Whey Beverage Emulsified System as Carrying Matrix of Fennel Seed Extract Obtained by Supercritical CO2 Extraction: Impact of Thermosonication Processing and Addition of Prebiotic Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients, Chemicals, and Reagents
2.2. Supercritical CO2 Extraction of Lipophilic Bioactive Compounds from Fennel Seeds
2.3. Manufacturing Fennel-Based Whey Beverages
2.4. Thermosonication Treatments
2.5. Experimental Design
2.6. Droplet Size Distribution
2.7. Microstructure
2.8. Kinetic Stability
2.9. Color Parameters
2.10. Chemical and Functional Properties
2.10.1. Emulsion Break-Up Procedure
2.10.2. Determination of Total Phenolic Content
2.10.3. In Vitro Antioxidant Capacity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Thermosonication Process Design
3.2. Technological Properties
3.2.1. Droplet Size Distribution and Microstructure
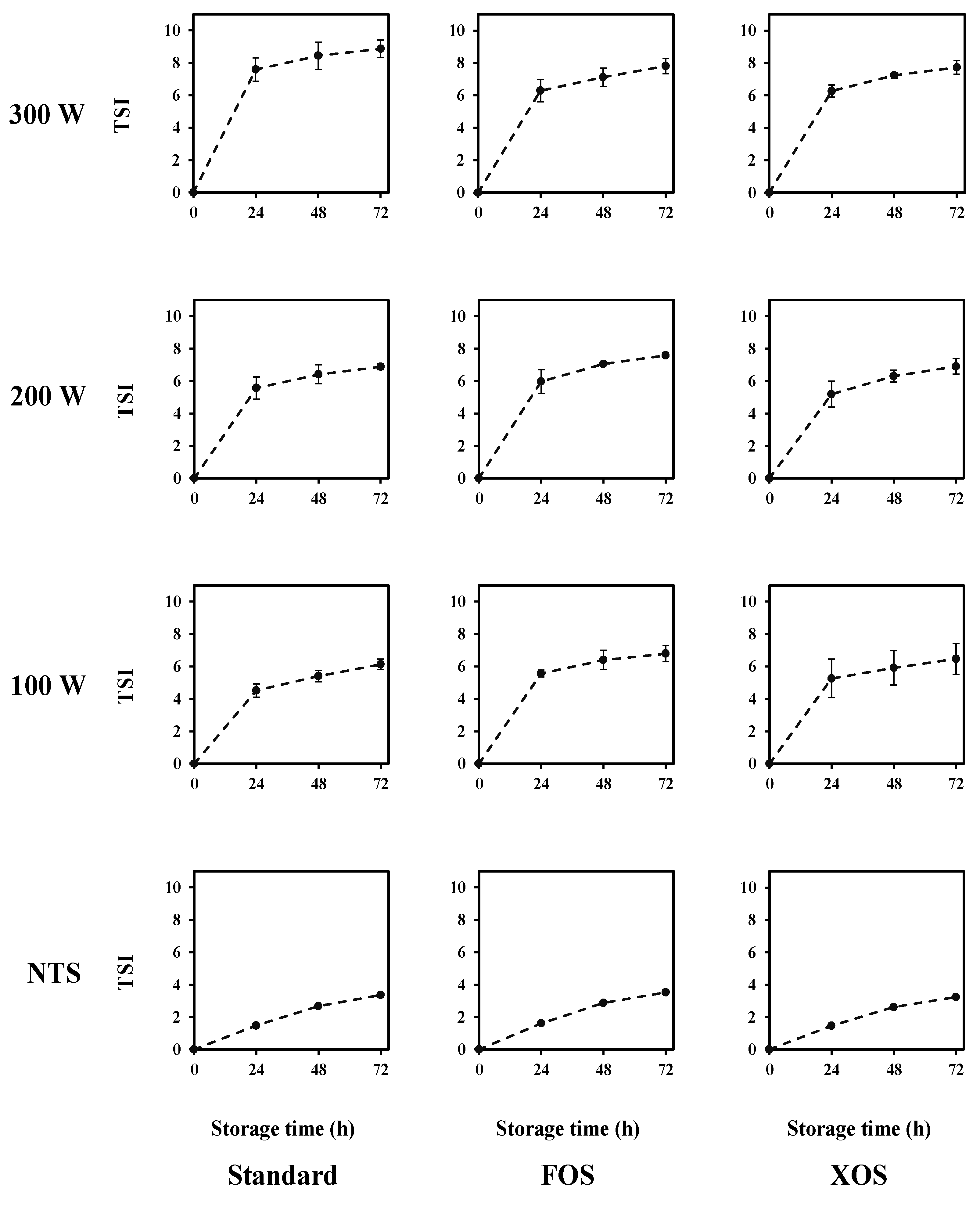
3.2.2. Kinetic Stability
3.2.3. Color
3.3. Chemical and Functional Properties
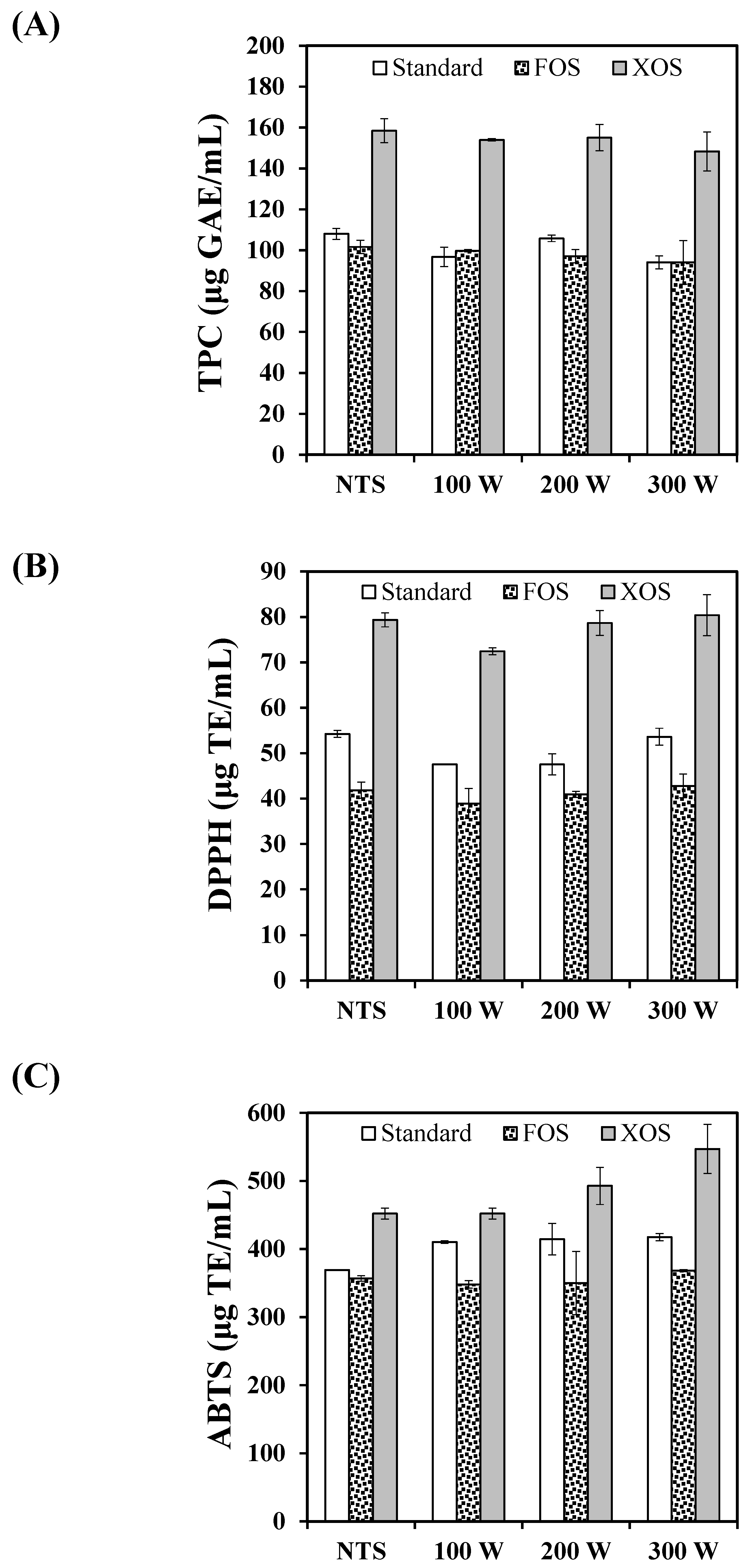
3.3.1. Phenolic Compounds
3.3.2. In Vitro Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Vijay Kumar, H.; Jagan Mohan Rao, L. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008, 41, 124–129. [Google Scholar] [CrossRef]
- Mao, Y.-L.; Wang, J.-Q.; Chen, G.-S.; Granato, D.; Zhang, L.; Fu, Y.-Q.; Gao, Y.; Yin, J.-F.; Luo, L.-X.; Xu, Y.-Q. Effect of chemical composition of black tea infusion on the color of milky tea. Food Res. Int. 2021, 139, 109945. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J. The effect of extrinsic cues on consumer perception: A study using milk tea products. Food Qual. Prefer. 2019, 71, 343–353. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- González-Martínez, C.; Becerra, M.; Cháfer, M.; Albors, A.; Carot, J.M.; Chiralt, A. Influence of substituting milk powder for whey powder on yoghurt quality. Trends Food Sci. Technol. 