Variation of Major Chemical Composition in Seed-Propagated Population of Wild Cocoa Tea Plant Camellia ptilophylla Chang
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Tea leaves
2.2.2. Determination of Total Polyphenols Content
2.2.3. Determination of Total Amino Acids Content
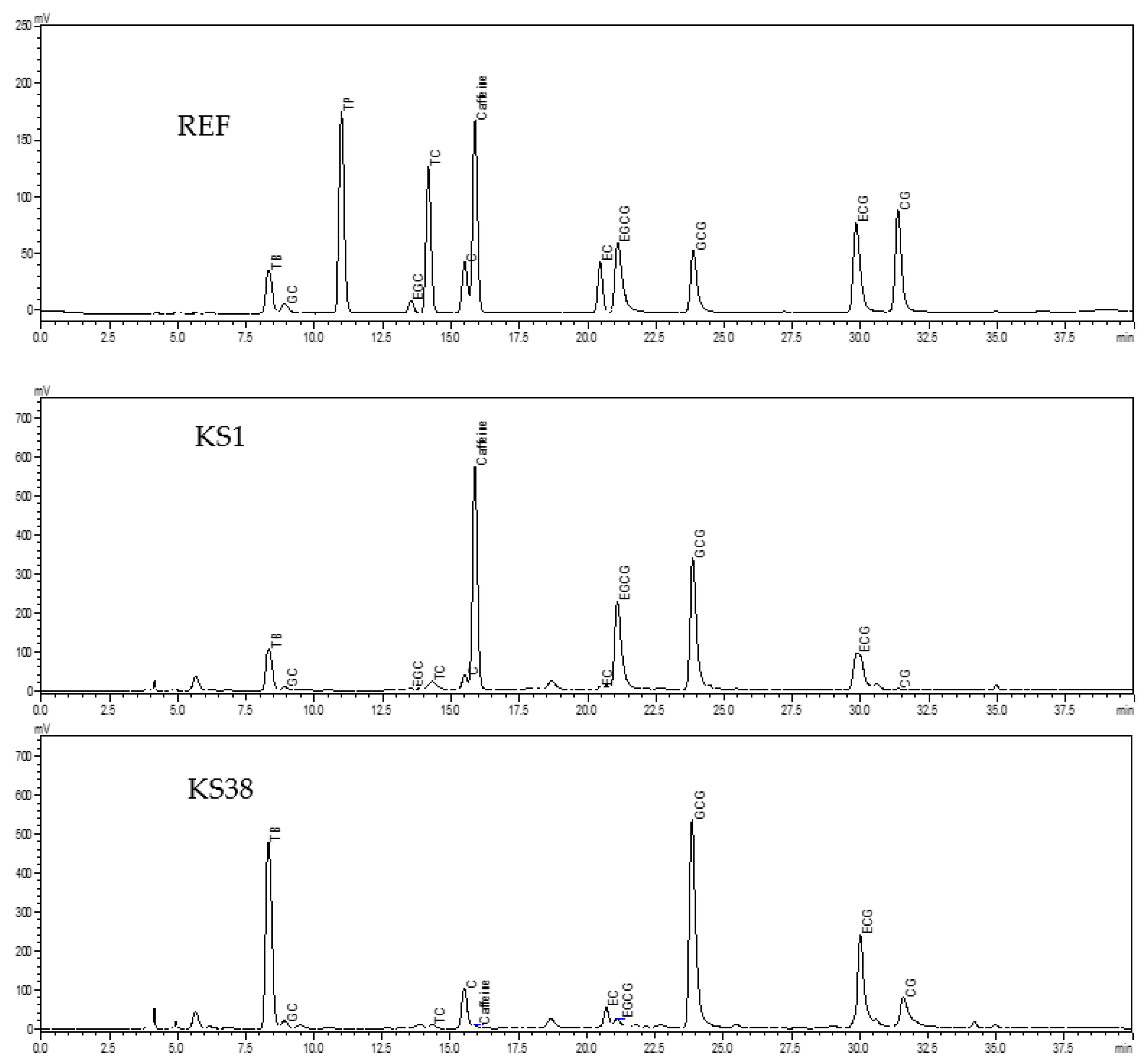
2.2.4. Determination of Alkaloids and Catechins
2.2.5. Statistical Analyses
3. Results
3.1. Contents of Total Polyphenols and Amino Acids
3.2. Contents of Alkaloids
3.3. Contents of Catechins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic effects of tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Y.; Xiang, J.; Lu, J.L.; Ye, J.H.; Chen, Z.J.; Zhao, J.W.; Liang, Y.R.; Zheng, X.Q. Protective effects of gallocatechin gallate against ultraviolet B induced skin damages in hairless mice. Sci. Rep. 2022, 12, 1310. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Q.; Yao, M.Z.; Ma, C.L.; Ma, J.Q.; Chen, L. Association mapping of caffeine content with TCS1 in tea plant and its related species. Plant Physiol. Biochem. 2016, 105, 251. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Melita, H.; Manolis, A.S. The cardiovascular benefits of caffeinated beverages: Real or surreal? “Metron ariston-all in moderation”. Curr. Medi. Chem. 2022, 29, 2235. [Google Scholar] [CrossRef]
- Gramza-michałowska, A. Caffeine in tea Camellia sinensis—Content, absorption, benefits and risks of consumption. J. Nutr. Health Aging 2014, 18, 114. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ahuja, P.S. Towards generating caffeine-free tea by metabolic engineering. Plant Food Hum. Nutr. 2007, 62, 185. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Kumar, V.; Yadav, S.K. Tea caffeine: Metabolism, functions, and reduction strategies. Food Sci. Biotechnol. 2010, 19, 275. [Google Scholar] [CrossRef]
- Shimbo, M.; Nakamura, K.; Shi, H.J.; Kizuki, M.; Seino, K.; Inose, T.; Takano, T. Green tea consumption in everyday life and mental health. Public Health Nutr. 2005, 8, 1300. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Black tea and improvement of attention: Evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924. EFSA J. 2018, 16, 5266. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, F.; Xin, N.; Yang, L.; Zhu, P. Association of coffee, tea, and caffeine consumption with all-cause risk and specific mortality for cardiovascular disease patients. Front. Nutr. 2022, 9, 842856. [Google Scholar] [CrossRef]
- Demir, F.; Kipcak, A.S.; Ozdemir, O.D.; Piskin, M.B.; Derun, E.M. Determination of lemon and carbonate effects on caffeine content of various teas and investigation of daily caffeine intakes. Turk. J. Biochem. 2016, 41, 308. [Google Scholar] [CrossRef]
