Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Moisture, Total Intramuscular Fat, Fatty Acid Profile and Lipid Oxidation
2.3. Evaluation of Volatile Profile of Cooked Meat
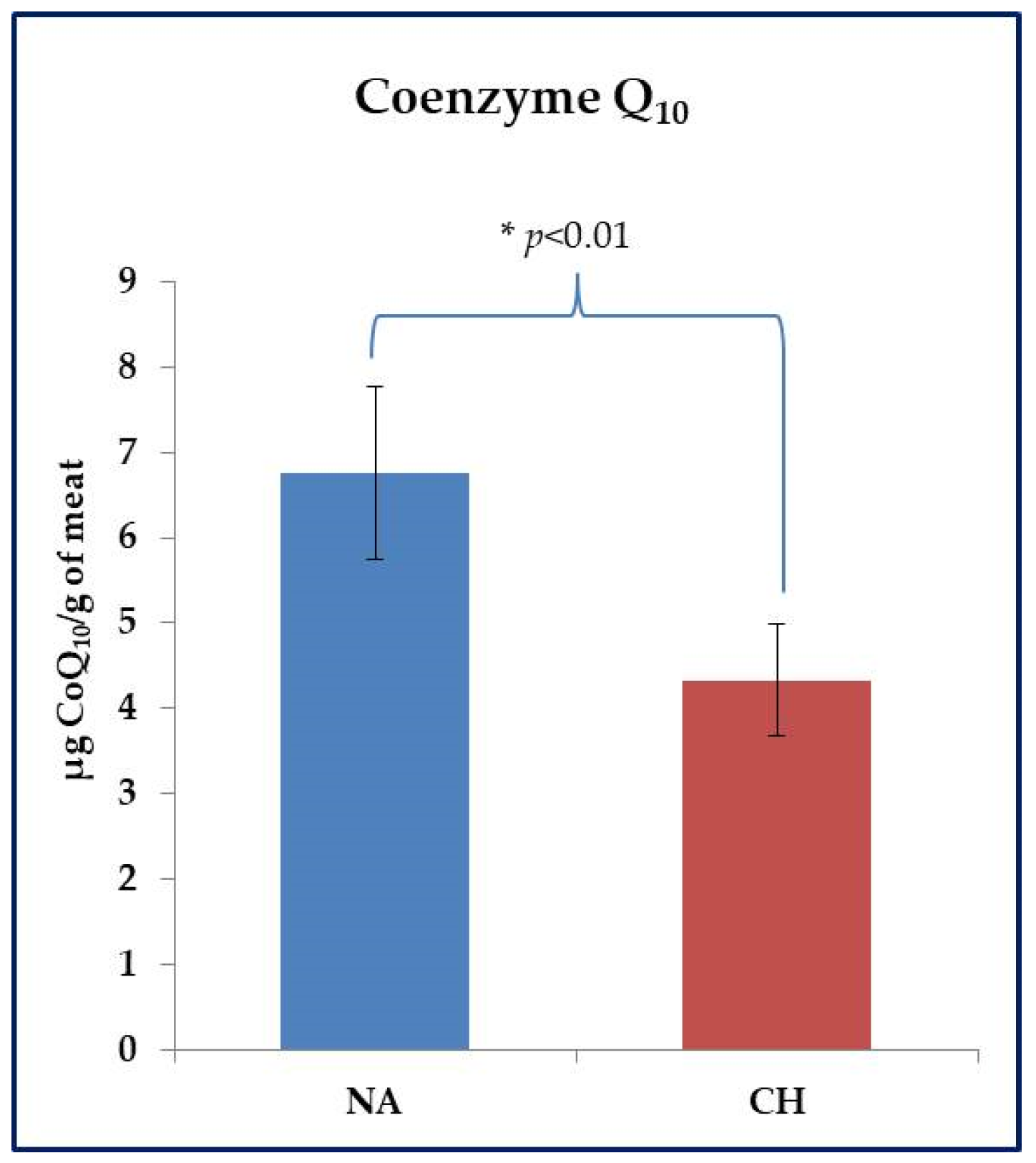
2.4. Determination of Coenzyme Q10
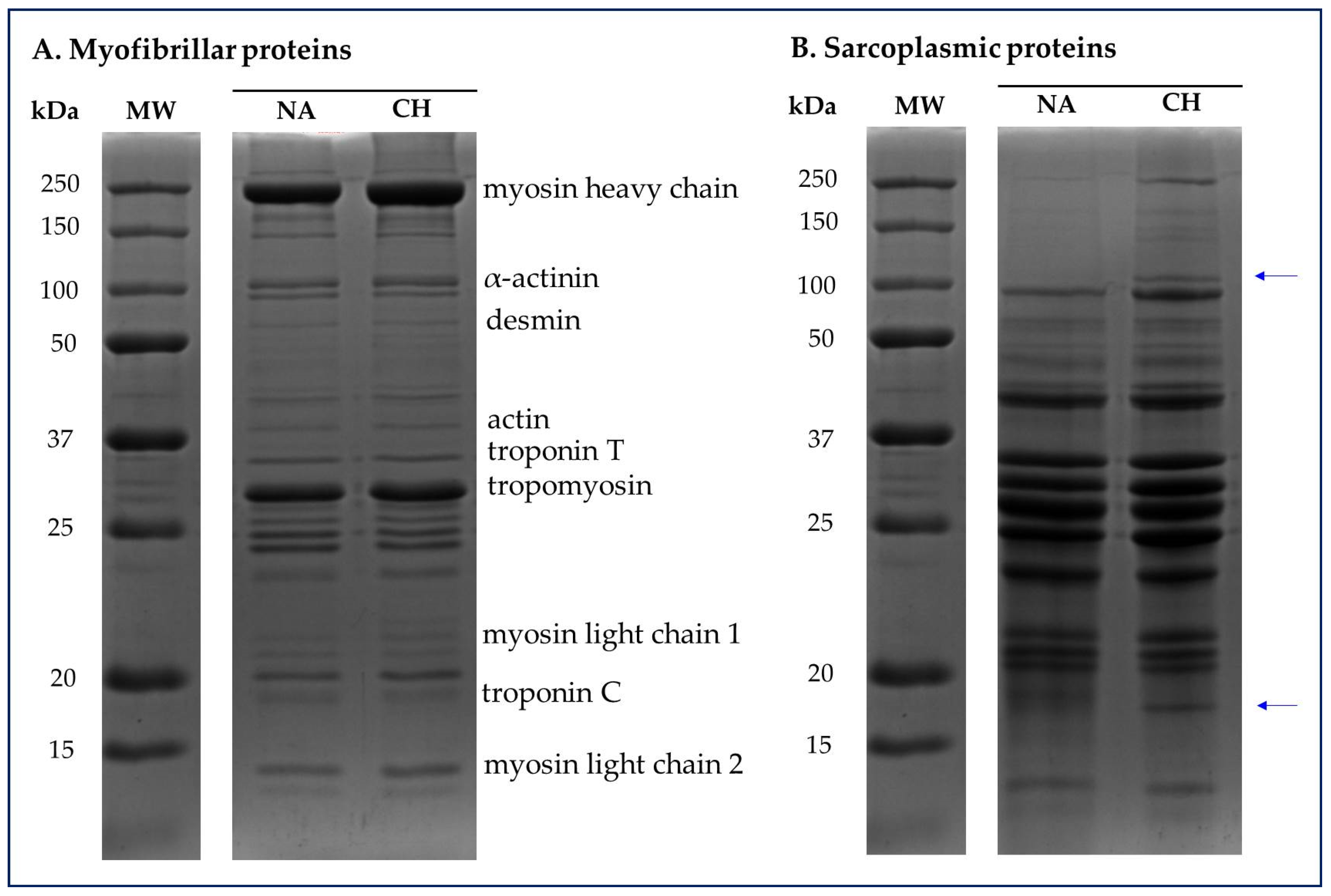
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Sarcoplasmic and Myofibrillar Proteins
2.6. Statistical Analysis
3. Results
3.1. Total Lipids, Fatty Acids Profile and Lipid Oxidative Stability
3.2. Identification of Volatile Compounds (VOC) in Cooked Meat
3.3. Dosage of Coenzyme Q10 in Muscle Tissue
3.4. Characterization of Electrophoretic Profile of Sarcoplasmic and Myofibrillar Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, S.J.; Bradley, D.G. Conserving livestock breed biodiversity. Trends Ecol. Evol. 1995, 10, 267–270. [Google Scholar] [CrossRef]
- Groenen, M.A.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Gaillard, C.L.; Park, C.; Milan, D.; Megens, H.J.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Galal, S. Biodiversity in goats. Small Rumin. Res. 2005, 60, 75–81. [Google Scholar] [CrossRef]
- Scintu, M.F.; Piredda, G. Typicity and biodiversity of goat and sheep milk products. Small Rumin. Res. 2007, 68, 221–231. [Google Scholar] [CrossRef]
- Ianni, A.; Bartolini, D.; Bennato, F.; Martino, G. Egg Quality from Nera Atriana, a Local Poultry Breed of the Abruzzo Region (Italy), and ISA Brown Hens Reared under Free Range Conditions. Animals 2021, 11, 257. [Google Scholar] [CrossRef]
- Lassaletta, L.; Estellés, F.; Beusen, A.H.; Bouwman, L.; Calvet, S.; Van Grinsven, H.J.; Doelman, J.C.; Stehfest, E.; Uwizeye, A.; Westhoek, H. Future global pig production systems according to the Shared Socioeconomic Pathways. Sci Total Environ. 2019, 665, 739–751. [Google Scholar] [CrossRef]
- OECD. Meat Consumption. 2021. Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 26 November 2021).