2002, 13, 334–340. [Google Scholar] [CrossRef]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Silva, M.; Anh Bui, T.H.; Dharmadana, D.; Zisu, B.; Chandrapala, J. Ultrasound-assisted formation of double emulsions stabilized by casein-whey protein mixtures. Food Hydrocoll. 2020, 109, 106143. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Ye, A. Gastric colloidal behaviour of milk protein as a tool for manipulating nutrient digestion in dairy products and protein emulsions. Food Hydrocoll. 2021, 115, 106599. [Google Scholar] [CrossRef]
- Chen, F.; Guo, Y.; Kang, J.; Yang, X.; Zhao, Z.; Liu, S.; Ma, Y.; Gao, W.; Luo, D. Insight into the essential oil isolation from Foeniculum vulgare Mill. fruits using double-condensed microwave-assisted hydrodistillation and evaluation of its antioxidant, antifungal and cytotoxic activity. Ind. Crops Prod. 2020, 144, 112052. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Moura, L.S.; Carvalho Jr, R.N.; Stefanini, M.B.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from fennel (Foeniculum vulgare): Global yield, composition and kinetic data. J. Supercrit. Fluids 2005, 35, 212–219. [Google Scholar] [CrossRef]
- Maitusong, J.; Aimila, A.; Zhang, J.; Mahinur, B.; Maiwulanjiang, M.; Aisa, H.A. Process optimization for the supercritical carbon dioxide extraction of Foeniculum vulgare Mill. seeds aromatic extract with respect to yield and trans-anethole contents using Box-Behnken design. Flavour Fragr. J. 2021, 36, 280–291. [Google Scholar] [CrossRef]
- Ben Abdesslem, S.; Ben Moussa, O.; Boulares, M.; Elbaz, M.; Chouaibi, M.; Ayachi, S.; Hassouna, M. Evaluation of the effect of fennel (Foeniculum vulgare Mill) essential oil addition on the quality parameters and shelf-life prediction of yoghurt. Int. J. Dairy Technol. 2020, 73, 403–410. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Meireles, M.A.A.; Pastore, G.M. Inulin thermal stability in prebiotic carbohydrate-enriched araticum whey beverage. LWT 2020, 128, 109418. [Google Scholar] [CrossRef]