- Tfouni, S.A.V.; Camara, M.M.; Kamikata, K.; Gomes, F.M.L.; Furlani, R.P.L. Caffeine in teas: Levels, transference to infusion and estimated intake. Food Sci. Technol. 2017, 38, 661. [Google Scholar] [CrossRef]
- Deng, W.W.; Li, M.; Gu, C.C.; Li, D.X.; Ma, L.L.; Jin, Y.; Wan, X.C. Low caffeine content in novel grafted tea with Camellia sinensis as scions and Camellia oleifera as stocks. Nat. Prod. Commun. 2015, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Koenig, J. Caffeine intake and its sources: A review of national representative studies. Crit. Rev. Food Sci. 2018, 58, 1250. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Liang, Y.R.; Wang, H. Decaffeination of fresh green tea leaf (Camellia sinensis) by hot water treatment. Food Chem. 2007, 101, 1451. [Google Scholar] [CrossRef]
- Ye, J.H.; Liang, Y.R.; Jin, J.; Liang, H.L.; Du, Y.Y.; Lu, J.L.; Ye, Q.; Lin, C. Preparation of partially decaffeinated instant green tea. J. Agri. Food Chem. 2007, 55, 3498. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.K.; Kim, K.H.; Kim, Y.S. Effect of supercritical carbon dioxide decaffeination on volatile components of green teas. J. Food Sci. 2007, 72, S497–S502. [Google Scholar] [CrossRef]
- Chowaniak, M.; Niemiec, M.; Zhu, Z.Q.; Rashidov, N.; Grodek-Szostak, Z.; Szelag-Sikora, A.; Sikora, J.; Kubon, M.; Fayzullo, S.A.; Mahmadyorzoda, U.M.; et al. Quality assessment of wild and cultivated green tea from different regions of China. Molecules 2021, 26, 3620. [Google Scholar] [CrossRef]
- Jin, J.Q.; Chai, Y.F.; Liu, Y.F.; Zhang, J.; Yao, M.Z.; Chen, L. Hongyacha, a naturally caffeine-free tea plant from Fujian, China. J. Agri. Food Chem. 2018, 66, 11311. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Kumar, V.; Ahuja, P.S.; Yadav, S.K. Agrobacterium-mediated silencing of caffeine synthesis through root transformation in Camellia sinensis L. Mol. Biotechnol. 2011, 48, 235. [Google Scholar] [CrossRef]
- Zeng, W.; Zeng, Z.; Teng, J.; Rothenberg, D.O.; Zhou, M.; Lai, R.; Lai, X.; Zhao, W.; Li, D.; Yan, C.; et al. Comparative analysis of purine alkaloids and main quality components of the three Camellia species in China. Foods 2022, 11, 627. [Google Scholar] [CrossRef]
- Jiang, C.K.; Zhao, W.F.; Zeng, Z.; Lai, X.F.; Wu, C.L.; Yuan, S.S.; Huang, Y.H.; Zhang, X. A treasure reservoir of genetic resource of tea plant (Camellia sinensis) in Dayao Mountain. Genet. Resour. Crop Ev. 2018, 65, 217. [Google Scholar] [CrossRef]
- He, R.R.; Xie, G.; Yao, X.S.; Kurihara, H. Effect of cocoa tea (Camellia ptilophylla) co-administrated with green tea on ambulatory behaviors. Biosci. Biotechnol. Biochem. 2009, 73, 957. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, X.R.; Ho, C.T.; Zhang, Y.Y.; Li, B.; Chen, Z.Z. Chemical composition and anti-inflammatory activity of water extract from black cocoa tea (Camellia ptilophylla). Food Res. Int. 2022, 161, 111831. [Google Scholar] [CrossRef]
