Risk–Benefit Assessment of an Increase in the Iodine Fortification Level of Foods in Denmark—A Pilot Study
Abstract
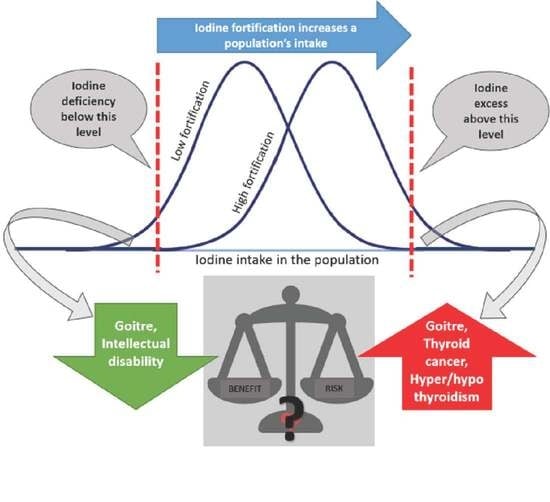
1. Introduction
2. Materials and Methods
2.1. Model Framework
Definition of Iodine Deficiency and Iodine Excessive Intake
2.2. Selection of Health Effects
2.3. Prevalence of Exposure
2.4. Estimating Changes in Incidence of Selected Health Outcomes
2.4.1. Fetus IQ
2.4.2. Goiter
2.5. DALY and DALY Difference
2.6. Scenario Analyses
3. Results
3.1. Iodine Intake and Prevalence of Exposure
3.2. Incidence
3.3. Risk–Benefit Assessment in Terms of DALY
3.4. Scenario Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| AR 1 | UL 2 | Mean (sd) | 5th Percentile | Median | 95th Percentile | |
|---|---|---|---|---|---|---|
| 13 ppm | ||||||
| Boys (4–6 years) | 65 | 250 | 179 (42) | 116 | 176 | 253 |
| Boys (7–10 years) | 65 | 300 | 191 (58) | 102 | 185 | 278 |
| Boys (11–14 years) | 75 | 450 | 203 (60) | 120 | 201 | 304 |
| Boys (15–17 years) | 100 | 500 | 220 (95) | 103 | 201 | 380 |
| Girls (4–6 years) | 65 | 250 | 161 (45) | 90 | 160 | 228 |
| Girls (7–10 years) | 65 | 300 | 177 (53) | 105 | 172 | 255 |
| Girls (11–14 years) | 75 | 450 | 162 (61) | 88 | 153 | 253 |
| Girls (15–17 years) | 100 | 500 | 168 (80) | 77 | 169 | 300 |
| 20 ppm | ||||||
| Boys (4–6 years) | 65 | 250 | 204 (45) | 134 | 203 | 277 |
| Boys (7–10 years) | 65 | 300 | 220 (64) | 124 | 217 | 321 |
| Boys (11–14 years) | 75 | 450 | 237 (65) | 144 | 233 | 344 |
| Boys (15–17 years) | 100 | 500 | 259 (104) | 118 | 239 | 455 |
| Girls (4–6 years) | 65 | 250 | 184 (47) | 111 | 183 | 259 |
| Girls (7–10 years) | 65 | 300 | 204 (55) | 128 | 196 | 286 |
| Girls (11–14 years) | 75 | 450 | 190 (66) | 111 | 184 | 293 |
| Girls (15–17 years) | 100 | 500 | 194 (84) | 97 | 195 | 325 |
References
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- World Health Organzation. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; WHO Press: Geneva, Switzerland, 2007; ISBN 9789241595827. [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T.; Vander, A. Vander’s Human Physiology: The Mechnisms of Body Function (The Endocrine System), 12th ed.; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Blerichrodt, N.; Born, M.P. Meta-Analysis of Reasearch on Iodine and Its Relationship to Cognitive Development. In The Damaged Brain of Iodine Deficiency; Stanbury, J.B., Ed.; Cognizant Communication Corporation: New York, NY, USA, 1994; pp. 195–200. [Google Scholar]
- WHO. Iodin Deficiency in Europe—A Continuing Public Health Problem; WHO Press: Geneva, Switzerland, 2007; ISBN 9789241593960. [Google Scholar]
- Rasmussen, L.B.; Ovesen, L.; Bülow, I.; Jørgensen, T.; Knudsen, N.; Laurberg, P.; Perrild, H. Dietary iodine intake and urinary iodine excretion in a Danish population: Effect of geography, supplements and food choice. Br. J. Nutr. 2002, 87, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Andersson, G.; Haraldsdóttir, J.; Kristiansen, E.; Molsted, K.; Laurberg, P.; Overvad, K.; Perrild, H.; Ovesen, L. Iodine. Do we need an enrichment program in Denmark? Int. J. Food Sci. Nutr. 1996, 47, 377–381. [Google Scholar] [CrossRef]
- Miljø- og Fødevareministeriet Bekendtgørelse om Tilsætning af jod til Husholdningssalt og Salt i Brød og Almindeligt Bagværk m.v. Available online: https://www.retsinformation.dk/eli/lta/2019/613 (accessed on 8 November 2021).
