Determination of the Most Efficient Household Technique for the Reduction of Pesticide Residues from Raw Fish Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling of Fishes
2.3. Treatment of the Raw Fishes with Pesticides and Household Decontamination Methods: Analysis
2.3.1. Washing with Running Water (T1)
2.3.2. Soaking in Stable Normal Water (T2)
2.3.3. Soaking in 2% NaCl Solution (T3)
2.3.4. Soaking in 2% Vinegar Solution (T4)
2.3.5. Soaking in 0.1% Sodium Bicarbonate Solution (T5)
2.3.6. Soaking in 0.1% Sodium Bicarbonate Solution + 2% NaCl Solution+ 2% Vinegar + Lemon Juice (T6)
2.4. Freezing Storage Duration after the Treatments
2.5. Extraction and Clean-Up of the Samples

2.6. GC-ECD Instrumentation
2.7. Quality Assurance of the Analytical Method
2.7.1. Formulation of Mixed Standard Solution from the Reference Pesticides
2.7.2. Calibration Curves
2.7.3. Assessment of Calibration Curve and Linearity
2.7.4. Assessment of the Accuracy and Precision Using (%) Recovery of Reference Pesticides
2.7.5. Assessment of Limit of Detection (LOD) and Limit of Quantification (LOQ)
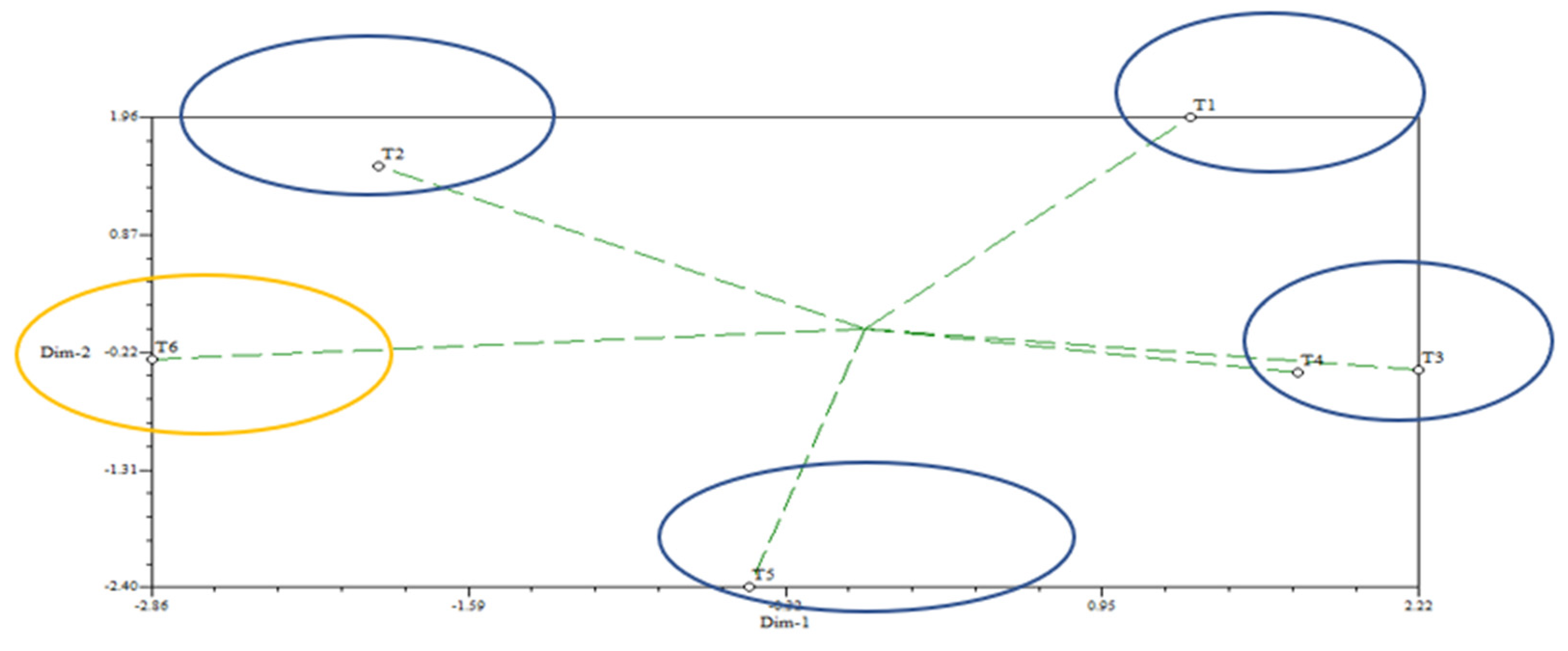
2.8. Statistical Analysis
3. Result
3.1. Quality Assurance of the Analytical Method
3.2. Reduction in Pesticide Residues (%) from African Catfish (Clarias gariepinus) by Various Household Techniques
3.3. Reduction in Pesticide Residues (%) from Snakehead Fish (Channa striata) Muscle by Household Processing Methods
3.4. Reduction in Pesticide Residues (%) from Climbing Perch (Anabus testudineus) Muscle by Household Processing Methods
3.5. Reduction in Pesticide Residues (%) from Three-Spot Gourami (Trichogaster trichopterus) Fish Muscle by Household Treatment Processing Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Ecobichon, D.J. Pesticide uses in developing countries. Toxicology 2001, 160, 27–33. [Google Scholar] [CrossRef]
- Muhammad, S.A. Analysis of Persistent Organic Pollutants in Fish: Health Risk Assessment Through Dietary Intake. Master’s Thesis, Universiti Sains Malaysia, Penang, Malaysia, March 2006. [Google Scholar]
- Hossain, M.M. Fate of Organochlorine Pesticide Residues (OCPs) in Sediment and in the Marine Food Chain. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, April 2001. [Google Scholar]
- Pal, P.; Shah, P.G. Effect of storage and processing on dissipation of five insecticides on wheat. Pestic. Res. J. 2008, 20, 253–258. [Google Scholar]
- Youssef, M.M.; Abdel, A.; Radwan, M.A. Removal of pirimiphos–methyl and chlorpyrifos–methyl residues from treated tomatoes and broad beans by commercial and home preparative procedures. Alex. Sci. Exch. 1995, 16, 461–469. [Google Scholar]
- Soliman, K.M. Changes in concentration of pesticide residues in potatoes during washing and home preparation. Food Chem. Toxicol. 2001, 39, 887–891. [Google Scholar] [CrossRef]
- Zohair, A. Behaviour of some organophosphorus and organochlorine pesticides in potatoes during soaking in different solutions. Food Addit. Contam. 2001, 39, 751–755. [Google Scholar] [CrossRef]
- Krol, W.J.; Arsenault, T.L.; Pylypiw, H.M.; Incorvia-Mattina, M.J. Reduction of pesticide residues on produce by rinsing. J. Agric. Food Chem. 2000, 48, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Seyler, L.A. EXTOXNET: Extension Toxicology Network: Pesticide information notebook [; H00 a]. 1994. Available online: https://agris.fao.org/agris-search/search.do?recordID=US9604421 (accessed on 16 April 2022).
