; Thong, K.L. Prevalence, antibiotic resistance and plasmid profiling of Salmonella in catfish (Clarias gariepinus) and tilapia (Tilapia mossambica) obtained from wet markets and ponds in Malaysia. Aquaculture 2013, 372, 127–133. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Tiu, L.; Wang, H.H. Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front. Microbiol. 2015, 6, 914. [Google Scholar] [CrossRef]
- Ranjbar, R.; Salighehzadeh, R.; Sharifiyazdi, H. Antimicrobial resistance and incidence of integrons in Aeromonas species isolated from diseased freshwater animals and water samples in Iran. Antibiotics 2019, 8, 198. [Google Scholar] [CrossRef]
- Tyagi, A.; Singh, B.; Thammegowda, N.K.B.; Singh, N.K. Shotgun metagenomics offers novel insights into taxonomic compositions, metabolic pathways and antibiotic resistance genes in fish gut microbiome. Arch. Microbiol. 2019, 201, 295–303. [Google Scholar] [CrossRef]
- Novotny, L.; Dvorska, L.; Lorencova, A.; Beran, V.; Pavlik, I. Fish: A potential source of bacterial pathogens for human beings. Vet. Med. 2004, 49, 343–358. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Emran, T.B.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Probiotics and Immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.N.; Duc, P.M.; Hatai, K. Overview of the use of probiotics in aquaculture. Int. J. Res. Fish Aquac. 2013, 3, 89–97. [Google Scholar]
- Jahangiri, L.; Esteban, M.Á. Administration of Probiotics in the water in finfish aquaculture systems: A review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent studies on probiotics as beneficial mediator in aquaculture: A review. JoBAZ 2020, 81, 53. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martíenez-Córdova, L.R.; Herández-Mendoza, A.; Cicala, F.; Lago-Lestón, A.; Martínez-Porchas, M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Yousuf, S.; Tyagi, A.; Singh, R. Probiotic supplementation as an emerging alternative to chemical therapeutics in finfish aquaculture: A Review. Probiotics Antimicrob. Prot. 2022, 1–18. [Google Scholar] [CrossRef]
- Ringø, E.; Li, X.; Doan, H.; Ghosh, K. Interesting probiotic bacteria other than the more widely used lactic acid bacteria and bacilli in finfish. Front. Mar. Sci. 2022, 9, 848037. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report; FAO: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A Review on the application of Bacillus as probiotics in Aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Matei, B.; Diguta, C.F.; Matei, F.; Popa, O. Antimicrobial potential of Kombucha bacterial biopolymer. Sci. Bull. Ser. F Biotechnol. 2020, 24, 133–137. [Google Scholar]
- Soltani, M.; Ghosh, K.; Hoseinifar, S.H.; Kumar, V.; Lymbery, A.; Roy, S.; Ringø, E. Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation in fish and shellfish. Rev. Fish. Sci. Aquac. 2019, 27, 331–379. [Google Scholar] [CrossRef]
- Diguță, F.C.; Nițoi, G.D.; Matei, F.; Luță, G.; Cornea, C.P. The Biotechnological potential of Pediococcus spp. isolated from Kombucha microbial consortium. Foods 2020, 9, 1780. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Yang, Z.; Li, M.; Liu, J.; Song, J. Effects of dietary live yeast Hanseniaspora opuntiae C21 on the immune and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shelfish Immunol. 2013, 34, 66–73. [Google Scholar] [CrossRef]
- Navarrete, P.; Tovar-Ramírez, D. Use of yeasts as probiotics in fish aquaculture. Sustain. Aquac. Tech. 2014, 1, 57196. [Google Scholar]
- Sharifuzzaman, S.M.; Austin, B. Probiotics for disease control in aquaculture. In Diagnosis and Control of Diseases of Fish and Shellfish; Austin, B., Newaj-Fyzul, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 189–222. [Google Scholar]
