Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crude extracts Analysis
2.2. Chromatographic Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Olive Oil—Detailed Information on the Market Situation, Price Developments, Balance Sheets, Production and Trade. 2022. Available online: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/prices/price-monitoring-sector/plant-products/olive-oil_en (accessed on 1 April 2022).
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive Oil History, Production and by-Product Management. Rev. Environ. Sci. Biotechnol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature Review on Production Process To Obtain Extra Virgin Olive Oil Enriched in Bioactive Compounds. Potential Use of Byproducts as Alternative Sources of Polyphenols. J. Agric. Food Chem. 2013, 61, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and Cardioprotective Activities of Polar Lipids of Olive Pomace, Olive Pomace-Enriched Fish Feed and Olive Pomace Fed Gilthead Sea Bream (Sparus Aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and Antiatherosclerotic Properties of Olive Oil and Olive Pomace Polar Extracts in Rabbits. Mediat. Inflamm. 2007, 2007, 36204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavčič Višnjevec, A.; Baker, P.; Charlton, A.; Preskett, D.; Peeters, K.; Tavzes, Č.; Kramberger, K.; Schwarzkopf, M. Developing an Olive Biorefinery in Slovenia: Analysis of Phenolic Compounds Found in Olive Mill Pomace and Wastewater. Molecules 2020, 26, 7. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated Fats from Plant and Animal Sources in Relation to Risk of Coronary Heart Disease among US Men and Women. Am. J. Clin. Nutr. 2018, 107, 445–453. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional Compounds from Olive Pomace to Obtain High-added Value Foods—A Review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0432 (accessed on 1 April 2022).
- Suárez-Eiroa, B.; Fernández, E.; Méndez-Martínez, G.; Soto-Oñate, D. Operational Principles of Circular Economy for Sustainable Development: Linking Theory and Practice. J. Clean. Prod. 2019, 214, 952–961. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of Pulsed Electric Fields and High Pressure on Improved Recovery of High-added-value Compounds from Olive Pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Čepo, D.V.; Albahari, P.; Končić, M.Z.; Radić, K.; Jurmanović, S.; Jug, M. Solvent extraction and chromatographic determination of polyphenols in olive pomace. Hrana U Zdr. I Boles. Znan.-Stručni Časopis Za Nutr. I Dijetetiku 2017, 8, 7–14. [Google Scholar]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque de Castro, M.D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography–Tandem Mass Spectrometry with a Quadrupole–Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound Increases the Aqueous Extraction of Phenolic Compounds with High Antioxidant Activity from Olive Pomace. LWT 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [Green Version]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; de Moura Bell, J.M.L.N. Integrated Microwave- and Enzyme-Assisted Extraction of Phenolic Compounds from Olive Pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of Olive Pomace by a Green Integrated Approach Applying Sustainable Extraction and Membrane-Assisted Concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Alburquerque, J. Agrochemical Characterisation of “Alperujo”, a Solid by-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and Other Health Properties of Olive Pomace Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as Sustainable Lipophilic Solvent to Substitute Hexane for Green Extraction of Natural Products. Properties, Applications, and Perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef] [PubMed]
- Gharby, S.; Ravi, H.K.; Guillaume, D.; Abert Vian, M.; Chemat, F.; Charrouf, Z. 2-Methyloxolane as Alternative Solvent for Lipid Extraction and Its Effect on the Cactus (Opuntia Ficus-Indica L.) Seed Oil Fractions. OCL 2020, 27, 27. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is It Possible to Substitute Hexane with Green Solvents for Extraction of Carotenoids? A Theoretical versus Experimental Solubility Study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef] [Green Version]
- Carré, P. About Solvents Used in the Preparation of Oils for Cosmetic Products Complying with the Cosmos Standard. OCL 2021, 28, 16. [Google Scholar] [CrossRef]
- Scientific Committee for Food. Reports of the Scientific Committee for Food, 35th ed.; European Commission: Brussels, Belgium, 1996. [Google Scholar]
- Parris, P.; Duncan, J.N.; Fleetwood, A.; Beierschmitt, W.P. Calculation of a Permitted Daily Exposure Value for the Solvent 2-Methyltetrahydrofuran. Regul. Toxicol. Pharmacol. 2017, 87, 54–63. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Toxicological Review of N-Hexane. 2005. Available online: https://iris.epa.gov/static/pdfs/0486tr.pdf (accessed on 1 February 2022).