- Gao, K.; Wang, D.M.; Zhao, Y.P.; Zheng, X.Q.; Zhang, H.H.; Deng, Y.Z.; Li, F.X.; Ye, C.X. Variation of main chemical ingredients of Cocoa tea after cultivation and comparison with traditional tea. Nat. Prod. Devel. 2004, 16, 552–556. (In Chinese) [Google Scholar]
- Ou, C.S.; Ou, Y.X.; Kong, Q.; Sun, H.R.; Luo, Y.M.; Wu, F.; Li, M.N.; Zeng, J.; Liang, Y.R.; Zheng, X.Q. Variation of major quality-related chemical components in leaves of seed-propagated offspring in ‘Cocoa tea’. J. Tea 2022, 48, 169–172. (In Chinese) [Google Scholar]
- Ye, C.X.; Zhu Huang, W.J. A study on which two hormones affected producing callus and roots of cuttings Ptilophylla Chang. Ecol. Sci. 1991, 1, 56–61. (In Chinese) [Google Scholar]
- He, Y.M.; Peng, L.; Li, C.R.; Li, J.X.; Huang, H.L.; Qiao, X.Y.; Yan, C.Y.; Wu, H.L.; Ye, C.X.; Song, X.H. Research on the biochemical ingredients of cultivated varieties of Cocoa tea. Guangdong Agric. Sci. 2011, 6, 10–13. (In Chinese) [Google Scholar]
- Lin, X.R.; Chen, Z.Z.; Zhang, Y.Y.; Gao, X.; Luo, W.; Li, B. Interactions among cehmical components of Cocoa tea (Camellia ptilophylla Chang), a naturally low caffeine-containing tea species. Food Funct. 2014, 5, 1175–1185. [Google Scholar] [CrossRef]
- Zeynep İlbay, Z.; Şahin, S.; Kirbaşlar, S.I. Investigation of polyhenolic content of rose hip (Rosa canina L.) tea extracts: A comparative study. Foods 2013, 2, 43–52. [Google Scholar] [CrossRef]
- Liang, Y.R.; Wu, Y.; Lu, J.L.; Zhang, L.Y. Application of chemical composition and infusion colour difference analysis to quality estimation of jasmine-scented tea. Intl. J. Food Sci. Technol. 2007, 42, 459. [Google Scholar] [CrossRef]
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. Int. J. Food Prop. 2008, 11, 258. [Google Scholar] [CrossRef]
- Yamada, M.; Sasaki, S.; Murakami, K.; Takahashi, Y.; Okubo, H.; Hirota, N.; Notsu, A.; Todoriki, H.; Miura, A.; Fukui, M.; et al. Estimation of caffeine intake in Japanese adults using 16 d weighed diet records based on a food composition database newly developed for Japanese populations. Public Health Nutr. 2010, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Zhao, Z.; Chen, C.S.; Yu, Y.; Jeyaraj, A.; Zhuang, J.; Arkorful, E.; Kuberan, T.; Periakaruppan, R.; Kou, X.B.; et al. Characterization of self-incompatibility and expression profiles of CsMCU2 related to pollination in different varieties of tea plants. Sci Hortic. 2022, 293, 110693. [Google Scholar] [CrossRef]
- Yuan, C.C.; Shi, S.H.; Ye, C.X. Phylogentic relationship of Camellia ptilophylla Chang with its allied species and disintegration of its population. ACTA Sci. Nat. Univ. Sunyatseni 1999, 38, 72–76. (In Chinese) [Google Scholar]