- Ferronato, G.; Corrado, S.; De Laurentiis, V.; Sala, S. The Italian meat production and consumption system assessed combining material flow analysis and life cycle assessment. J. Clean. Prod. 2021, 321, 128705. [Google Scholar] [CrossRef]
- Lebret, B.; Čandek-Potokar, M. Pork quality attributes from farm to fork. Part I. Carcass and fresh meat. Animal 2021, 16, 100402. [Google Scholar] [CrossRef]
- Davoli, R.; Zambonelli, P.; San-Cristobal, M.; Scotti, E.; Fontanesi, L.; Colombo, M.; Dall’Olio, S.; Braglia, S.; Russo, V. SNPs and microsatellite markers for genetic diversity study in Italian pig breeds. In Proceedings of the 6th International Symposium on the Mediterranean Pig, Capo d’Orlando, Italy, 11–13 October 2007; Nanni Costa, L., Zambonelli, P., Russo, V., Eds.; Università di Bologna: Bologna, Italy, 2007; pp. 46–53. [Google Scholar]
- Madonia, G.; Diaferia, C.; Moretti, V.M.; Margiotta, S.; Manganelli, E.; Pruiti, V.; Caprino, F.; D’Amico, A. Nero Siciliano pigs proposed as a traditional quality product: Comparison between salami made from black pig’s meat and white pig’s meat. Option Mediterr. 2007, 76, 251–257. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis; Williams, S., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984. [Google Scholar]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Ianni, A.; Di Luca, A.; Martino, C.; Bennato, F.; Marone, E.; Grotta, L.; Cichelli, A.; Martino, G. Dietary supplementation of dried grape pomace increases the amount of linoleic acid in beef, reduces the lipid oxidation and modifies the volatile profile. Animals 2019, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of Friesian cows: Effect on chemical-nutritional composition and aromatic profile of dairy products. J. Dairy Sci. 2019, 102, 2918–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ianni, A.; Martino, C.; Pomilio, F.; Di Luca, A.; Martino, G. Dietary selenium intake in lactating dairy cows modifies fatty acid composition and volatile profile of milk and 30-day-ripened caciotta cheese. Eur. Food Res. Technol. 2019, 245, 2113–2121. [Google Scholar] [CrossRef]
- Martino, G.; Mugnai, C.; Compagnone, D.; Grotta, L.; Del Carlo, M.; Sarti, F. Comparison of performance, meat lipids and oxidative status of pigs from commercial breed and organic crossbreed. Animals 2014, 4, 348–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- He, F. Bradford protein assay. Bio-Protocol 2011, 6, e45. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; López, R.C. Physico-chemical characteristics of M. Longissimus dorsi from three lines of free-range reared Iberian pigs slaughtered at 90 kg live-weight and commercial pigs: A comparative study. Meat Sci. 2003, 64, 499–506. [Google Scholar] [CrossRef]
- Cava, R.; Estévez, M.; Ruiz, J.; Morcuende, D. Physicochemical characteristics of three muscles from free-range reared Iberian pigs slaughtered at 90 kg live weight. Meat Sci. 2003, 63, 533–541. [Google Scholar] [CrossRef]
- Serra, X.; Gil, F.; Pérez-Enciso, M.; Oliver, M.A.; Vázquez, J.M.; Gispert, M.; Diaz, I.; Moreno, F.; Latorre, R.; Noguera, J.L. A comparison of carcass, meat quality and histochemical characteristics of Iberian (Guadyerbas line) and Landrace pigs. Livest Prod. Sci. 1998, 56, 215–223. [Google Scholar] [CrossRef]
