Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Primary Extraction of C-Phycocyanin
2.2.1. Pretreatment of Arthrospira platensis
2.2.2. Swelling Extraction Process
2.2.3. High-Pressure Cell Disruption
2.2.4. Freezing and Thawing
2.2.5. Ultrasonication with Freezing and Thawing
2.3. Purification of CP
2.4. Color Measurement
2.5. UV-Vis Spectra
2.6. Fluorescence Spectra
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Thermogravimetric Analysis (TGA)
2.9. ζ Potential and Particle Size
2.10. Protein Solubility
2.11. Antioxidant Activity
2.11.1. DPPH Scavenging Activity
2.11.2. ABTS Scavenging Activity
2.11.3. Reducing Power
2.12. Statistical Analysis
3. Results and Discussion
3.1. Yield, Purity, and Color of CP
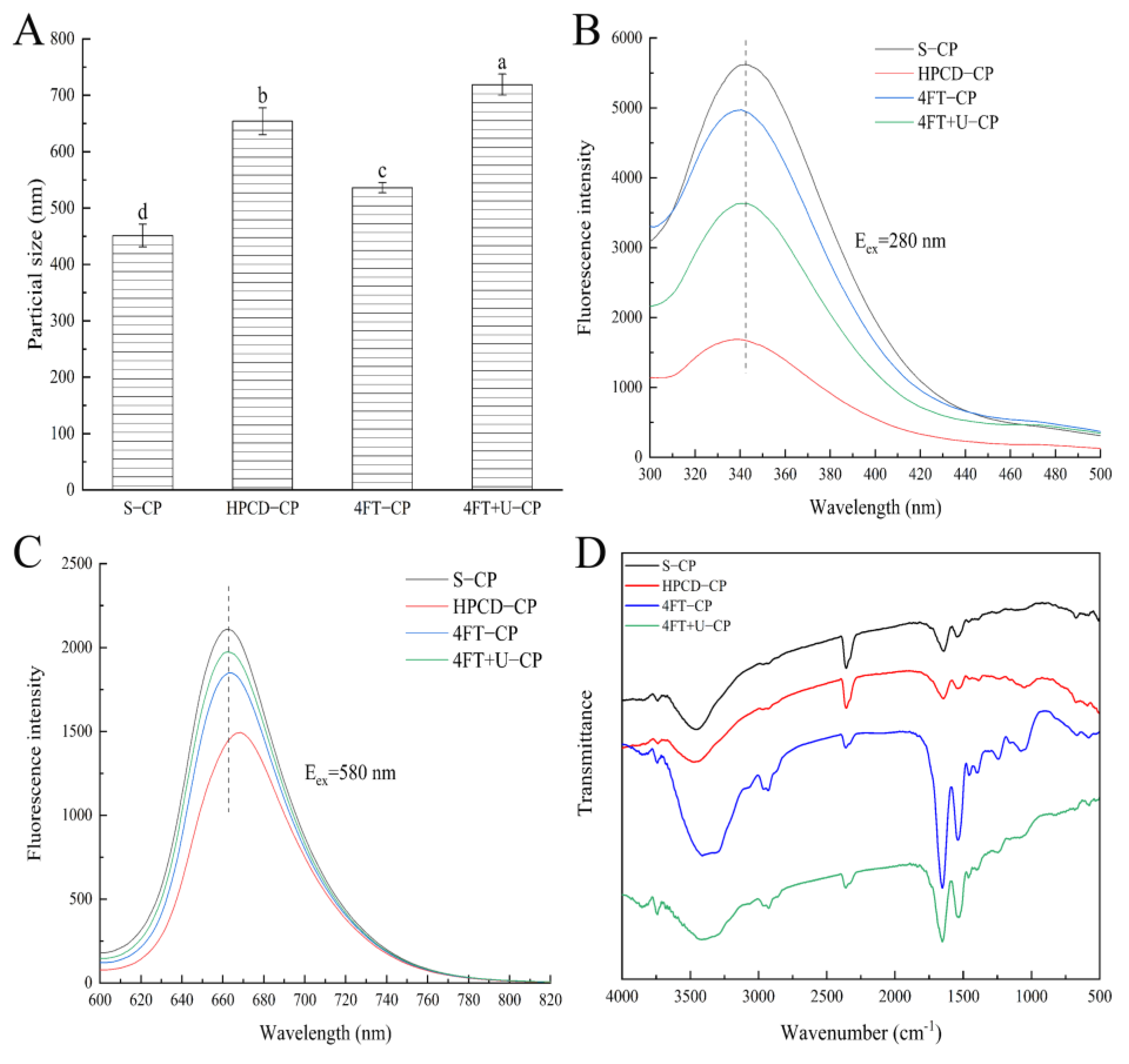
3.2. ζ Potential and Particle Size
3.3. Spectrophotometric of CP
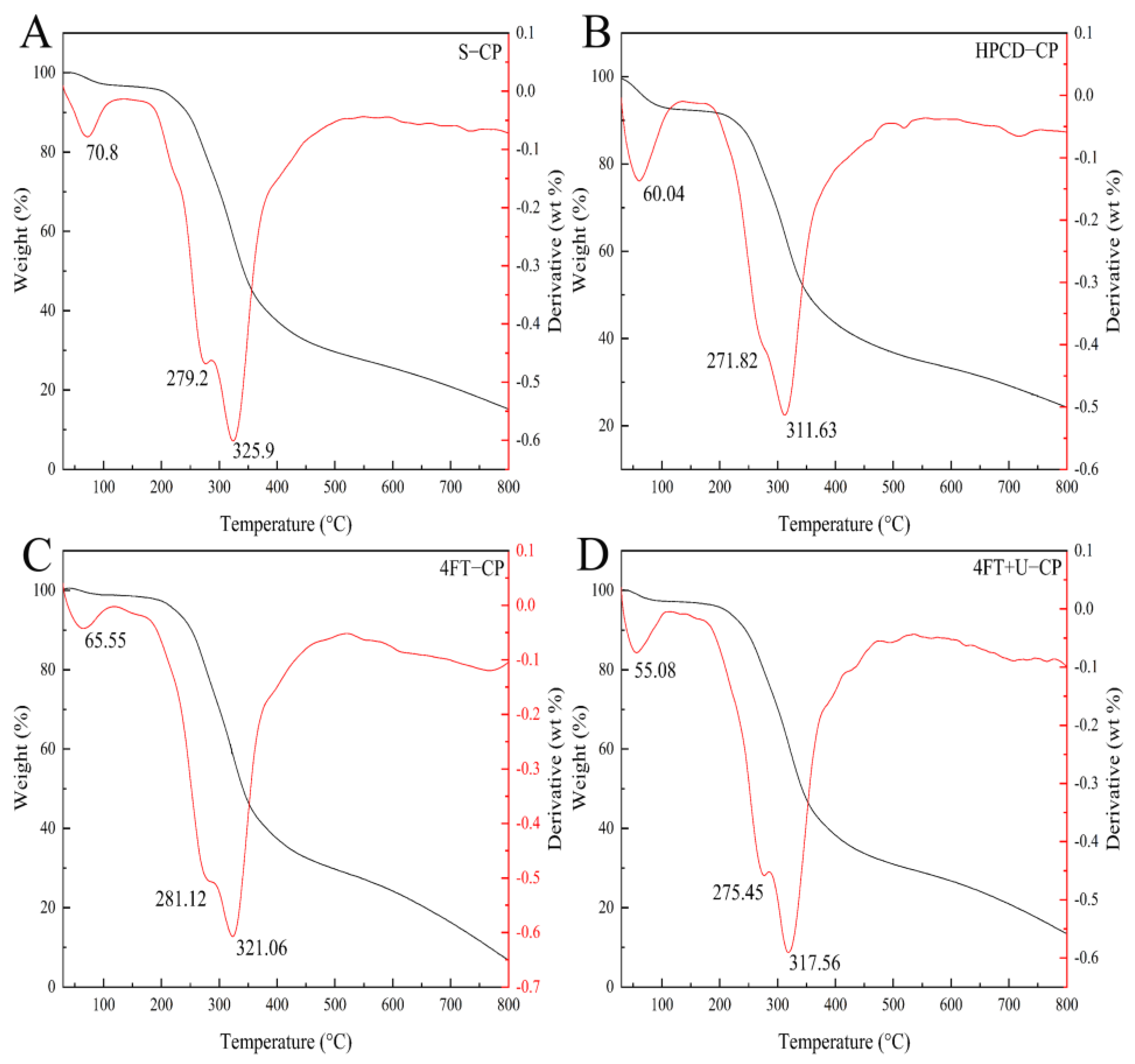
3.4. Thermogravimetric Analysis (TGA)
3.5. Solubility of CP
3.6. Antioxidant Activity of CPs
3.6.1. DPPH Radical Scavenging Activities
3.6.2. ABTS Radical Scavenging Activities
3.6.3. Reducing Power
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Qahtani, H.W.; Binobead, M.A. Anti-inflammatory, antioxidant and antihepatotoxic effects of Spirulina platensis against D-galactosamine induced hepatotoxicity in rats. Saudi J. Biol. Sci. 2019, 26, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, P.; Palanisamy, S.; Anjali, R.; Vinosha, M.; Elakkiya, M.; Marudhupandi, T.; Tabarsa, M.; You, S.G.; Prabhu, N.M. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int. J. Biol. Macromol. 2019, 141, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zainoddin, H.A.H.; Hamzah, A.; Jamari, Z.; Omar, W.A.W. Chemical profiles of methanolic extracts from two species of microalgae, Nannochloropsis sp. and Spirulina sp. Pertanika J. Trop. Agric. Sci. 2018, 41, 1085–1096. [Google Scholar]
- Bennett, A.; Bogor Ad, L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry 1971, 10, 3625. [Google Scholar] [CrossRef]
- Bermejo, R.; Talavera, E.M.; Alvarez-Pez, J.; Orte, J.C. Chromatographic purification of biliproteins from Spirulina platensis. High-performance liquid chromatographic separation of their α and β subunits. J. Chromatogr. A 1997, 778, 441–450. [Google Scholar]