- Peng, L.; Wang, X.J.; Shi, X.G.; Li, C.R.; Ye, C.X.; Song, X.H. Characterization of the constituents and antioxidative activity of cocoa tea (Camellia ptilophylla). Food Chem. 2011, 129, 1475–1482. [Google Scholar] [CrossRef]
- Yang, X.R.; Wang, X.J.; Li, K.K.; Li, J.; Li, C.R.; Shi, X.G.; Ko, C.H.; Leung, P.C.; Ye, C.X.; Song, X.H. Cocoa tea (Camellia ptilophylla Chang), a natural decaffeinated species of tea—Recommendations on the proper way of preparation for consumption. J. Funct. Foods 2011, 2, 305–312. [Google Scholar] [CrossRef]
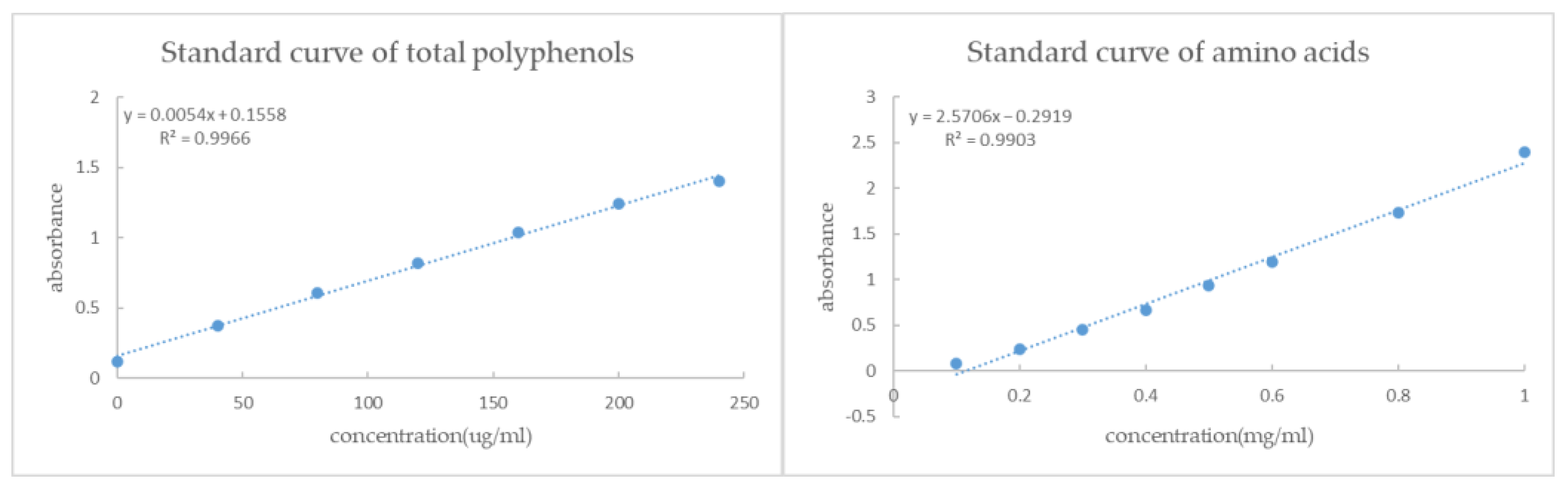
| Plant No. | Total Polyphenols (EGCG Equivalent) | Amino Acids |
|---|---|---|
| KS1 | 31.96 ± 0.81 cdef① | 6.75 ± 0.23 ijk |
| KS2 | 33.74 ± 1.13 b | 4.15 ± 0.03 u |
| KS2B | 27.87 ± 0.74 mn | 7.32 ± 0.12 gh |
| KS3 | 31.36 ± 0.17 efghi | 6.22 ± 0.14 lmn |
| KS4 | 28.99 ± 0.26 jklm | 6.51 ± 0.21 klm |
| KS6 | 30.28 ± 0.52 ghijk | 6.97 ± 0.05 hij |
| KS7 | 36.03 ± 0.95 a | 3.97 ± 0.11 u |
| KS8 | 18.33 ± 0.54 q | 3.42 ± 0.05 v |
| KS10 | 28.69 ± 0.57 klm | 5.66 ± 0.03 op |
| KS17 | 27.00 ± 0.37 no | 8.25 ± 0.02 d |
| KS21 | 27.90 ± 0.5 mn | 4.53 ± 0.16 t |
| KS22 | 29.84 ± 0.36 ijkl | 6.60 ± 0.11 jkl |
| KS23A | 27.42 ± 0.36 mn | 10.40 ± 0.33 a |
| KS27 | 30.06 ± 0.3 hijkl | 6.66 ± 0.32 ijk |
| KS31 | 28.08 ± 0.55 mn | 5.91 ± 0.40 no |
| KS32 | 31.54 ± 0.18 defgh | 7.98 ± 0.08 de |
| KS33 | 32.50 ± 0.57 bcde | 6.25 ± 0.08 lmn |
| KS34 | 35.44 ± 1.69 a | 5.42 ± 0.10 pq |
| KS35 | 27.72 ± 1.11 mn | 3.25 ± 0.03 v |
| KS36 | 18.32 ± 0.34 q | 3.41 ± 0.03 v |
| KS37 | 25.86 ± 0.61 op | 4.89 ± 0.12 rst |
| KS38 | 33.25 ± 0.82 bc | 4.59 ± 0.08 st |
| KS40 | 32.05 ± 0.9 cdef | 5.10 ± 0.15 qr |
| KS41 | 28.74 ± 0.74 klm | 8.91 ± 0.26 c |
| KS43 | 25.50 ± 0.24 p | 6.02 ± 0.08 no |
| KS44 | 31.75 ± 0.59 cdefg | 6.90 ± 0.25 ijk |
| KS45 | 32.00 ± 0.55 cdef | 5.44 ± 0.15 pq |
| KS47 | 29.84 ± 1.02 ijkl | 5.49 ± 0.08 pq |
| KS48 | 28.54 ± 0.55 lmn | 4.96 ± 0.11 rs |
| KS49 | 25.27 ± 0.35 p | 5.98 ± 0.19 no |
| KS50 | 24.35 ± 0.08 p | 6.71 ± 0.13 ijk |
| KS51 | 33.07 ± 1.16 bcd | 6.14 ± 0.13 mn |
| KS53 | 28.58 ± 1.05 lmn | 7.06 ± 0.41 hi |
| KS54 | 32.90 ± 0.16 bcde | 6.56 ± 0.18 jkl |
| KS55A | 30.40 ± 0.39 fghij | 7.45 ± 0.14 fg |
| KS55B | 25.16 ± 0.83 p | 8.08 ± 0.21 de |
| KS59 | 33.78 ± 1.06 b | 6.06 ± 0.06 no |
| KS62 | 30.70 ± 0.94 fghi | 7.76 ± 0.16 ef |
| KS65 | 32.67 ± 0.3 bcde | 9.56 ± 0.42 b |
| Mean | 29.42 ± 3.88 | 6.24 ± 1.63 |
| CV (%) ② | 13.20 | 26.10 |
| Plant No. | Caffeine | Theacrine | Theobromine | Theophylline | Total |
|---|---|---|---|---|---|
| KS1 | 52.69 ± 3.54 a① | 6.09 ± 0.44 e | 11.66 ± 0.73 op | 0 | 70.45 ± 4.69 a |