- Laurberg, P.; Perrild, H.; Jørgensen, T.; Ovesen, L.; Rasmussen, L. Fødevareberigelse Med jod:Den Danske Monitorering (DanThyr); Dansk Fødevareforskning: Copenhagen, Denmark, 2009; ISBN 87-91587-22-0. [Google Scholar]
- Rasmussen, L.B.; Carlé, A.; Jørgensen, T.; Knudsen, N.; Laurberg, P.; Pedersen, I.B.; Perrild, H.; Vejbjerg, P.; Ovesen, L. Iodine intake before and after mandatory iodization in Denmark: Results from the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr) study. Br. J. Nutr. 2008, 100, 166–173. [Google Scholar] [CrossRef]
- Laurberg, P.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Knudsen, N.; Pedersen, I.B.; Rasmussen, L.B.; Carlé, A.; Vejbjerg, P. The Danish investigation on iodine intake and thyroid disease, DanThyr: Status and perspectives. Eur. J. Endocrinol. 2006, 155, 219–228. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Jørgensen, T.; Perrild, H.; Knudsen, N.; Krejbjerg, A.; Laurberg, P.; Pedersen, I.B.; Bjergved, L.; Ovesen, L. Mandatory iodine fortification of bread and salt increases iodine excretion in adults in Denmark: A 11-year follow-up study. Clin. Nutr. 2014, 33, 1033–1040. [Google Scholar] [CrossRef]
- Andersen, S.L.; Sørensen, L.K.; Krejbjerg, A.; Møller, M.; Laurberg, P. Iodine deficiency in Danish pregnant women. Dan. Med. J. 2013, 60, 9. [Google Scholar]
- Kirkegaard-Klitbo, D.M.; Perslev, K.; Andersen, S.L.; Perrild, H.; Knudsen, N.; Weber, T.; Rasmussen, L.B.; Laurberg, P. Iodine deficiency in pregnancy is prevalent in vulnerable groups in Denmark. Dan. Med. J. 2016, 63, 1–5. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 1–57. [Google Scholar] [CrossRef]
- Nordic Counsil of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed.; Nordic Counsil of Ministers: Copenhagen, Denmark, 2014; ISBN 978-92-893-2670-4. [Google Scholar]
- Hoekstra, J.; Hart, A.; Boobis, A.; Claupein, E.; Cockburn, A.; Hunt, A.; Knudsen, I.; Richardson, D.; Schilter, B.; Schütte, K.; et al. BRAFO tiered approach for benefit—Risk assessment of foods. Food Chem. Toxicol. 2012, 50, S684–S698. [Google Scholar] [CrossRef]
- Farakos, S.S.; Membre, J.M.; Nauta, M. Risk-benefit analysis in food safety and nutrition. Curr. Opin. Food Sci. 2021, 36, 76–82. [Google Scholar] [CrossRef]
- Devleesschauwer, B.; Havelaar, A.H.; Maertens, C. DALY calculation in practice: A stepwise approach. Int. J. Public Health 2014, 59, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Lopez, A.D. (Eds.) The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Summary; Harvard School of Public Health on Behalf of the World Health Organization and the World Bank: Cambridge, MA, USA, 1996. [Google Scholar]
- Hoekstra, J.; Verkaik-Kloosterman, J.; Rompelberg, C.; van Kranen, H.; Zeilmaker, M.; Verhagen, H.; de Jong, N. Integrated risk–benefit analyses: Method development with folic acid as example. Food Chem. Toxicol. 2008, 46, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J.; Mugambi, G.; Verkaik-Kloosterman, J.; Hoekstra, J.; Kraemer, K.; Osendarp, S.; Melse-Boonstra, A.; Gallagher, A.M.; Verhagen, H. Addressing the risk of inadequate and excessive micronutrient intakes: Traditional versus new approaches to setting adequate and safe micronutrient levels in foods. Food Nutr. Res. 2015, 59, 1–10. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S. Grading quality of evidence and strength of recommendations. Br. Med. J. (Clin. Res. Ed). 2004, 328, 1490. [Google Scholar]
- Bougma, K.; Aboud, F.E.; Harding, K.B.; Marquis, G.S. Iodine and mental development of children 5 years old and under: A systematic review and meta-analysis. Nutrients 2013, 5, 1387–1416. [Google Scholar] [CrossRef]
- Yu, X.; Fan, C.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Chong, W.; Yang, F.; et al. A five-year follow-up study of goiter and thyroid nodules in three regions with different iodine intakes in China. J. Endocrinol. Investig. 2008, 31, 243–250. [Google Scholar] [CrossRef]