- Tesfaye, W.; Suarez-Lepe, J.A.; Loira, I.; Palomero, F.; Morata, A. Dairy and Nondairy-Based Beverages as a Vehicle for Probiotics, Prebiotics, and Symbiotics: Alternatives to Health Versus Disease Binomial Approach Through Food. In Milk-Based Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 473–520. [Google Scholar] [CrossRef]
- Licht, T.R.; Ebersbach, T.; Frøkiær, H. Prebiotics for prevention of gut infections. Trends Food Sci. Technol. 2012, 23, 70–82. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Costa, A.L.R.; Cunha, R.L.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Manufacturing a prebiotic whey beverage exploring the influence of degree of inulin polymerization. Food Hydrocoll. 2018, 77, 787–795. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Novel thermal and non-thermal processing of watermelon juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Awuah, G.B.; Ramaswamy, H.S.; Economides, A. Thermal processing and quality: Principles and overview. Chem. Eng. Processing Process Intensif. 2007, 46, 584–602. [Google Scholar] [CrossRef]
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Režek Jambrak, A.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Bursać Kovačević, D. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Aguilar, K.; Garvín, A.; Ibarz, A.; Augusto, P.E.D. Ascorbic acid stability in fruit juices during thermosonication. Ultrason. Sonochem. 2017, 37, 375–381. [Google Scholar] [CrossRef]