| KS2 | 1.24 ± 0.05 m | 4.17 ± 0.04 j | 52.49 ± 1.26 c | 0 | 57.89 ± 1.36 cd |
| KS2B | 28.33 ± 0.28 ij | 3.79 ± 0.03 k | 9.71 ± 0.16 q | 0 | 41.83 ± 0.46 op |
| KS3 | 39.44 ± 0.31 fg | 1.71 ± 0.02 pqr | 14.69 ± 0.17 mn | 0 | 55.84 ± 0.46 de |
| KS4 | 34.06 ± 0.23 h | 5.77 ± 0.03 fg | 16.18 ± 0.14 m | 0 | 56.02 ± 0.38 de |
| KS6 | 1.39 ± 0.03 m | 1.09 ± 0.01 t | 55.45 ± 1.12 b | 0 | 57.93 ± 1.11 cd |
| KS7 | 0.98 ± 0.03 m | 2.34 ± 0.04 o | 38.69 ± 0.52 ij | 0 | 42.01 ± 0.58 op |
| KS8 | 21.37 ± 0.97 l | 5.08 ± 0.20 h | 0.93 ± 0.01 u | 0.11 ± 0.05 a | 27.50 ± 1.22 r |
| KS10 | 45.74 ± 2.53 b | 2.96 ± 0.02 n | 6.94 ± 0.12 r | 0.08 ± 0.12 ab | 55.73 ± 2.50 de |
| KS17 | 35.60 ± 0.87 h | 2.59 ± 0.05 o | 9.99 ± 0.24 pq | 0 | 48.17 ± 1.15 jklm |
| KS21 | 0.96 ± 0.01 m | 1.81 ± 0.09 pq | 37.03 ± 0.21 jk | 0 | 39.80 ± 0.28 p |
| KS22 | 38.34 ± 1.42 g | 3.29 ± 0.16 lm | 18.71 ± 0.25 l | 0 | 60.34 ± 1.61 c |
| KS23A | 0.60 ± 0.08 m | 4.83 ± 0.17 hi | 39.13 ± 1.16 i | 0 | 44.55 ± 1.25 no |
| KS27 | 0 | 1.61 ± 0.02 pqr | 47.55 ± 0.50 ef | 0 | 49.17 ± 0.48 ijkl |
| KS31 | 33.46 ± 0.09 h | 1.73 ± 0.03 pqr | 13.08 ± 0.10 no | 0 | 48.28 ± 0.12 jklm |
| KS32 | 45.09 ± 0.28 bc | 5.78 ± 0.01 f | 3.72 ± 0.01 s | 0 | 54.59 ± 0.28 defg |
| KS33 | 0.80 ± 0.01 m | 3.55 ± 0.03 kl | 47.03 ± 0.43 fg | 0 | 51.38 ± 0.47 ghij |
| KS34 | 42.69 ± 1.52 de | 5.46 ± 0.24 g | 15.29 ± 0.56 m | 0 | 63.45 ± 2.32 b |
| KS35 | 24.76 ± 2.66 k | 3.65 ± 0.18 k | 1.02 ± 0.05 u | 0.15 ± 0.01 a | 29.57 ± 2.70 r |
| KS36 | 0 | 1.12 ± 0.07 st | 38.06 ± 2.95 ij | 0 | 39.18 ± 3.02 p |
| KS37 | 0 | 0.94 ± 0.08 t | 43.34 ± 0.42 h | 0.12 ± 0.09 a | 44.40 ± 0.26 no |
| KS38 | 0.27 ± 0.38 m | 1.92 ± 0.06 p | 45.45 ± 1.60 g | 0 | 47.64 ± 1.99 klmn |
| KS40 | 1.90 ± 0.04 m | 1.48 ± 0.01 qr | 43.54 ± 0.74 h | 0 | 46.92 ± 0.78 lmn |
| KS41 | 40.73 ± 1.90 ef | 1.84 ± 0.08 pq | 3.39 ± 0.17 st | 0 | 45.96 ± 2.16 lmn |
| KS43 | 43.37 ± 1.37 cd | 4.68 ± 0.12 i | 6.07 ± 0.17 r | 0 | 54.13 ± 1.66 efgh |
| KS44 | 38.06 ± 0.70 g | 4.12 ± 0.05 j | 2.49 ± 0.03 stu | 0 | 44.68 ± 0.77 no |
| KS45 | 0.62 ± 0.01 m | 7.77 ± 0.12 b | 36.2 ± 0.58 k | 0 | 44.59 ± 0.71 no |
| KS47 | 1.11 ± 0.03 m | 1.50 ± 0.05 qr | 50.48 ± 1.24 d | 0.11 ± 0.15 a | 53.19 ± 1.12 efgh |
| KS48 | 1.51 ± 0.04 m | 1.49 ± 0.03 qr | 42.09 ± 1.02 h | 0 | 45.09 ± 1.08 mno |
| KS49 | 30.18 ± 0.40 i | 1.03 ± 0.23 t | 2.09 ± 0.12 stu | 0.09 ± 0.12 ab | 33.38 ± 0.56 q |
| KS50 | 27.69 ± 0.23 j | 0.91 ± 0.14 t | 1.69 ± 0.04 tu | 0 | 30.28 ± 0.15 r |
| KS51 | 38.81 ± 0.63 fg | 7.07 ± 0.10 c | 5.94 ± 0.30 r | 0 | 51.82 ± 0.92 fghi |
| KS53 | 42.99 ± 1.45 cde | 6.57 ± 0.23 d | 1.32 ± 0.05 u | 0 | 50.89 ± 1.73 hijk |
| KS54 | 0 | 3.20 ± 0.06 mn | 49.06 ± 0.72 de | 0 | 52.26 ± 0.78 fghi |