- Guardia-Rubio, M.; Ayora-Canada, M.J.; Ruiz-Medina, A. Effect of washing on pesticide residues in olives. J. Food Sci. 2007, 72, 139–143. [Google Scholar] [CrossRef]
- Lopez-Fern’andez, O.; Rial-Otero, R.; Simal-G’andara, J. Factors governing the removal of mancozeb residues from lettuces with washing solutions. Food Control 2013, 34, 530–538. [Google Scholar] [CrossRef]
- Klinhom, P.; Halee, A.; Methawiwat, S. The effectiveness of household chemicals in residue removal of methomyl and carbaryl pesticides on Chinese kale. Kasesart J. 2008, 42, 136–143. [Google Scholar]
- Zhang, Z.Y.; Liu, X.J.; Hong, X.Y. Effects of home preparation on pesticide residues in cabbage. Food Control 2007, 18, 1484–1487. [Google Scholar] [CrossRef]
- Radwan, M.A.; Abu-Elamayem, M.M.; Shiboob, M.H.; Abdel-Aal, A. Residual behaviour of profenofos on some field-grown vegetables and its removal using various washing solutions and household processing. Food Chem. Toxicol. 2005, 43, 553–557. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of microorganisms and pesticides from fresh fruits and vegetables: A comprehensive review from common household processes to modern techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef]
- Rao, C.S.; Bhushan, V.S.; Reddy, H.; Darsi, R.; Aruna, M.; Ramesh, B. Risk mitigation methods for removal of pesticide residues in brinjal for food safety. Univers. J. Agric. Res. 2014, 2, 279–283. [Google Scholar] [CrossRef]
- Tomer, V.; Sangha, J.K.; Singh, B.; Takkar, R. Efficacy of processing treatments on cypermethrin residues in okra (Abelmoschus esculentus). Nutr. Food Sci. 2014, 44, 545–553. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Kanchanamayoon, O.; Phopin, K.; Prachayasittikul, V. Food safety in Thailand 2: Pesticide residues found in Chinese kale (Brassica oleracea), a commonly consumed vegetable in Asian countries. Sci. Total Environ. 2015, 532, 447–455. [Google Scholar] [CrossRef]
- Vijayasree, V.; Bai, H.; Beevi, S.N.; Mathew, T.B.; Kumar, V.; George, T.; Xavier, G. Persistence and effects of processing on reduction of chlorantraniliprole residues on cowpea fruits. Bull. Environ. Contam. Toxicol. 2013, 90, 494–498. [Google Scholar] [CrossRef]
- Mekonen, S.; Ambelu, A.; Spanoghe, P. Reduction of pesticide residues from teff (Eragrostis tef) flour spiked with selected pesticides using household food processing steps. Heliyon 2019, 5, e01740. [Google Scholar] [CrossRef] [PubMed]
- Zaidon, S.Z.; Ho, Y.B.; Hashim, Z.; Saari, N.; Praveena, S.M. Pesticides contamination and analytical methods of determination in environmental matrices in Malaysia and their potential human health effects–A review. Malays. J. Med. Health Sci. 2018, 14, 81–88. [Google Scholar]
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food-a review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, R.S.; Narendra, R.C.; Shashi, V. Decontamination methods utilizing house hold practices for removing pesticides on field bean for food safety. J. Nutr. Health Food Eng. 2018, 8, 260–267. [Google Scholar] [CrossRef][Green Version]
- Alaboudi, A.R.; Almashhadany, D.A.; Jarrah, B.S. Effect of cooking and freezing on levels of pesticides residues in local fresh fish. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2021, 78, 28–36. [Google Scholar] [CrossRef]