- Envigo CRS Ltd. MeTHF: Toxicity Study by Inhalation Administration to Han Wistar Rats for 13 Weeks (#YB26GY); Envigo CRS Ltd.: Indianapolis, IN, USA, 2019. [Google Scholar]
- European Chemicals Agency (ECHA). N-Hexane—REACH Registration Dossier. Available online: https://echa.europa.eu/en/registration-dossier/-/registered-dossier/15741/6/2/8 (accessed on 1 February 2022).
- European Chemicals Agency (ECHA). Tetrahydro-2-Methylfuran-REACH Registration Dossier. Available online: https://echa.europa.eu/en/registrationdossier/-/registered-dossier/13699/6/4/2 (accessed on 1 February 2022).
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Impurities: Guideline for Residual Solvents Q3C (R4). 2009. Available online: https://www.tga.gov.au/sites/default/files/ich28395enrev4.pdf (accessed on 1 February 2022).
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Impurities: Guideline for Residual Solvents Q3C (R8)—PDE for 2-Methyltetrahydrofuran, Cylcopentyl Methyl Ether and Tertiary-Butyl Alcohol (Draft Version). 2020. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ich-guideline-q3c-r8-impurities-guideline-residual-solvents-step-5_en.pdf (accessed on 1 February 2022).
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Bourgou, S.; Detry, P.; Aidi Wannes, W.; Kenny, T.; Riadh, K.; Sellami, I.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Claux, O.; Rapinel, V.; Goupy, P.; Patouillard, N.; Vian, M.A.; Jacques, L.; Chemat, F. Dry and Aqueous 2-Methyloxolane as Green Solvents for Simultaneous Production of Soybean Oil and Defatted Meal. ACS Sustain. Chem. Eng. 2021, 9, 7211–7223. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative Solvents for Lipid Extraction and Their Effect on Protein Quality in Black Soldier Fly (Hermetia Illucens) Larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef] [PubMed]
- Queimada, A.J.; Mota, F.L.; Pinho, S.P.; Macedo, E.A. Solubilities of Biologically Active Phenolic Compounds: Measurements and Modeling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Agricultural Chemists; Horwitz, W. Official Methods of Analysis, 19th ed.; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- MacLean, W.C.; Warwick, P.; Food and Agriculture Organization of the United Nations (Eds.) Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop, Rome, 3–6 December 2002; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-105014-9. Available online: https://www.sennutricion.org/media/Docs_Consenso/Food_energy_methods_of_analysis_and_conversion_factors-FAO_2002.pdf (accessed on 1 October 2021).
- International Organization for Standardization (ISO). ISO 659 -Oilseeds—Determination of Oil Content (Reference Method). 2009. Available online: https://www.iso.org/standard/43169.html (accessed on 1 October 2021).
- Uribe, E.; Pasten, A.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Ortiz, J.; Di Scala, K. Comparison of Chemical Composition, Bioactive Compounds and Antioxidant Activity of Three Olive-Waste Cakes. J. Food Biochem. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant Activity, Phenolic Compounds and Anthocyanins Content of Eighteen Strains of Mexican Maize. LWT—Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Tontisirin, K. Chapter 2: Methods of food analysis. In Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop, Rome, 3–6 December 2002; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 7–17. [Google Scholar]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization (ISO). ISO 9936—Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 1 January 2022).
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I. Color-Pigment Correlation in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of Extraction Methods for the Identification and Quantification of Polyphenols in Virgin Olive Oil by Ultra-HPLC-QToF Mass Spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Determination of Biophenos in Olive Oils by HPLC-COI-/T.20/Doc No 29. International Olive Council. 2009. Available online: http://www.internationaloliveoil.org/ (accessed on 1 October 2021).