- Ribeiro, M.d.M.; Valdramidis, V.P.; Nunes, C.A.; de Souza, V.R. Synergistic effect of thermosonication to reduce enzymatic activity in coconut water. Innov. Food Sci. Emerg. Technol. 2017, 41, 404–410. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Adeboyejo, F.O.; Okekunbi, T.A.; Aderibigbe, O.R. Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice. Ultrason. Sonochem. 2021, 70, 105316. [Google Scholar] [CrossRef]
- Owusu-Ansah, P.; Yu, X.; Osae, R.; Mustapha, A.T.; Zhang, R.; Zhou, C. Inactivation of Bacillus cereus from pork by thermal, non-thermal and single-frequency/multi-frequency thermosonication: Modelling and effects on physicochemical properties. LWT 2020, 133, 109939. [Google Scholar] [CrossRef]
- Tremarin, A.; Canbaz, E.A.; Brandão, T.R.S.; Silva, C.L.M. Modelling Alicyclobacillus acidoterrestris inactivation in apple juice using thermosonication treatments. LWT 2019, 102, 159–163. [Google Scholar] [CrossRef]
- Evelyn, E.; Silva, F.V.M. Thermosonication versus thermal processing of skim milk and beef slurry: Modeling the inactivation kinetics of psychrotrophic Bacillus cereus spores. Food Res. Int. 2015, 67, 67–74. [Google Scholar] [CrossRef]
- Parreiras, P.M.; Vieira Nogueira, J.A.; Rodrigues da Cunha, L.; Passos, M.C.; Gomes, N.R.; Breguez, G.S.; Falco, T.S.; Bearzoti, E.; Menezes, C.C. Effect of thermosonication on microorganisms, the antioxidant activity and the retinol level of human milk. Food Control 2020, 113, 107172. [Google Scholar] [CrossRef]
- Evelyn; Milani, E.; Silva, F.V.M. Comparing high pressure thermal processing and thermosonication with thermal processing for the inactivation of bacteria, moulds, and yeasts spores in foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar] [CrossRef]
- Erkaya, T.; Başlar, M.; Şengül, M.; Ertugay, M.F. Effect of thermosonication on physicochemical, microbiological and sensorial characteristics of ayran during storage. Ultrason. Sonochem. 2015, 23, 406–412. [Google Scholar] [CrossRef]