- Abass, D.A.; Obirikorang, K.A.; Campion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus). Aquac. Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- Rawling, M.D.; Pontefract, N.; Rodiles, A.; Anagnostara, I.; Leclercq, E.; Schiavone, M.; Castex, M.; Merrifield, D.L. The effect of feeding a novel multistrain yeast fraction on European seabass (Dicentrachus labrax) intestinal health and growth performance. J. World Aquac. Soc. 2019, 50, 1108–1122. [Google Scholar] [CrossRef]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic yeast enhanced growth performance and disease resistance in freshwater catfish gulsa tengra (Mystus cavasius). Aquac. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Mahdy, M.A.; Jamal, M.T.; Al-Harb, M.; Al-Mur, B.A.; Haque, M.F. Use of yeasts in aquaculture nutrition and immunostimulation: A review. J. Appl. Biol. Biotech. 2022, 10, 59–65. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts—Characteristics and food application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic yeast Saccharomyces: Back to nature to improve human health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of singlecell ingredients in aquaculture feeds—A review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Łukaszewicz, M. Saccharomyces cerevisiae var. boulardii—Probiotic yeast. In Probiotics; IntechOpen: London, UK, 2012. [Google Scholar]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- Ansari, F.; Samakkhah, S.A.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Ochangco, H.S.; Gamero, A.; Smith, I.M.; Christensen, J.E.; Jespersen, L.; Arneborg, N. In vitro investigation of Debaryomyces hansenii strains for potential probiotic properties. World J. Microbiol. Biotechnol. 2016, 32, 141. [Google Scholar] [CrossRef] [PubMed]
- Gil-Rodríguez, A.M.; Carrascosa, A.V.; Requena, T. Yeasts in foods and beverages: In vitro characterisation of probiotic traits. LWT-Food Sci. Technol. 2015, 64, 1156–1162. [Google Scholar] [CrossRef]
- Rodriguez, P.F.-P.; Arévalo-Villena, M.; Rosa, I.Z.; Perez, A.B. Selection of potential non-Saccharomyces probiotic yeasts from food origin by a step-by-step approach. Food Res. Int. 2018, 112, 143–151. [Google Scholar] [CrossRef]
- Guluarte, C.; Reyes-Becerril, M.; Gonzalez-Silvera, D.; Cuesta, A.; Angulo, C.; Esteban, M.Á. Probiotic properties and fatty acid composition of the yeast Kluyveromyces lactis M3. In vivo immunomodulatory activities in gilthead seabream (Sparus aurata). Fish Shelfish Immunol. 2019, 94, 389–397. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Pintado, C.; Briones Pérez, A.; Arévalo-Villena, M. Potential probiotic strains of Saccharomyces and non-Saccharomyces: Functional and biotechnological characteristics. J. Fungi 2021, 7, 177. [Google Scholar] [CrossRef]
- Reyes-Becerril, E.; Alamillo, C. Angulo. Probiotic and immunomodulatory activity of marine yeast Yarrowia lipolytica strains and response against Vibrio parahaemolyticus in fish. Probiotics Antimicrob. Proteins 2021, 13, 1292–1305. [Google Scholar] [CrossRef]
- Wendy, M.S.; Zachariah, B.C.; Brett, T.H.; Thomas, J.; O’Neill, J.B.; Stephanie, S.B. Evaluation of the ability of Pichia guilliermondii to improve growth performance and disease resistance in rainbow trout (Oncorhynchus mykiss). JWAS 2022, 3, 411–423. [Google Scholar]
- Shewale, R.N.; Sawale, P.D.; Khedkar, C.D.; Singh, A. Selection criteria for probiotics: A review. Int. J. Probiotics Prebiotics 2014, 9, 17–22. [Google Scholar]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Byakika, S.; Mukisa, I.; Byenkya, Y.; Muyanja, C.; Byenkya Byaruhanga, Y. A review of criteria and methods for evaluating the probiotic potential of microorganisms. Food Rev. Int. 2019, 35, 427–466. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of yeasts as potential probiotics: A review of gastrointestinal tract conditions and investigation methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V.; Kiessling, A.; Lundh, T. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquac. Nutr. 2019, 26, 275–286. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- CLSI. CLSI Supplement M100S. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Corbu, V.; Vassu, T.; Csutak, O. Pichia (Kodamaea) ohmeri CMGB-ST19-A new strain with complex biotechnological properties. AgroLife Sci. J. 2019, 8, 77–86. [Google Scholar]
- Fadahunsi, I.F.; Olubodun, S. Antagonistic pattern of yeast species against some selected food-borne pathogens. Bull. Natl. Res. Cent. 2021, 45, 34. [Google Scholar] [CrossRef]