- Pedersen, A.; Christensen, T.; Knudsen, J.; Matthiessen, V.; Rosenlund-Sørensen, M.; Biltoft-Jensen, A. Danskernes Kostvaner 2011–2013. Hovedresultater [Dietary Habits in Denmark 2011–2013. Main Results]; DTU Library: Søborg, Denmark, 2015. [Google Scholar]
- Outzen, M.; Lund, C.E.; Christensen, T.; Trolle, E.; Ravn, G. Assessment of iodine fortification of salt in the Danish population. Eur. J. Nutr. 2022. [Google Scholar] [CrossRef]
- Salomon, J.A.; Haagsma, J.A.; Davis, A.; de Noordhout, C.M.; Polinder, S.; Havelaar, A.H.; Cassini, A.; Devleesschauwer, B.; Kretzschmar, M.; Speybroeck, N.; et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob. Health 2015, 3, e712–e723. [Google Scholar] [CrossRef]
- Krejbjerg, A.; Bjergved, L.; Pedersen, I.B.; Knudsen, N.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. Thyroid nodules in an 11-year DanThyr follow-up study. J. Clin. Endocrinol. Metab. 2014, 99, 4749–4757. [Google Scholar] [CrossRef][Green Version]
- Völzke, H.; Ittermann, T.; Albers, M.; Friedrich, N.; Nauck, M.; Below, H.; Kramer, A. Five-year change in morphological and functional alterations of the thyroid gland: The study of health in pomerania. Thyroid 2012, 22, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, W.M.G.; Evered, D.C.; Hall, R.; Appelton, D.; Brewis, M.; Clark, F.; Grimley Evans, J.; Young, E.; Bird, T.; Smith, P.A. The spectrum of thyroid disease in a community: The Wickham Survey. Clin. Endocrinol. 1977, 7, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.; Ho, J. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2015; WHO Press: Geneva, Switzerland, 2017; Volume 1. [Google Scholar]
- Díez, J.J. Goiter in adult patients aged 55 years and older: Etiology and clinical features in 634 patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 920–923. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurberg, P.; Cerqueira, C.; Ovesen, L.; Rasmussen, L.B.; Perrild, H.; Andersen, S.; Pedersen, I.B.; Carlé, A. Iodine intake as a determinant of thyroid disorders in populations. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 13–27. [Google Scholar] [CrossRef]
- Bjergved, L.; Jørgensen, T.; Perrild, H.; Carlé, A.; Cerqueira, C.; Krejbjerg, A.; Laurberg, P.; Ovesen, L.; Pedersen, I.B.; Rasmussen, L.B.; et al. Predictors of change in serum TSH after iodine fortification: An 11-year follow-up to the DanThyr study. J. Clin. Endocrinol. Metab. 2012, 97, 4022–4029. [Google Scholar] [CrossRef]
- Møllehave, L.T.; Linneberg, A.; Skaaby, T.; Knudsen, N.; Jørgensen, T.; Thuesen, B.H. Trends in treatments of thyroid disease following iodine fortification in Denmark: A nationwide register-based study. Clin. Epidemiol. 2018, 10, 763–770. [Google Scholar] [CrossRef]
- Rochau, U.; Qerimi Rushaj, V.; Schaffner, M.; Schönhensch, M.; Stojkov, I.; Jahn, B.; Hubalewska-Dydejczyk, A.; Erlund, I.; Thuesen, B.H.; Zimmermann, M.; et al. Decision-Analytic Modeling Studies in Prevention and Treatment of Iodine Deficiency and Thyroid Disorders: A Systematic Overview. Thyroid 2020, 30, 746–758. [Google Scholar] [CrossRef]
- Schaffner, M.; Mühlberger, N.; Conrads-Frank, A.; Qerimi Rushaj, V.; Sroczynski, G.; Koukkou, E.; Heinsbaek Thuesen, B.; Völzke, H.; Oberaigner, W.; Siebert, U.; et al. Benefits and Harms of a Prevention Program for Iodine Deficiency Disorders: Predictions of the Decision-Analytic EUthyroid Model. Thyroid 2021, 31, 494–508. [Google Scholar] [CrossRef]
- Norwegian Scientific Committee for Food and Environment (VKM). Benefit and Risk Assessment of Iodization of Household Salt and Salt Used in Bread and Bakery Products. Scientific Opinion of the Panel on Nutrition, Dietetic Products, Novel Food and Allergy of the Norwegian Scientific Committee for Food and Environment; VKM: Oslo, Norway, 2020; ISBN 9788282593434. [Google Scholar]
- Mensa Danmark. Mensa—Ofte Stillede Sporgsm°al. 2015. Available online: http://mensa.dk/om-mensa/faq (accessed on 8 November 2021).