- Ragab, E.S.; Lu, J.; Pang, X.Y.; Nassar, K.S.; Yang, B.Y.; Zhang, S.W.; Lv, J.P. Effect of thermosonication process on physicochemical properties and microbial load of goat’s milk. J. Food Sci. Technol. 2019, 56, 5309–5316. [Google Scholar] [CrossRef]
- Strieder, M.M.; Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Low-frequency and high-power ultrasound-assisted production of natural blue colorant from the milk and unripe Genipa americana L. Ultrason. Sonochem. 2020, 66, 105068. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Saldaña, M.D.A. High-intensity ultrasound-assisted recovery of cinnamyl alcohol glycosides from Rhodiola rosea roots: Effect of probe diameter on the ultrasound energy performance for the extraction of bioactive compounds. Food Bioprod. Processing 2020, 122, 245–253. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, C.; Pu, Z.; Yu, H.; Li, D. Effect of low-frequency ultrasonic field at different power on the dynamics of a single bubble near a rigid wall. Ultrason. Sonochem. 2019, 58, 104704. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Zabot, G.L.; Cazarin, C.B.B.; Maróstica, M.R.; Meireles, M.A.A. Biopolymer-prebiotic carbohydrate blends and their effects on the retention of bioactive compounds and maintenance of antioxidant activity. Carbohydr. Polym. 2016, 144, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pachekrepapol, U.; Somboonchai, N.; Krimjai, W. Physicochemical, rheological, and microbiological properties of lactose-free functional yogurt supplemented with fructooligosaccharides. J. Food Process. Preserv. 2021, 45, e15017. [Google Scholar] [CrossRef]
- Jardines, A.P.; Arjona-Román, J.L.; Severiano-Pérez, P.; Totosaus-Sánchez, A.; Fiszman, S.; Escalona-Buendía, H.B. Agave fructans as fat and sugar replacers in ice cream: Sensory, thermal and texture properties. Food Hydrocoll. 2020, 108, 106032. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shin, W.