- Moraes, C.C.; Mazutti, M.A.; Maugeri, F.; Kalil, S.J. Modeling of ion exchange expanded-bed chromatography for the purification of C-phycocyanin. J. Chromatogr. A 2013, 1281, 73–78. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Wang, Z.; Zhang, C.; Lu, S.; Liu, J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011, 126, 261–269. [Google Scholar] [CrossRef]
- Sintra, T.E.; Bagagem, S.S.; Ahsaie, F.G.; Fernandes, A.; Martins, M.; Macário, I.P.E.; Pereira, J.L.; Gonçalves, F.J.M.; Pazuki, G.; Coutinho, J.A.P.; et al. Sequential recovery of C-phycocyanin and chlorophylls from Anabaena cylindrica. Sep. Purif. Technol. 2021, 255, 117538. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, S.; Schievano, A.; Ida, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from Spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebiba, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal. Res. 2014, 3, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Vernes, L.; Abert-Vian, M.; el Maataoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of phycocyanin-A natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Chen, C.Y.; Jesisca, C.; Hsieh, D.J.; Lee, C.H.; Chang, J.S. Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour Technol. 2016, 200, 500–505. [Google Scholar]
- Pan-utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Drévillon, L.; Koubaa, M.; Vorobiev, E. Lipid extraction from Yarrowia lipolytica biomass using high-pressure homogenization. Biomass Bioenergy 2018, 115, 143–150. [Google Scholar] [CrossRef]
- Antecka, A.; Klepacz-Smółka, A.; Szeląg, R.; Pietrzyk, D.; Ledakowicz, S. Comparison of three methods for thermostable C-phycocyanin separation and purification. Chem. Eng. Processing-Process Intensif. 2022, 171, 108563. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Yan, Y.; Ramaswamy, H.S.; Sun, J.; Bai, W. The impact of ultrasonic treatment on blueberry wine anthocyanin color and its In-vitro anti-oxidant capacity. Food Chem. 2020, 333, 127455. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Ersus Bilek, S. Enzyme-assisted extraction of stabilized chlorophyll from spinach. Food Chem. 2015, 176, 152–157. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; Silva, J.; Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef]