| KS55A | 42.49 ± 0.89 de | 1.00 ± 0.01 t | 11.35 ± 0.36 opq | 0.06 ± 0.05 ab | 54.91 ± 1.20 def |
| KS55B | 24.03 ± 0.88 k | 1.42 ± 0.01 rs | 1.58 ± 0.04 tu | 0 | 27.03 ± 0.90 r |
| KS59 | 0.10 ± 0.02 m | 15.92 ± 0.57 a | 53.57 ± 1.04 c | 0 | 69.60 ± 1.58 a |
| KS62 | 0 | 1.70 ± 0.04 pqr | 42.72 ± 1.32 h | 0 | 44.42 ± 1.35 no |
| KS65 | 1.23 ± 0.03 m | 1.49 ± 0.03 qr | 65.39 ± 1.54 a | 0 | 68.10 ± 1.60 a |
| Mean | 20.07 ± 19.11 | 3.45 ± 2.83 | 25.26 ± 20.50 | 0.02 ± 0.04 | 48.79 ± 10.70 |
| CV (%) ② | 95.25 | 81.96 | 81.16 | 225.58 | 21.94 |
| Plant No. | GCG | EGCG | ECG | C | CG | GC | EGC | EC | Total Catechins |
|---|---|---|---|---|---|---|---|---|---|
| KS1 | 110.16 ± 8.85 fgh① | 54.81 ± 4.04 d | 26.76 ± 1.98 e | 13.18 ± 0.73 st | 1.06 ± 0.17 rst | 18.78 ± 1.14 h | 10.87 ± 0.91 de | 4.15 ± 0.26 defgh | 239.78 ± 17.96 c |
| KS2 | 108.37 ± 3.50 ghi | 32.23 ± 0.79 i | 17.12 ± 0.63 l | 17.65 ± 0.16 pq | 3.75 ± 0.13 no | 2.94 ± 0.03 p | 1.67 ± 0.04 hi | 3.16 ± 0.18 fghij | 186.88 ± 5.04 jklmn |
| KS2B | 44.24 ± 0.65 op | 25.41 ± 0.24 k | 15.42 ± 0.17 m | 14.24 ± 0.13 rst | 16.38 ± 0.17 e | 18.88 ± 0.06 h | 0 | 1.79 ± 0.16 ijkl | 136.38 ± 1.16 rs |
| KS3 | 135.73 ± 1.89 cd | 4.38 ± 0.06 nop | 21.94 ± 0.38 g | 21.01 ± 0.05 klm | 23.46 ± 0.20 b | 32.51 ± 0.21 c | 0 | 0.97 ± 0.04 jkl | 239.99 ± 2.56 c |
| KS4 | 81.02 ± 0.52 l | 36.14 ± 0.29 gh | 10.92 ± 0.15 n | 11.28 ± 0.06 u | 1.99 ± 0.08 pq | 16.31 ± 0.02 i | 8.79 ± 0.15 f | 3.65 ± 0.07 efghi | 170.10 ± 1.11 op |
| KS6 | 136.36 ± 2.76 cd | 3.69 ± 0.06 nop | 33.32 ± 0.88 b | 24.57 ± 0.16 gh | 25.78 ± 0.66 a | 1.88 ± 0.02 p | 0 | 0 | 225.60 ± 4.52 def |
| KS7 | 141.86 ± 3.38 c | 6.09 ± 0.10 n | 40.32 ± 1.06 a | 28.92 ± 0.44 d | 17.41 ± 0.29 d | 20.27 ± 0.38 g | 0 | 2.25 ± 0.06 ghijk | 257.11 ± 5.52 b |
| KS8 | 17.71 ± 0.96 r | 16.95 ± 1.02 l | 6.88 ± 0.24 o | 2.57 ± 0.10 x | 0.82 ± 0.05 st | 8.53 ± 0.23 m | 11.00 ± 0.71 d | 6.12 ± 0.30 d | 70.58 ± 2.24 u |
| KS10 | 103.93 ± 1.77 hij | 16.09 ± 0.27 l | 26.32 ± 0.65 e | 16.85 ± 0.15 q | 10.37 ± 0.15 fg | 16.23 ± 0.16 i | 2.79 ± 2.80 h | 4.39 ± 5.45 defg | 190.45 ± 13.68 jkl |
| KS17 | 97.48 ± 2.33 jk | 17.95 ± 0.21 l | 10.93 ± 0.22 n | 19.48 ± 0.61 mno | 3.12 ± 0.09 o | 19.76 ± 0.37 gh | 6.00 ± 0.11 g | 0.80 ± 0.10 kl | 175.54 ± 3.78 nop |
| KS21 | 84.08 ± 0.65 l | 3.75 ± 0.16 nop | 15.15 ± 0.24 m | 23.58 ± 0.48 hi | 10.72 ± 0.19 f | 10.96 ± 0.26 l | 0 | 2.00 ± 0.29 hijkl | 150.25 ± 0.53 q |
| KS22 | 92.33 ± 2.59 k | 28.56 ± 1.04 j | 15.05 ± 0.95 m | 19.16 ± 0.42 no | 10.05 ± 0.20 gh | 11.55 ± 0.93 l | 10.31 ± 1.07 de | 1.66 ± 0.06 ijkl | 188.66 ± 6.49 jklmn |
| KS23A | 46.66 ± 1.68 o | 25.85 ± 1.00 k | 17.34 ± 0.75 kl | 16.95 ± 0.46 q | 7.87 ± 0.34 j | 13.20 ± 0.35 k | 0 | 3.29 ± 0.14 fghi | 131.16 ± 4.46 rs |