- Danmarks Statistik. Statistikbanken—Befolkning og Valg. 2020. Available online: https://www.statistikbanken.dk/statbank5a/default.asp?w=1440 (accessed on 8 November 2021).
- Medscape. How Does the Incidence of Goiter Vary by Sex. 2020. Available online: https://www.medscape.com/answers/120034-102152/how-does-the-incidence-of-goiter-vary-by-sex (accessed on 8 November 2021).
- Knudsen, N.; Bülow, I.; Jørgensen, T.; Laurberg, P.; Ovesen, L.; Perrild, H. Goitre prevalence and thyroid abnormalities at ultrasonography: A comparative epidemiological study in two regions with slightly different iodine status. Clin. Endocrinol. 2000, 53, 479–485. [Google Scholar] [CrossRef]
- World Health Organization; World Bank; Harvard School of Public Health. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2019; World Health Organization: Geneva, Switzerland, 2020; Available online: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_daly-methods.pdf?sfvrsn=31b25009_7 (accessed on 8 November 2021).
- Sheila, S.A. Iodine deficiency in pregnancy: The effect on neurodevelopment in the child. Nutrients 2011, 3, 265–273. [Google Scholar] [CrossRef]
- Taylor, P.N.; Okosieme, O.E.; Dayan, C.M.; Lazarus, J.H. Impact of iodine supplementation in mild-to-moderate iodine deficiency: Systematic review and meta-analysis. Eur. J. Endocrinol. 2014, 170, R1–R15. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Krejbjerg, A.; Laurberg, P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 465–479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Data Sources and Inclusion Criteria for the Database on Iodine Deficiency. Available online: https://www.who.int/vmnis/iodine/data/sources/iodine_data_sources/en/ (accessed on 17 May 2021).
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]


| Health Effect | Level of Evidence | Target Population | Dose-Response (95% CI) | Reference |
|---|---|---|---|---|
| Fetus IQ | Convincing | Women of childbearing age (15–49) | Average lower fetus IQ of 7.4 (6.9–10.2) IQ-points due to maternal ID. | [24] |
| Goiter | Convincing | Total adult population (18–75) | OR for mild ID = 1.83 (1.26; 2.65) OR for chronic IE = 1.46 (1.01; 2.11). | [25] |
| Health Outcome | Disability Weight 1 [95% UI] 2 | Duration (Years) |
|---|---|---|
| Fetal IQ | IQ (>85) = 0 | 81.5 3 |
| IQ (70–85) = 0.011 [0.000; 0.020] | ||
| IQ (50–69) = 0.043 [0.026; 0.064] | ||
| IQ (35–49) = 0.100 [0.066; 0.142] | ||
| IQ (20–34) = 0.160 [0.107; 0.226] | ||
| IQ (<20) = 0.200 [0.133; 0.283] | ||
| Goiter | Goiter grade 1 = 0.001 | Men 4 = 2 |
| Goiter grade 2 = 0.025 | Women 4 = 5 |
| Mean (SD) | 5th Percentile | 50th Percentile | 95th Percentile | |
|---|---|---|---|---|
| 13 ppm | ||||
| Adult men (18–75 years) | 230 (84) | 118 | 220 | 363 |
| Adult women (18–75 years) | 200 (75) | 102 | 191 | 328 |
| Women of childbearing age (15–49) | 198 (80) | 102 | 184 | 339 |
| 20 ppm 1 | ||||
| Adult men (18–75 years) | 267 (90) | 142 | 259 | 409 |