- Bera, M.B.; Mukherjee, K.R. Solubility, emulsifying, and foaming properties of rice bran protein concentrates. J. Food Sci. 1989, 54, 142–145. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Nunez, L.; Amador, D. Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. CHAPTER 3-Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 59–94. [Google Scholar] [CrossRef]
- Tian, R.; Zhu, G.; Feng, J.; Tian, B.; Sui, X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020, 68, 105202. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules 2019, 24, 3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajid, A.M.; Al-Lohedan, H.A. Spectroscopic and computational evaluation on the binding of safranal with human serum albumin: Role of inner filter effect in fluorescence spectral correction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 434–442. [Google Scholar]
- Zhou, B.; Zhang, M.; Fang, Z.-x.; Liu, Y. Effects of ultrasound and microwave pretreatments on the ultrafiltration desalination of salted duck egg white protein. Food Bioprod. Processing 2015, 96, 306–313. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Barreto, P.; Pires, A.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.-S. A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Isolation, screening and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: A sustainable approach. Bioresour. Technol. 2019, 293, 121998. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Zhang, W.; Xu, W.; Hu, Z. Effects and mechanism of dilute acid soaking with ultrasound pretreatment on rice bran protein extraction. J. Cereal Sci. 2019, 87, 318–324. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Rayas-Duarte, P. The effect of mixing and wheat protein/gluten on the gelatinization of wheat starch. Food Chem. 2003, 81, 533–545. [Google Scholar] [CrossRef]
- Fuertes, S.; Laca, A.; Oulego, P.; Paredes, B.; Rendueles, M.; Díaz, M. Development and characterization of egg yolk and egg yolk fractions edible films. Food Hydrocoll. 2017, 70, 229–239. [Google Scholar] [CrossRef]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Cavonius, L.R.; Albers, E.; Undeland, I. pH-shift processing of Nannochloropsis oculata microalgal biomass to obtain a protein-enriched food or feed ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.M.; Lisboa, C.R.; Costa, J.A.V. High protein ingredients of microalgal origin: Obtainment and functional properties. Innov. Food Sci. Emerg. 2018, 47, 187–194. [Google Scholar] [CrossRef]