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid Hydrolysis of Secoiridoid Aglycons during Storage of Virgin Olive Oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing Analytical Requirements To Support Health Claims on “Olive Oil Polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guérère, M. Triacylglycerol and Fatty Acid Compositions of French Virgin Olive Oils. Characterization by Chemometrics. J. Agric. Food Chem. 2003, 51, 5723–5731. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Domingues, M.; Domingues, P. Polar Lipids from Olives and Olive Oil: A Review on Their Identification, Significance and Potential Biotechnological Applications. Foods 2018, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.; Coimbra, M.A. Characterisation of Phenolic Extracts from Olive Pulp and Olive Pomace by Electrospray Mass Spectrometry. J. Sci. Food Agric. 2005, 85, 21–32. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic Composition and Antimicrobial Activity of Algerian Olive Products and By-Products. LWT 2018, 93, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Jia, X.; Zheng, Z.; Lu, X.; Zheng, Y.; Zheng, B.; Xiao, J. Chemical Composition and Nutritional Function of Olive (Olea Europaea L.): A Review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Flórez, A.; Sinausia Nieva, L.; Sánchez-Ortíz, A.; Beltrán, G.; Perona, J.S. The Fatty Acid Composition of Virgin Olive Oil from Different Cultivars Is Determinant for Foam Cell Formation by Macrophages. J. Agric. Food Chem. 2015, 63, 6731–6738. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Ben Kaab, S.; Hammami, M.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Isoda, H.; Ksouri, R.; Fauconnier, M.-L. Green Solvent to Substitute Hexane for Bioactive Lipids Extraction from Black Cumin and Basil Seeds. Foods 2021, 10, 1493. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of By-Products from the Olive Oil Processing: Revalorization Strategies Based on Target Molecules and Green Extraction Technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-Promoting Properties of Oleocanthal and Oleacein: Two Secoiridoids from Extra-Virgin Olive Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
- THE 17 GOALS|Sustainable Development [WWW Document]. Available online: https://sdgs.un.org/goals (accessed on 16 January 2022).
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]



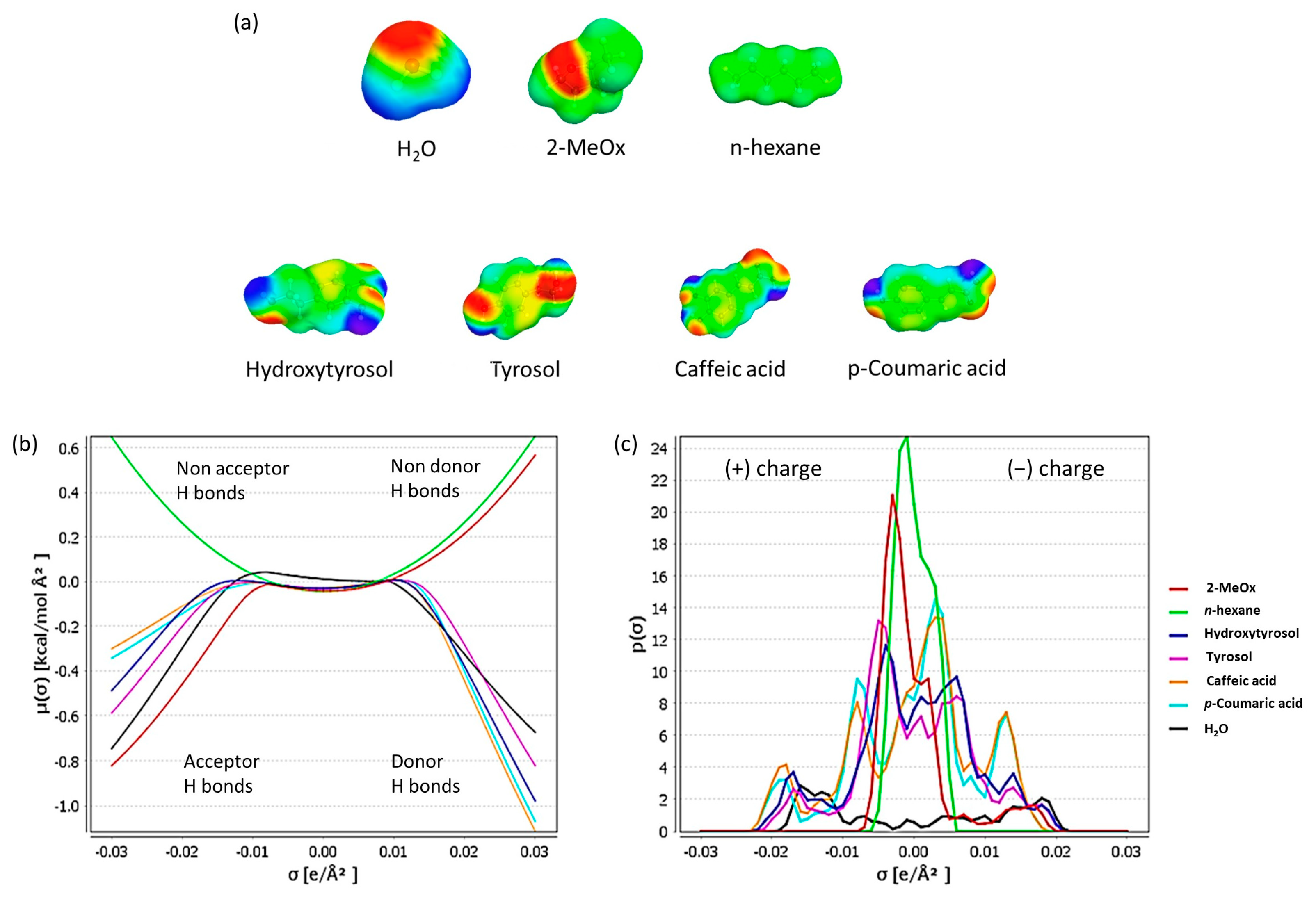
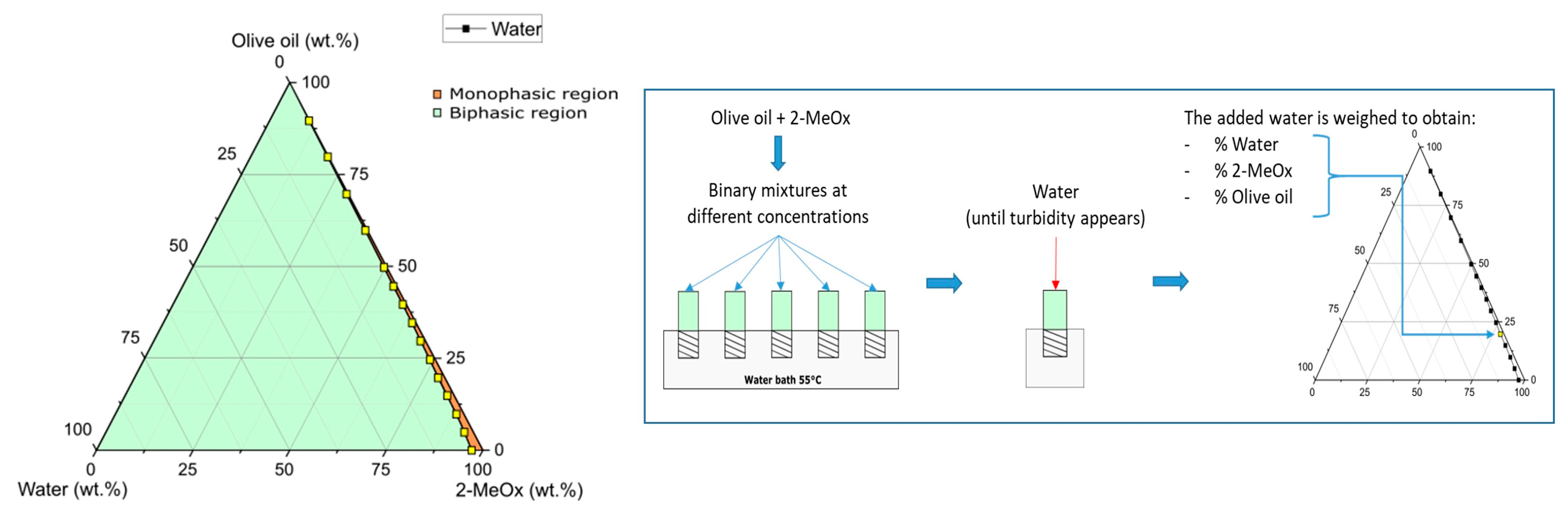
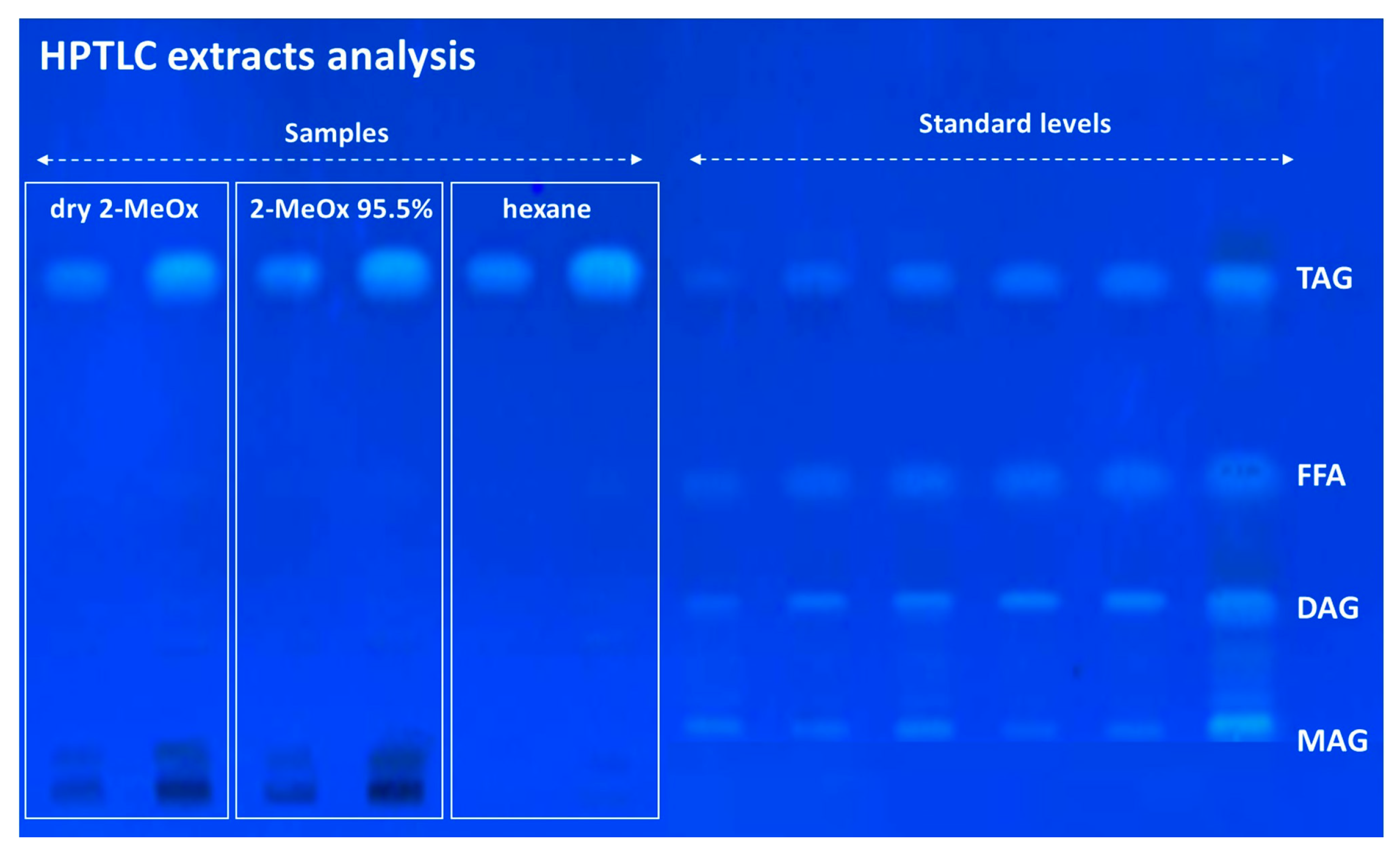

| n-Hexane | Dry 2-MeOx | 2-MeOx 95.5% | |||||
|---|---|---|---|---|---|---|---|
| COSMO-RS (Log10 (xsolub)) | Experimental Solubility (g/L) | COSMO-RS (log10) (xsolub)) | Experimental Solubility (g/L) | COSMO-RS (Log10 (xsolub)) | Experimental Solubility (g/L) | ||