| KS27 | 130.99 ± 2.16 d | 3.79 ± 0.09 nop | 20.21 ± 0.34 hi | 35.21 ± 0.28 c | 19.09 ± 0.35 c | 30.30 ± 0.33 d | 0 | 14.27 ± 0.10 bc | 253.86 ± 2.84 b |
| KS31 | 134.19 ± 0.99 d | 3.40 ± 0.27 op | 15.21 ± 0.12 m | 17.30 ± 0.15 q | 9.48 ± 0.11 hi | 20.03 ± 0.07 gh | 0 | 0 | 199.61 ± 0.72 hij |
| KS32 | 78.23 ± 0.33 l | 31.28 ± 0.11 i | 14.20 ± 0.09 m | 15.12 ± 0.11 r | 1.16 ± 0.01 rst | 20.33 ± 0.11 g | 9.62 ± 0.02 ef | 2.29 ± 0.02 ghijk | 174.38 ± 3.34 nop |
| KS33 | 112.42 ± 0.94 fg | 32.46 ± 0.35 i | 23.83 ± 0.26 f | 14.66 ± 0.14 rs | 5.74 ± 0.06 l | 2.19 ± 0.09 p | 1.21 ± 0.04 ij | 2.10 ± 0.16 hijkl | 194.62 ± 1.49 ijk |
| KS34 | 108.80 ± 5.69 ghi | 34.97 ± 1.69 h | 20.69 ± 1.10 gh | 19.05 ± 0.73 op | 3.73 ± 0.21 no | 22.93 ± 0.87 f | 10.06 ± 0.55 de | 2.41 ± 0.23 ghijk | 225.61 ± 13.08 def |
| KS35 | 28.00 ± 1.16 q | 11.16 ± 0.52 m | 15.04 ± 0.66 m | 7.03 ± 0.31 w | 1.45 ± 0.19 qrs | 6.54 ± 0.36 n | 0 | 5.55 ± 0.07 de | 76.38 ± 4.20 u |
| KS36 | 68.96 ± 5.02 m | 2.75 ± 0.20 op | 6.27 ± 0.47 o | 19.85 ± 1.23 mno | 5.41 ± 0.38 l | 10.93 ± 0.69 l | 0 | 1.87 ± 0.17 ijkl | 116.05 ± 8.07 t |
| KS37 | 123.17 ± 0.39 e | 9.14 ± 0.02 m | 14.40 ± 0.09 m | 21.44 ± 0.07 jkl | 4.59 ± 0.10 m | 48.64 ± 1.11 b | 6.18 ± 0.52 g | 2.54 ± 0.16 fghijk | 230.11 ± 1.55 cde |
| KS38 | 153.94 ± 4.88 b | 4.97 ± 0.20 no | 41.46 ± 1.19 a | 36.53 ± 2.10 c | 25.33 ± 0.59 a | 33.56 ± 1.05 c | 0 | 21.00 ± 0.74 a | 316.79 ± 7.15 a |
| KS40 | 113.30 ± 2.27 fg | 3.85 ± 0.02 nop | 31.66 ± 0.69 c | 22.33 ± 0.21 ijk | 16.98 ± 0.36 de | 1.88 ± 0.05 p | 0 | 0 | 190.01 ± 3.49 jklm |
| KS41 | 116.86 ± 6.07 f | 4.89 ± 0.23 no | 16.90 ± 0.97 l | 26.43 ± 1.25 ef | 16.46 ± 0.89 e | 21.97 ± 0.89 f | 0 | 15.44 ± 0.83 b | 218.95 ± 11.11 efg |
| KS43 | 79.94 ± 2.58 l | 38.32 ± 1.24 g | 18.96 ± 0.57 ij | 10.23 ± 0.21 uv | 2.19 ± 0.11 p | 13.48 ± 0.36 k | 12.74 ± 0.22 c | 1.74 ± 0.12 ijkl | 175.74 ± 7.40 mnop |
| KS44 | 82.33 ± 1.98 l | 26.60 ± 0.52 jk | 14.90 ± 0.31 m | 21.75 ± 0.36 jkl | 1.40 ± 0.04 qrs | 14.64 ± 0.26 j | 6.08 ± 0.12 g | 4.68 ± 0.13 def | 172.38 ± 3.64 op |
| KS45 | 39.26 ± 0.63 p | 47.32 ± 0.86 e | 20.43 ± 0.34 h | 8.95 ± 0.10 v | 1.64 ± 0.02 pqr | 13.06 ± 0.16 k | 7.31 ± 0.29 g | 5.68 ± 0.07 de | 143.65 ± 2.32 qr |
| KS47 | 114.27 ± 3.37 fg | 2.65 ± 0.08 op | 18.88 ± 0.77 ijk | 28.87 ± 0.29 d | 19.59 ± 0.57 c | 25.43 ± 0.60 e | 0 | 0 | 209.70 ± 5.54 gh |
| KS48 | 113.22 ± 3.43 fg | 3.15 ± 0.10 op | 12.35 ± 0.5 n | 22.95 ± 0.19 ij | 7.17 ± 0.28 k | 18.97 ± 0.15 h | 0 | 0 | 177.81 ± 4.43 lmnop |
| KS49 | 2.12 ± 0.06 s | 79.15 ± 1.62 b | 18.83 ± 0.46 ijk | 0.97 ± 0.14 y | 0.14 ± 0.01 u | 3.06 ± 1.05 p | 49.99 ± 1.37 a | 12.59 ± 0.34 c | 166.86 ± 4.53 op |
| KS50 | 2.02 ± 0.03 s | 74.3 ± 1.67 c | 17.72 ± 0.28 jkl | 1.13 ± 0.21 y | 0.13 ± 0.01 u | 5.16 ± 0.56 o | 50.41 ± 1.09 a | 12.97 ± 0.49 c | 163.84 ± 4.19 p |