- Djurichkovic, L.D.; Donelson, J.M.; Fowler, A.M.; Feary, D.A.; Booth, D.J. The effects of water temperature on the juvenile performance of two tropical damselfishes expatriating to temperate reefs. Sci. Rep. 2019, 9, 13937. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 496–1508. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.P.; Oliveira, D.R.; Lopes, A.C.A.; de Abreu, L.R.; Duarte, W.F. Survival of Kluyveromyces lactis and Torulaspora delbrueckii to simulated gastrointestinal conditions and their use as single and mixed inoculum for cheese production. Food Res. Int. 2019, 125, 108620. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Hernández-Sánchez, H.; Ribas-Aparicio, R.M.; Cauich-Sánchez, P.I.; Dávila-Ortiz, G. Evaluation of the probiotic potential of Saccharomyces cerevisiae strain (C41) Isolated from Tibicos by in vitro studies. Probiotics Antimicrob. Proteins 2019, 11, 794–800. [Google Scholar] [CrossRef]
- Agarbati, L.C.; Marini, E.E.Z.; Ciani, M.F.C. Potential probiotic yeasts sourced from natural environmental and spontaneous processed foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1217–1235. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics-Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Rajkowska, K.; Kunicka-Styczynska, A. Probiotic properties of yeasts isolated from chicken feces and kefirs. Pol. J. Microbiol. 2010, 59, 257–263. [Google Scholar] [CrossRef]
- Syal, P.; Vohra, A. Probiotic potential of yeasts isolated from traditional Indian fermented foods. Int. J. Microbiol. Res. 2013, 5, 390–398. [Google Scholar] [CrossRef]
- Helmy, E.A.; Soliman, S.A.; Abdel-Ghany, T.M.; Ganash, M. Evaluation of potentially probiotic attributes of certain dairy yeast isolated from buffalo sweetened Karish cheese. Heliyon 2019, 5, e01649. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, G.; Dopcea, I.; Nicolae, A.-M.; Stelian, P.; Matei, F. Conservation methods used for yeast isolated from vineyards—Lyophilisation advantage. Sci. Bull. Biotechnol. 2010, 14, 31–36. [Google Scholar]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT—Food Sci. Technol. 2015, 63, 685–690. [Google Scholar] [CrossRef]



| Yeast Strains | Auto-Aggregation (%) | Hydrophobicity (%) | Antioxidant Activity (%) | |||
|---|---|---|---|---|---|---|
| 2 h | 4 h | 24 h | Hexane | Xylene | ||
| S. cerevisiae BB06 | 60.51 ± 3.03 a | 81.01 ± 1.35 a | 92.08 ± 1.49 a | 53.43 ± 1.09 a | 24.36 ± 0.36 b | 55.97 ± 1.62 b |
| M. pulcherrima OBT05 | 45.70 ± 1.59 b | 65.88 ± 1.43 b | 73.44 ± 1.58 b | 37.76 ± 1.08 b | 38.08 ± 0.90 a | 57.14 ± 2.85 ab |
| T. delbrueckii MT07 | 47.24 ± 2.14 b | 64.68 ± 3.42 b | 74.14 ± 0.27 b | 5.93 ± 1.54 d | 19.03 ± 3.87 c | 60.61 ± 1.32 a |
| boulardii | 60.99 ± 2.77 a | 80.03 ± 0.67 a | 89.96 ± 1.55 a | 32.84 ± 3.27 c | 34.73 ± 0.99 a | 60.46 ± 0.80 a |
| Yeast Strains | Catalase Activity | Hemolytic Activity | Antibiotics Susceptibility | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM- 10 | CL- 30 | C- 30 | E- 10 | L- 10 | NA-30 | VA-10 | CTM-10 | FLU-10 | ITR- 10 | KCA- 10 | MCL-10 | NS- 100 | |||
| cerevisiae BB06 | + | Gamma | R | R | R | R | R | R | R | R | R | R | R | R | S |
| pulcherrima OBT05 | ++ | Gamma | R | R | R | R | R | R | R | S | S | S | S | S | S |
| delbrueckii MT07 | ++ | Gamma | R | R | R | R | R | R | R | R | R | R | R | R | S |
| S. boulardii | + | Gamma | R | R | R | R | R | R | R | R | R | R | R | R | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods 2023, 12, 124. https://doi.org/10.3390/foods12010124
Diguță CF, Mihai C, Toma RC, Cîmpeanu C, Matei F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods. 2023; 12(1):124. https://doi.org/10.3390/foods12010124
Chicago/Turabian StyleDiguță, Camelia Filofteia, Constanța Mihai, Radu Cristian Toma, Carmen Cîmpeanu, and Florentina Matei. 2023. "In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use" Foods 12, no. 1: 124. https://doi.org/10.3390/foods12010124
APA StyleDiguță, C. F., Mihai, C., Toma, R. C., Cîmpeanu, C., & Matei, F. (2023). In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods, 12(1), 124. https://doi.org/10.3390/foods12010124