| Phenolic compounds | Hydroxytyrosol | −5.64 | <0.01 | 0.00 | 858.05 ± 6.99 | 0.00 | 2204.16 ± 34.11 |
| Tyrosol | −4.93 | 0.01 ± 0.001 | 0.00 | 379.38 ± 9.56 | 0.00 | 573.47 ± 6.80 | |
| Caffeic acid | −7.59 | nd ° | 0.00 | 46.04 ± 0.49 | 0.00 | 133.69 ± 0.96 | |
| p-Coumaric acid | −6.94 | <0.01 | 0.00 | 155.59 ± 0.32 | 0.00 | 237.11 ± 0.29 | |
| TAG a | Triolein (OOO) | 0.00 | ∞ | 0.00 | ∞ | 0.00 | ∞ |
| Content | |
|---|---|
| Ash (g/100 g DM) | 4.18 ± 0.66 |
| Oil (g/100 g DM) | 13.66 ± 1.01 |
| Protein (g/100 g DM) | 6.64 ± 0.39 |
Total phenolic content (g GAE/100 g DM)
| 2.24 ± 0.10
|
| Carbohydrates (g/100 g DM) | 73.28 ± 2.14 |
| Hexane | Dry 2-MeOx | 2-MeOx 95.5% | |
|---|---|---|---|
| Extraction yield (g/100 g DM) | 13.87 ± 0.50 | 15.68 ± 1.69 | 14.10 ± 0.34 |
| Fatty acid profile (relative %) | |||
| C14 | traces | traces | traces |
| C14:1 | traces | traces | traces |
| C16 | 15.20 ± 0.09 | 15.08 ± 0.02 | 15.19 ± 0.13 |
| C16:1 | 0.80 ± 0.46 | 1.07 ± 0.01 | 1.00 ± 0.07 |
| C18 | 2.72 ± 0.02 | 2.66 ± 0.03 | 2.77 ± 0.08 |
| C18:1 n-9 | 68.05 ± 0.28 | 67.65 ± 0.02 | 67.43 ± 0.20 |
| C18:2 n-6 cis | 11.77 ± 0.09 | 11.96 ± 0.09 | 11.34 ± 0.66 |
| C18:3 n-3 | 0.79 ± 0.02 | 0.91 ± 0.01 | 0.80 ± 0.10 |
| C20 | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| C20:1 n-9 | 0.33 ± 0.01 | 0.34 ± 0.11 | 1.13 ± 0.82 |
| Others | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| Σ SFA a | 18.20 ± 0.12 | 18.00 ± 0.02 | 18.24 ± 0.21 |