-S. Functional improvements in bovine serum albumin–fucoidan conjugate through the Maillard reaction. Food Chem. 2016, 190, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying properties of soy whey protein isolate–fenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll. 2013, 30, 691–697. [Google Scholar] [CrossRef]
- Wang, W.-D.; Li, C.; Bin, Z.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef]
- Chevalier, R.C.; Gomes, A.; Cunha, R.L. Tailoring W/O emulsions for application as inner phase of W/O/W emulsions: Modulation of the aqueous phase composition. J. Food Eng. 2021, 297, 110482. [Google Scholar] [CrossRef]
- Poletto, P.; Pereira, G.N.; Monteiro, C.R.M.; Pereira, M.A.F.; Bordignon, S.E.; de Oliveira, D. Xylooligosaccharides: Transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem. 2020, 91, 352–363. [Google Scholar] [CrossRef]
- Biely, P.; Singh, S.; Puchart, V. Towards enzymatic breakdown of complex plant xylan structures: State of the art. Biotechnol. Adv. 2016, 34, 1260–1274. [Google Scholar] [CrossRef]
- Bannikova, A.; Zyainitdinov, D.; Evteev, A.; Drevko, Y.; Evdokimov, I. Microencapsulation of polyphenols and xylooligosaccharides from oat bran in whey protein-maltodextrin complex coacervates: In-vitro evaluation and controlled release. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100236. [Google Scholar] [CrossRef]
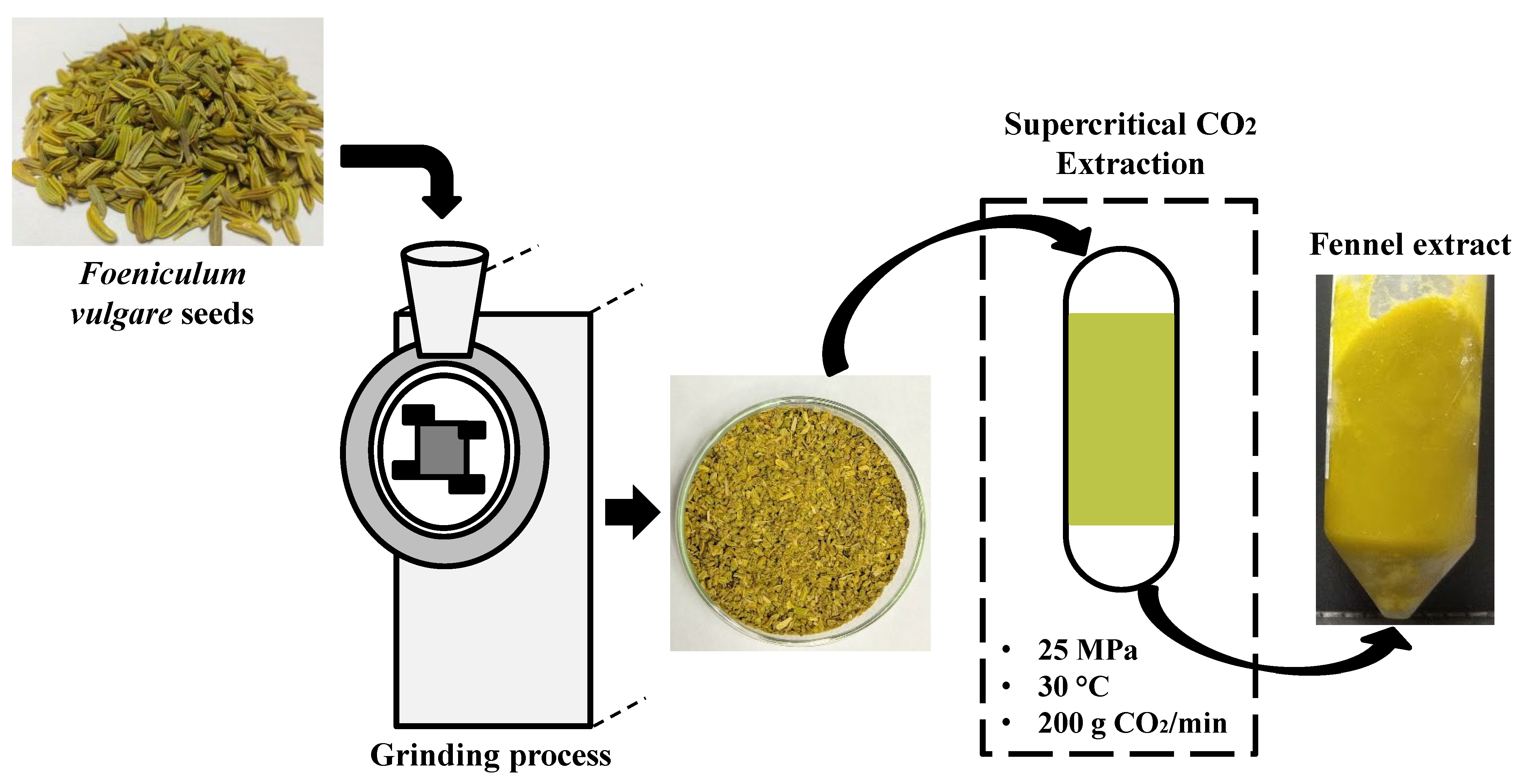
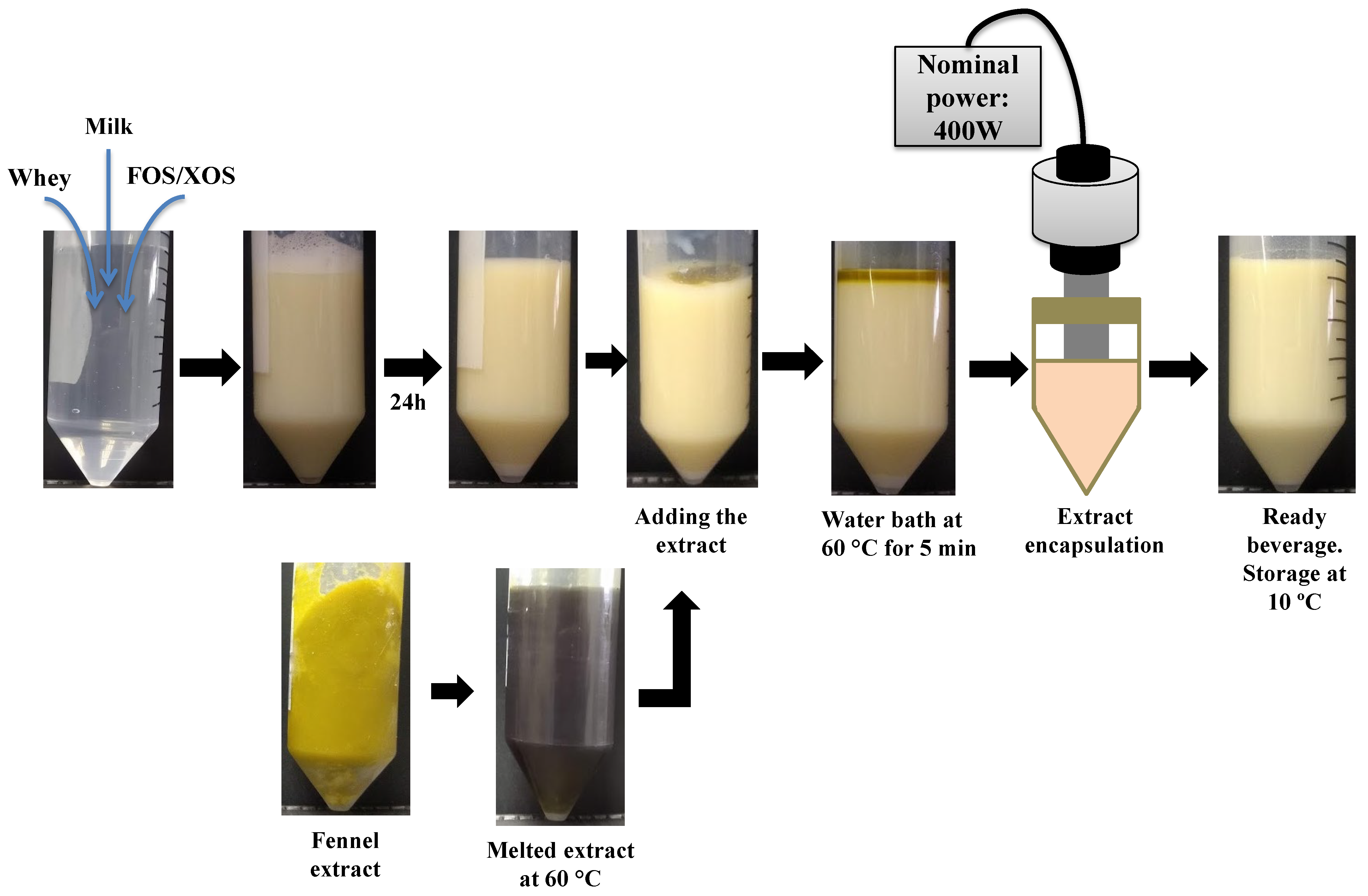

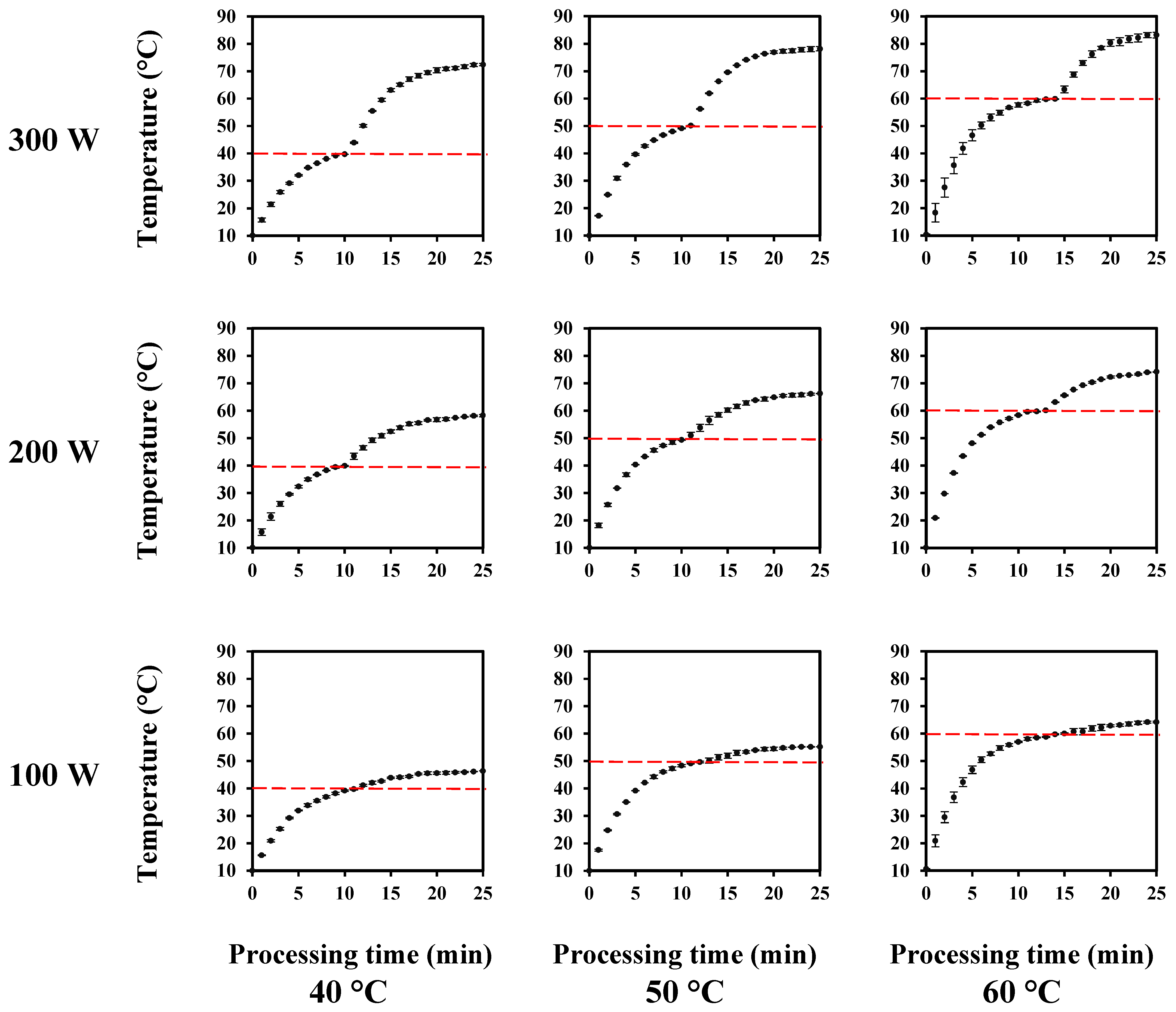
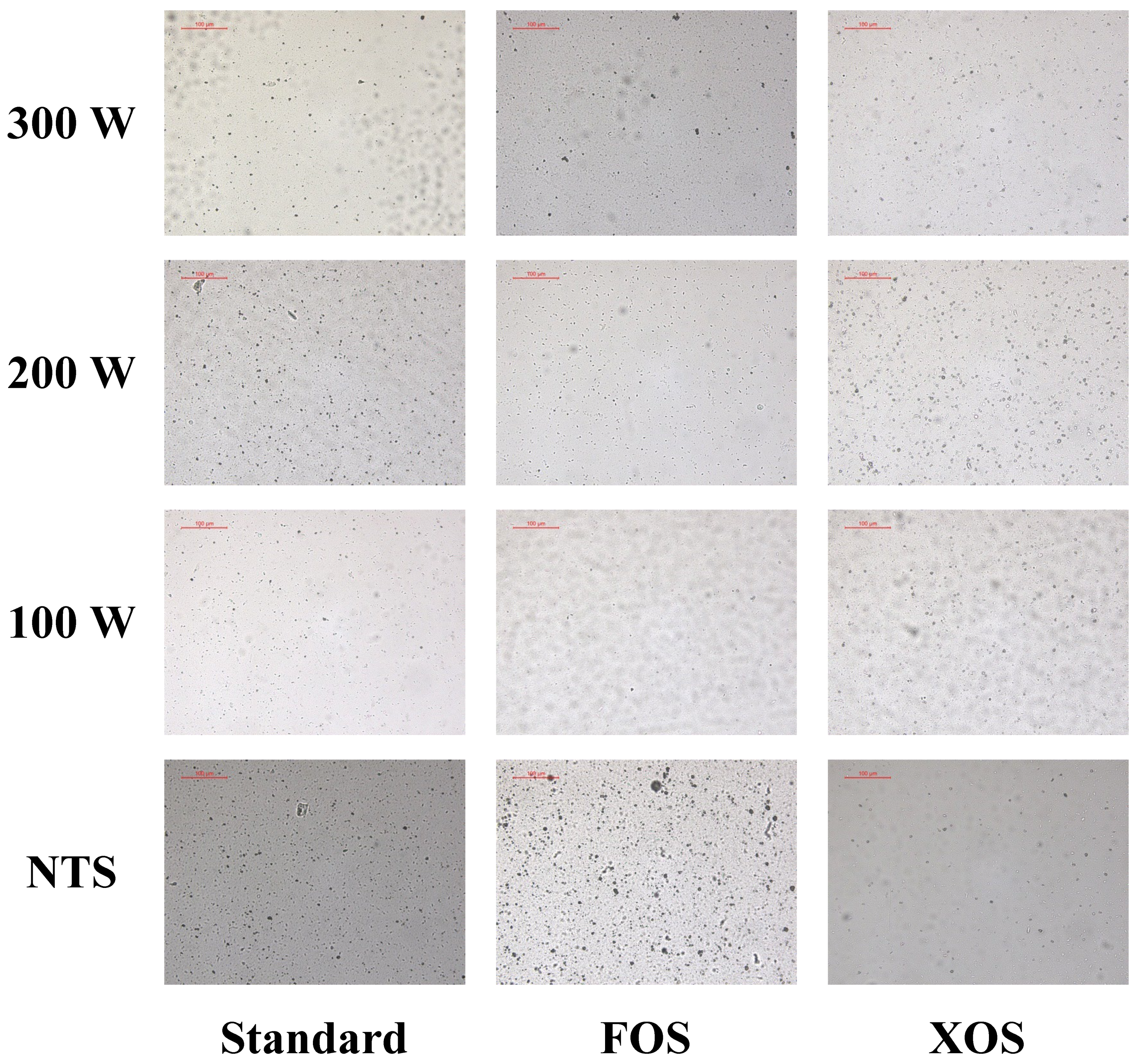


| Whey Beverage | Fennel Extract (g/100 g) | Whey Powder (g/100 g) | Skimmed Milk Powder (g/100 g) | FOS (g/100 g) | XOS (g/100 g) | Deionized Water (g/100 g) |
|---|---|---|---|---|---|---|
| Standard | 1 | 10 | 5 | - | - | 84 |
| FOS | 1 | 10 | 5 | 3 | - | 81 |
| XOS | 1 | 10 | 5 | - | 3 | 81 |
| Ultrasonic Power | Whey Beverage | ||||