| Adult women (18–75 years) | 228 (80) | 121 | 217 | 369 |
| Women of childbearing age (15–49) | 226 (84) | 120 | 212 | 376 |
| Target Population | Iodine Nutrition Status | Prevalence of Exposure at 13 ppm (%) | Prevalence of Exposure at 20 ppm (%) |
|---|---|---|---|
| Men (18–75) | ID (<100 µg/day) | 1.47 | 0.23 |
| IE (>600 µg/day) | 0.19 | 0.42 | |
| Women (18–75) | ID (<100 µg/day) | 4.19 | 1.23 |
| IE (>600 µg/day) | 0.07 | 0.13 | |
| Women of childbearing age (15–49) | ID (<125 µg/day) | 16.15 | 7.13 |
| Health Outcome | Incidence/100,000 at 20 ppm [95% UI] 1 | Incidence/100,000 at 20 ppm [95% UI] 1 | |
|---|---|---|---|
| Fetal IQ | IQ 70–85 | 25.3 [21.8; 30.3] | 11.2 [9.6; 13.4] |
| IQ 50–69 | 9.3 [7.5; 12.1] | 4.1 [3.3; 5.4] | |
| IQ 35–49 | 0.4 [0.3; 0.6] | 0.2 [0.1; 0.2] | |
| IQ 20–34 | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | |
| IQ < 20 | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | |
| Goiter | ID (Men) | 8.5 [2.5; 16.6] | 1.4 [0.4; 2.7] |
| ID (Women) | 94.0 [27.8; 180.4] | 28.4 [8.2; 55.5] | |
| IE (Men) | 0.6 [0.0; 1.5] | 1.4 [0.0; 3.2] | |
| IE (Women) | 0.9 [0.0; 2.0] | 1.7 [0.0; 4.0] | |
| Health Outcome | DALY/100,000 at 20 ppm | DALY/100,000 at 20 ppm | |
|---|---|---|---|
| Fetal IQ | IQ 70–85 | 24.8 [10.3; 42.7] | 10.9 [4.6; 18.8] |
| IQ 50–69 | 33.6 [18.5; 53.4] | 14.8 [8.1; 23.6] | |
| IQ 35–49 | 3.2 [1.8; 5.2] | 1.4 [0.8; 2.3] | |
| IQ 20–34 | 0.2 [0.1; 0.3] | 0.1 [0.0; 0.1] | |
| IQ < 20 | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | |
| Goiter | ID (Men) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] |
| ID (Women) | 0.6 [0.2; 1.1] | 0.2 [0.1; 0.3] | |
| IE (Men) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | |
| IE (Women) | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobsen, L.S.; Nielsen, J.O.; Paulsen, S.E.; Outzen, M.; Linneberg, A.; Møllehave, L.T.; Christensen, T.; Ravn-Haren, G. Risk–Benefit Assessment of an Increase in the Iodine Fortification Level of Foods in Denmark—A Pilot Study. Foods 2022, 11, 1281. https://doi.org/10.3390/foods11091281
Jakobsen LS, Nielsen JO, Paulsen SE, Outzen M, Linneberg A, Møllehave LT, Christensen T, Ravn-Haren G. Risk–Benefit Assessment of an Increase in the Iodine Fortification Level of Foods in Denmark—A Pilot Study. Foods. 2022; 11(9):1281. https://doi.org/10.3390/foods11091281
Chicago/Turabian StyleJakobsen, Lea Sletting, Josefine Ostenfeld Nielsen, Sophie Egesø Paulsen, Malene Outzen, Allan Linneberg, Line Tang Møllehave, Tue Christensen, and Gitte Ravn-Haren. 2022. "Risk–Benefit Assessment of an Increase in the Iodine Fortification Level of Foods in Denmark—A Pilot Study" Foods 11, no. 9: 1281. https://doi.org/10.3390/foods11091281
APA StyleJakobsen, L. S., Nielsen, J. O., Paulsen, S. E., Outzen, M., Linneberg, A., Møllehave, L. T., Christensen, T., & Ravn-Haren, G. (2022). Risk–Benefit Assessment of an Increase in the Iodine Fortification Level of Foods in Denmark—A Pilot Study. Foods, 11(9), 1281. https://doi.org/10.3390/foods11091281