| Σ MUFA b | 69.19 ± 0.21 | 69.06 ± 0.10 | 69.57 ± 0.55 |
| Σ PUFA c | 12.56 ± 0.11 | 12.87 ± 0.10 | 12.14 ± 0.76 |
| C18:1/C18:2 | 5.78 ± 0.03 | 5.66 ± 0.04 | 5.95 ± 0.33 |
| Hexane | Dry 2-MeOx | 2-MeOx 95.5% | |
|---|---|---|---|
| Tocopherol and Tocotrienol content (mg/kg extract) | |||
| α-tocopherol acetate | <5 | <5 | <5 |
| α-tocopherol | 288 | 234 | 201 |
| β-tocopherol | 4 | 3 | 3 |
| γ-tocopherol | 7 | 6 | 5 |
| δ-tocopherol | <2 | <2 | <2 |
| α-tocotrienol | <2 | <2 | <2 |
| β-tocotrienol | <2 | <2 | <2 |
| γ-Tocotrienol | <2 | <2 | <2 |
| δ-Tocotrienol | <2 | <2 | <2 |
| Total | 300 ± 45 | 243 ± 36 | 209 ± 31 |
| Vitamine E activity (mg α-TE a/kg extract) | 291 | 236 | 203 |
| Squalene and carotenoids content (mg/kg extract) | |||
| Squalene | 5810 | 4285 | 4033 |
| Carotenoids | 11.97 ± 0.32 | 134.39 ± 7.37 | 149.00 ± 2.50 |
| Hexane | Dry 2-MeOx | 2-MeOx 95.5% | |
|---|---|---|---|
| Hydroxytyrosol a | 7.12 ± 0.84 | 1729.78 ± 34.30 | 1843.50 ± 22.77 |
| Tyrosol a | 24.11 ± 2.14 | 771.46 ± 14.34 | 893.69 ± 20.33 |
| Oleacein a | 140.56 ± 14.17 | 6144.46 ± 11.34 | 7698.91 ± 34.07 |
| Oleocanthal a | 766.39 ± 10.34 | 3596.98 ± 46.55 | 3425.47 ± 22.34 |
| Caffeic acid b | nd ° | 287.10 ± 4.34 | 310.43 ± 7.07 |
| p-Coumaric acid b | nd ° | 698.42 ± 7.27 | 808.36 ± 14.59 |
| Hexane | Dry 2-MeOx | 2-MeOx 95.5% | |
|---|---|---|---|