- Tejeda, J.F.; Gandemer, G.; Antequera, T.; Viau, M.; García, C. Lipid traits of muscles as related to genotype and fattening diet in Iberian pigs: Total intramuscular lipids and triacylglicerols. Meat Sci. 2002, 60, 357–363. [Google Scholar] [CrossRef]
- Pugliese, C.; Madonia, G.; Chiofalo, V.; Margiotta, S.; Acciaioli, A.; Gandini, G. Comparison of the performances of Nero Siciliano pigs reared indoors and outdoors. 1. Growth and carcass composition. Meat Sci. 2003, 65, 825–831. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Ledward, D.A. Lawrie’s Meat Science; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Pérez, J.F.; Baucells, M.D.; Mourot, J.; Gasa, J. Comparative digestibility and lipogenic activity in Landrace and Iberian finishing pigs fed ad libitum corn- and corn–sorghum–acorn-based diets. Livest. Prod. Sci. 2002, 77, 195–205. [Google Scholar] [CrossRef]
- Green, C.D.; Ozguden-Akkoc, C.G.; Wang, Y.; Jump, D.B.; Olson, L.K. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species [S]. J. Lipid Res. 2010, 51, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Innosa, D.; Grotta, L.; D’Onofrio, A.; Martino, G. Chemical-nutritional characteristics and aromatic profile of milk and related dairy products obtained from goats fed with extruded linseed. Asian-Austral. J. Anim. Sci. 2020, 33, 148. [Google Scholar] [CrossRef] [Green Version]
- Innosa, D.; Ianni, A.; Faccia, M.; Martino, C.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Martino, G. Physical, nutritional, and sensory properties of cheese obtained from goats fed a dietary supplementation with olive leaves. Animals 2020, 10, 2238. [Google Scholar] [CrossRef]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta Mol. Basis Dis. 1995, 1271, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Forsmark-Andrée, P.; Lee, C.P.; Dallner, G.; Ernster, L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic. Biol. Med. 1997, 22, 391–400. [Google Scholar] [CrossRef]
- Nevrkla, P.; Kapelański, W.; Václavková, E.; Hadaš, Z.; Cebulska, A.; Horký, P. Meat quality and fatty acid profile of pork and backfat from an indigenous breed and a commercial hybrid of pigs. Ann. Anim. Sci. 2017, 17, 1215. [Google Scholar] [CrossRef] [Green Version]
- Tejerina, D.; García-Torres, S.; De Vaca, M.C.; Vázquez, F.M.; Cava, R. Effect of production system on physical–chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pig. Food Chem. 2021, 133, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Martın, L.; Timón, M.L.; Petrón, M.J.; Ventanas, J.; Antequera, T. Evolution of volatile aldehydes in Iberian ham matured under different processing conditions. Meat Sci. 2000, 54, 333–337. [Google Scholar] [CrossRef]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT Food Sci. Technol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Pegg, R.B. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Xiong, Y.L. Myofibrillar protein from different muscle fiber types: Implications of biochemical and functional properties in meat processing. Crit. Rev. Food Sci. Nutr. 1994, 3, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Sayd, T.; Morzel, M.; Chambon, C.; Franck, M.; Figwer, P.; Larzul, C.; Le Roy, P.; Monin, G.; Chérel, P.; Laville, E. Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: Implications on meat color development. J. Agric. Food Chem. 2006, 54, 2732–2737. [Google Scholar] [CrossRef]