|---|---|---|---|---|---|
| NTS | Standard | 1.06 ± 0.04 | 0.477 ± 0.005 | 1.93 ± 0.3 | 10.1 ± 0.7 |
| FOS | 1.05 ± 0.04 | 0.48 ± 0.01 | 1.73 ± 0.2 | 8.00 ± 0.05 | |
| XOS | 1.02 ± 0.01 | 0.473 ± 0.003 | 1.73 ± 0.1 | 8.30 ± 0.1 | |
| 100 W | Standard | 1.02 ± 0.03 | 0.47 ± 0.01 | 1.51 ± 0.6 | 8.8 ± 0.2 |
| FOS | 0.97 ± 0.01 | 0.438 ± 0.003 | 1.82 ± 0.1 | 9.48 ± 0.03 | |
| XOS | 0.98 ± 0.02 | 0.44 ± 0.01 | 1.95 ± 0.1 | 8.6 ± 0.1 | |
| 200 W | Standard | 1.17 ± 0.04 | 0.49 ± 0.01 | 2.39 ± 0.1 | 12.3 ± 0.1 |
| FOS | 0.76 ± 0.06 | 0.41 ± 0.02 | 0.67 ± 0.1 | 6.4 ± 0.6 | |
| XOS | 0.81 ± 0.03 | 0.417 ± 0.002 | 0.72 ± 0.03 | 6.8 ± 0.5 | |
| 300 W | Standard | 1.17 ± 0.07 | 0.49 ± 0.01 | 2.60 ± 0.3 | 13 ±1 |
| FOS | 0.73 ± 0.03 | 0.40 ± 0.01 | 0.64 ± 0.02 | 6.2 ± 0.6 | |
| XOS | 0.72 ± 0.02 | 0.40 ± 0.01 | 0.64 ± 0.01 | 5.9 ± 0.2 |
| Ultrasonic Power | Whey Beverage | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NTS | Standard | 6.40 ± <0.01 | 63.8 ± 0.4 | −4.84 ± 0.04 | 14.5 ± 0.1 | 15.3 ± 0.1 | 108.5 ± 0.3 | 19.0 ± 0.4 | − |
| FOS | 6.37 ± 0.03 | 64.4 ± 0.9 | −4.92 ± 0.08 | 14.7 ± 0.5 | 15.5 ± 0.4 | 108.5 ± 0.8 | 19.2 ± 0.7 | − | |
| XOS | 6.40 ± 0.01 | 63.6 ± 0.7 | −4.89 ± 0.05 | 14.9 ± 0.2 | 15.7 ± 0.2 | 108.158 ± 0.003 | 19.8 ± 0.5 | − | |
| 100 W | Standard | 6.33 ± 0.03 | 63.7 ± 0.9 | −4.9 ± 0.1 | 14.7 ± 0.2 | 15.5 ± 0.2 | 108.3 ± 0.2 | 19.5 ± 0.6 | 0.7 ± 0.2 |
| FOS | 6.3 ± 0.1 | 63.2 ± 0.2 | −4.83 ± 0.04 | 14.71 ± 0.07 | 15.48 ± 0.05 | 108.2 ± 0.2 | 19.7 ± 0.1 | 1.3 ± 0.2 | |
| XOS | 6.39 ± 0.02 | 63 ± 1 | −4.71 ± 0.04 | 14.9 ± 0.1 | 15.6 ± 0.1 | 107.53 ± <0.01 | 20.4 ± 0.1 | 1.1 ± 0.8 | |
| 200 W | Standard | 6.33 ± 0.05 | 63.2 ± 0.4 | −4.74 ± 0.06 | 15.1 ± 0.3 | 15.8 ± 0.2 | 107.5 ± 0.5 | 20.5 ± 0.7 | 0.8 ± 0.4 |
| FOS | 6.36 ± <0.01 | 63.2 ± 0.8 | −4.7 ± 0.1 | 15.2 ± 0.3 | 15.9 ± 0.2 | 107.2 ± 0.7 | 20.7 ± 0.4 | 1.5 ± 0.4 | |