| Total HT a (mg/kg extract product) | 194.42 ± 14.10 | 5585.06 ± 34.34 | 7293.06 ± 24.06 |
| Total T a (mg/kg extract product) | 523.69 ± 24.34 | 4934.31 ± 50.06 | 4618.25 ± 40.23 |
| Total HT, T and their derivates a,b (mg/kg extract product) | 1736 ± 45.16 | 24622 ± 99.17 | 27590 ± 75.54 |
| Total phenolic content (mg GAE/kg extract) | 862.51 ± 17.99 | 21989.22 ± 834.62 | 18487.30 ± 255.24 |
| Antioxidant activity (mg TE/kg extract) | 374.63 ± 79.45 | 26307.66 ± 849.86 | 26681.75 ± 452.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. https://doi.org/10.3390/foods11091357
Cravotto C, Fabiano-Tixier AS, Claux O, Rapinel V, Tomao V, Stathopoulos P, Skaltsounis AL, Tabasso S, Jacques L, Chemat F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods. 2022; 11(9):1357. https://doi.org/10.3390/foods11091357
Chicago/Turabian StyleCravotto, Christian, Anne Sylvie Fabiano-Tixier, Ombéline Claux, Vincent Rapinel, Valérie Tomao, Panagiotis Stathopoulos, Alexios Leandros Skaltsounis, Silvia Tabasso, Laurence Jacques, and Farid Chemat. 2022. "Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent" Foods 11, no. 9: 1357. https://doi.org/10.3390/foods11091357
APA StyleCravotto, C., Fabiano-Tixier, A. S., Claux, O., Rapinel, V., Tomao, V., Stathopoulos, P., Skaltsounis, A. L., Tabasso, S., Jacques, L., & Chemat, F. (2022). Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods, 11(9), 1357. https://doi.org/10.3390/foods11091357