| KS51 | 95.39 ± 2.67 k | 48.23 ± 1.10 e | 18.40 ± 0.39 jkl | 13.91 ± 0.13 rst | 0.91 ± 0.04 st | 20.40 ± 0.30 g | 8.56 ± 0.02 f | 6.06 ± 0.14 d | 213.47 ± 6.55 fg |
| KS53 | 66.19 ± 2.13 m | 44.97 ± 1.54 f | 24.43 ± 0.85 f | 12.99 ± 0.48 t | 0.94 ± 0.04 st | 10.53 ± 0.31 l | 7.05 ± 0.2 g | 3.21 ± 0.20 fghi | 170.32 ± 5.60 op |
| KS54 | 103.19 ± 1.64 ij | 25.47 ± 0.44 k | 11.98 ± 0.22 n | 27.38 ± 0.47 e | 5.58 ± 0.13 l | 2.23 ± 0.12 p | 0 | 2.96 ± 0.22 fghijk | 178.81 ± 2.96 lmno |
| KS55A | 14.29 ± 0.38 r | 122.63 ± 3.74 a | 29.84 ± 0.99 d | 2.79 ± 0.07 x | 0.65 ± 0.03 tu | 6.30 ± 0.15 no | 28.53 ± 0.43 b | 2.59 ± 0.28 fghijk | 207.62 ± 5.00 ghi |
| KS55B | 55.51 ± 2.38 n | 2.09 ± 0.03 p | 11.23 ± 0.61 n | 20.63 ± 0.42 lmn | 4.32 ± 0.25 mn | 30.22 ± 1.10 d | 0 | 1.55 ± 0.21 ijkl | 125.56 ± 4.61 st |
| KS59 | 54.47 ± 1.21 n | 2.70 ± 0.20 op | 2.10 ± 0.75 p | 44.23 ± 2.70 a | 19.47 ± 0.55 c | 57.02 ± 1.32 a | 0 | 0.85 ± 0.50 kl | 180.85 ± 4.36 klmno |
| KS62 | 101.88 ± 3.04 j | 3.49 ± 0.11 nop | 18.75 ± 0.65 ijk | 40.55 ± 0.96 b | 8.92 ± 0.41 i | 21.78 ± 0.60 f | 0 | 13.33 ± 0.35 c | 208.69 ± 6.12 gh |
| KS65 | 161.45 ± 4.30 a | 4.56 ± 0.09 nop | 33.53 ± 1.04 b | 25.82 ± 0.51 fg | 7.57 ± 0.22 jk | 0 | 0 | 0 | 232.93 ± 6.15 cd |
| Mean | 88.57 ± 41.69 | 24.11 ± 25.97 | 19.22 ± 8.67 | 19.17 ± 10.08 | 8.28 ± 7.78 | 16.75 ± 12.45 | 6.39 ±11.86 | 4.36 ± 5.00 | 186.85 ± 48.12 |
| CV(%)② | 47.07 | 107.71 | 45.12 | 52.59 | 93.94 | 74.29 | 185.55 | 114.82 | 25.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.-Q.; Dong, S.-L.; Li, Z.-Y.; Lu, J.-L.; Ye, J.-H.; Tao, S.-K.; Hu, Y.-P.; Liang, Y.-R. Variation of Major Chemical Composition in Seed-Propagated Population of Wild Cocoa Tea Plant Camellia ptilophylla Chang. Foods 2023, 12, 123. https://doi.org/10.3390/foods12010123
Zheng X-Q, Dong S-L, Li Z-Y, Lu J-L, Ye J-H, Tao S-K, Hu Y-P, Liang Y-R. Variation of Major Chemical Composition in Seed-Propagated Population of Wild Cocoa Tea Plant Camellia ptilophylla Chang. Foods. 2023; 12(1):123. https://doi.org/10.3390/foods12010123
Chicago/Turabian StyleZheng, Xin-Qiang, Shu-Ling Dong, Ze-Yu Li, Jian-Liang Lu, Jian-Hui Ye, Shi-Ke Tao, Yan-Ping Hu, and Yue-Rong Liang. 2023. "Variation of Major Chemical Composition in Seed-Propagated Population of Wild Cocoa Tea Plant Camellia ptilophylla Chang" Foods 12, no. 1: 123. https://doi.org/10.3390/foods12010123
APA StyleZheng, X.-Q., Dong, S.-L., Li, Z.-Y., Lu, J.-L., Ye, J.-H., Tao, S.-K., Hu, Y.-P., & Liang, Y.-R. (2023). Variation of Major Chemical Composition in Seed-Propagated Population of Wild Cocoa Tea Plant Camellia ptilophylla Chang. Foods, 12(1), 123. https://doi.org/10.3390/foods12010123