- Picariello, G.; De Martino, A.; Mamone, G.; Ferranti, P.; Addeo, F.; Faccia, M.; Musso, S.S.; Di Luccia, A. Proteomic study of muscle sarcoplasmic proteins using AUT-PAGE/SDS-PAGE as two-dimensional gel electrophoresis. J. Chromatogr. B 2006, 833, 101–108. [Google Scholar] [CrossRef]


| Fatty Acids 1 | NA | CH | p-Value |
|---|---|---|---|
| C14:0 | 1.59 ± 0.18 | 1.77 ± 0.22 | ns |
| C16:0 | 28.81 ± 1.75 | 27.32 ± 1.41 | * |
| C18:0 | 12.31 ± 0.77 | 13.78 ± 0.81 | * |
| SFA | 42.71 ± 3.55 | 42.87 ± 3.27 | ns |
| C14:1 | 0.09 ± 0.02 | 0.06 ± 0.01 | ns |
| C16:1 | 2.87 ± 0.33 | 2.91 ± 0.41 | ns |
| C18:1 cis9 | 40.3 ± 2.91 | 39.56 ± 3.08 | ns |
| C18:1 cis11 | 1.59 ± 0.18 | 1.70 ± 0.20 | ns |
| MUFA | 44.85 ± 3.64 | 44.23 ± 3.76 | ns |
| C18:2 cis9, 12 | 9.02 ± 0.88 | 8.86 ± 0.91 | ns |
| C18:3 cis9, 12, 15 | 0.92 ± 0.11 | 0.75 ± 0.09 | ** |
| PUFA | 9.94 ± 0.82 | 9.61 ± 0.93 | ns |
| Others 2 | 2.50 ± 0.24 | 3.29 ± 0.29 | * |
| AI | 0.64 ± 0.07 | 0.64 ± 0.08 | ns |
| TI | 1.43 ± 0.12 | 1.48 ± 0.11 | ns |
| VOC 1 | NA | CH | p |
|---|---|---|---|
| Aldehydes | |||
| butanal 3-methyl | 0.92 ± 0.11 | 0.81 ± 0.28 | ns |
| butanal 2-methyl | 0.09 ± 0.04 | 0.11 ± 0.02 | ns |
| pentanal | 2.68 ± 0.79 | 2.39 ± 0.44 | ns |
| hexanal | 72.27 ± 1.97 | 75.06 ± 2.21 | * |
| heptanal | 2.46 ± 0.22 | 3.62 ± 0.41 | ** |
| 2-heptanal | 1.04 ± 0.23 | 0.92 ± 0.11 | ns |
| 2-nonenal | 0.21 ± 0.04 | 0.13 ± 0.03 | ** |
| 2-decenal | 0.55 ± 0.08 | 0.37 ± 0.06 | ns |
| octanal | 2.31 ± 0.38 | 1.61 ± 0.23 | ns |
| nonanal | 2.57 ± 0.16 | 2.31 ± 0.25 | ns |
| Ketones | |||
| 3-hydroxy-2-butanone | 2.10 ± 0.20 | 2.00 ± 0.19 | ns |
| Alcohols | |||
| 1-butanol 3-methyl | 0.65 ± 0.08 | 0.54 ± 0.07 | ns |
| 1-pentanol | 0.61 ± 0.09 | 0.54 ± 0.06 | ns |
| 1-hexanol | 2.98 ± 0.21 | 2.65 ± 0.31 | ns |
| 1-octyn-3-ol | 0.73 ± 0.11 | 0.57 ± 0.08 | ns |
| 2-octen-1-ol | 1.62 ± 0.22 | 1.47 ± 0.21 | ns |
| 1-octanol-2-butyl | 6.21 ± 1.42 | 4.90 ± 1.63 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianni, A.; Bennato, F.; Martino, C.; Odoardi, M.; Sacchetti, A.; Martino, G. Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo. Foods 2022, 11, 1297. https://doi.org/10.3390/foods11091297
Ianni A, Bennato F, Martino C, Odoardi M, Sacchetti A, Martino G. Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo. Foods. 2022; 11(9):1297. https://doi.org/10.3390/foods11091297
Chicago/Turabian StyleIanni, Andrea, Francesca Bennato, Camillo Martino, Maurizio Odoardi, Agostino Sacchetti, and Giuseppe Martino. 2022. "Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo" Foods 11, no. 9: 1297. https://doi.org/10.3390/foods11091297
APA StyleIanni, A., Bennato, F., Martino, C., Odoardi, M., Sacchetti, A., & Martino, G. (2022). Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo. Foods, 11(9), 1297. https://doi.org/10.3390/foods11091297