- Constantinides, A.; Adu-Amankwa, B. Enzymatic modification of vegetable protein: Mechanism, kinetics, and production of soluble and partially soluble protein in a batch reactor. Biotechnol. Bioeng. 1980, 22, 1543–1565. [Google Scholar] [CrossRef]
- Madhyastha, H.K.; Sivashankari, S.; Vatsala, T.M. C-phycocyanin from Spirulina fussiformis exposed to blue light demonstrates higher efficacy of in vitro antioxidant activity. Biochem. Eng. J. 2009, 43, 221–224. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Q.; Lu, T.; Ma, H.; Chen, X. The effects of ultrasonication on the phytochemicals, antioxidant, and polyphenol oxidase and peroxidase activities in coffee leaves. Food Chem. 2021, 373, 131480. [Google Scholar] [CrossRef]


| CP Content (wt%) | Protein Content (wt%) | Yield (%) | |
|---|---|---|---|
| S-CP | 10.9 ± 0.00 c | 79.1 ± 0.7 b | 15.9 |
| HPCD-CP | 15.3 ± 0.00 a | 77.8 ± 0.2 b | 5.8 |
| 4FT-CP | 13.6 ± 0.53 b | 83.7 ± 2.2 a | 9.8 |
| 4FT+U-CP | 12.2 ± 0.45 c | 81.1 ± 0.5 ab | 15.92 |
| Sample | Area (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| 1660–1650 cm−1 | 1640–1600 cm−1 | 1670–1660 cm−1 | 1650–1640 cm−1 | |
| 1690–1670 cm−1 | 1700–1690 cm−1 | |||
| S-CP | 19.16 | 54.14 | 26.70 | 0 |
| HPCD-CP | 0 | 55.46 | 22.08 | 22.46 |
| 4FT-CP | 28.31 | 49.50 | 22.19 | 0 |
| 4FT+U-CP | 28.66 | 49.84 | 21.50 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, S.; Xiong, H.; Zhao, Q. Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis. Foods 2022, 11, 1296. https://doi.org/10.3390/foods11091296
Chen Q, Li S, Xiong H, Zhao Q. Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis. Foods. 2022; 11(9):1296. https://doi.org/10.3390/foods11091296
Chicago/Turabian StyleChen, Qian, Shuhui Li, Hua Xiong, and Qiang Zhao. 2022. "Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis" Foods 11, no. 9: 1296. https://doi.org/10.3390/foods11091296
APA StyleChen, Q., Li, S., Xiong, H., & Zhao, Q. (2022). Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis. Foods, 11(9), 1296. https://doi.org/10.3390/foods11091296