| XOS | 6.3 ± 0.2 | 62.56 ± 0.02 | −4.5 ± 0.1 | 15.6 ± 0.2 | 16.2 ± 0.2 | 106.2 ± 0.2 | 22.0 ± 0.2 | 1.74 ± 0.05 | |
| 300 W | Standard | 6.32 ± 0.06 | 63.93 ± 0.04 | −4.50 ± 0.08 | 15.5 ± 0.1 | 16.2 ± 0.1 | 106.1 ± 0.4 | 21.4 ± 0.4 | 1.1 ± 0.2 |
| FOS | 6.32 ± 0.01 | 63.3 ± 0.7 | −4.4 ± 0.2 | 15.9 ± 0.2 | 16.5 ± 0.2 | 105.6 ± 0.4 | 22.4 ± 0.5 | 1.8 ± 0.5 | |
| XOS | 6.3 ± 0.1 | 61.8 ± 0.6 | −4.17 ± 0.04 | 15.5 ± 0.4 | 16.1 ± 0.4 | 105.1 ± 0.2 | 22.7 ± 0.5 | 2.1 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urango, A.C.M.; Neves, M.I.L.; Meireles, M.A.A.; Silva, E.K. Whey Beverage Emulsified System as Carrying Matrix of Fennel Seed Extract Obtained by Supercritical CO2 Extraction: Impact of Thermosonication Processing and Addition of Prebiotic Fibers. Foods 2022, 11, 1332. https://doi.org/10.3390/foods11091332
Urango ACM, Neves MIL, Meireles MAA, Silva EK. Whey Beverage Emulsified System as Carrying Matrix of Fennel Seed Extract Obtained by Supercritical CO2 Extraction: Impact of Thermosonication Processing and Addition of Prebiotic Fibers. Foods. 2022; 11(9):1332. https://doi.org/10.3390/foods11091332
Chicago/Turabian StyleUrango, Adela Cristina Martinez, Maria Isabel Landim Neves, Maria Angela A. Meireles, and Eric Keven Silva. 2022. "Whey Beverage Emulsified System as Carrying Matrix of Fennel Seed Extract Obtained by Supercritical CO2 Extraction: Impact of Thermosonication Processing and Addition of Prebiotic Fibers" Foods 11, no. 9: 1332. https://doi.org/10.3390/foods11091332
APA StyleUrango, A. C. M., Neves, M. I. L., Meireles, M. A. A., & Silva, E. K. (2022). Whey Beverage Emulsified System as Carrying Matrix of Fennel Seed Extract Obtained by Supercritical CO2 Extraction: Impact of Thermosonication Processing and Addition of Prebiotic Fibers. Foods, 11(9), 1332. https://doi.org/10.3390/foods11091332