- Prodhan, M.D.H.; Alam, S.N. Determination of multiple organochlorine pesticide residues in shrimp using modified QuEChERS extraction and gas chromatography. SAARC J. Agri. 2018, 16, 81–93. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Mekonen, S.; Ambelu, A.; Spanoghe, P. Pesticide residue evaluation in major staple food items of Ethiopia using the QuEChERS method: A case study from the Jimma Zone: Pesticide residues in food items. Environ. Toxicol. Chem. 2014, 33, 1294–1302. [Google Scholar] [CrossRef]
- Ogbeide, O.; Tongo, I.; Ezemonye, L. Risk assessment of agricultural pesticides in water, sediment, and fish from Owan River, Edo State, Nigeria. Environ. Monit. Assess. 2015, 187, 654. [Google Scholar] [CrossRef]
- EU (European Commission). Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/11813/2017). 2017. Available online: http://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 1 January 2020).
- Pihlström, T.; Fernández-Alba, A.R.; Gamón, M.; Amate, C.F.; Poulsen, M.E.; Lippold, R.; Anastassiades, M. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed. SANTE 2017, 11813, 21–22. [Google Scholar]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; SANTE/12682/2019; DG SANTE: Bruxelles, Belgium, 2019. [Google Scholar]
- Liang, Y.; Wang, W.; Shen, Y. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 2012, 133, 636–640. [Google Scholar] [CrossRef]
- Demirci, A.; Ngadi, M.O. (Eds.) Microbial Decontamination in the Food Industry: Novel Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Geetha, P. Survey on Pesticide Usage, Monitoring of Pesticide Residues and Decontamination Methods in Spinach (Spinacia oleracea L.). Master’s Thesis, Professor Jayashankar Telangana State Agricultural University, Hyderabad, India, 2015. [Google Scholar]
- Nowowi, M.F.M.; Ishak, M.A.M.; Ismail, K.; Zakaria, S.R. Study on the effectiveness of five cleaning solutions in removing chlorpyrifos residues in cauliflower (Brassica oleracea). J. Environ. Chem. Ecotoxicol. 2016, 8, 69–72. [Google Scholar]
- Zhang, Y.S.; Li, X.P.; Liu, H.M. Study on universal cleaning solution in removing blended pesticide residues in Chinese cabbage. J. Environ. Chem. Ecotoxicol. 2013, 5, 202–207. [Google Scholar]
- Ratna, K.B.; Ranga, R.G.V.; Sahrawat, K.L.; Rajasekhar, P. Occurrence of insecticide residues in selected crops and natural resources. Bull. Environ. Contam. Toxicol. 2012, 89, 187–192. [Google Scholar] [CrossRef]
- Molina-Ruiz, J.M.; Cieslik, E.; Cieslik, I.; Walkowska, I. Determination of pesticide residues in fish tissues by modified QuEChERS method and dual-d-SPE clean-up coupled to gas chromatography-mass spectrometry. Env. Sci. Pollut. Res. 2015, 22, 369–378. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K. Effect of Ras cheese manufacturing on the stability of DDT and its metabolites. Food Chem. 1997, 59, 115–119. [Google Scholar] [CrossRef]
- Radwan, M.A.; Shiboob, M.H.; Abu-Elamayem, M.M. Residues of Pirimiphos-methyl and profenofos on green pepper and eggplant fruit and their effects on some quality properties. Emir. J. Agric. Sci. 2004, 16, 32–42. [Google Scholar] [CrossRef]
- Food, U.S. Drug Administration (USFDA). Clinical Trial Requirement Guidelines; USFDA: Silver Spring, MD, USA, 2009; Volume 1, pp. 1–65. [Google Scholar]
| Reference Pesticides Standards | % Recovery of Reference Pesticides (n = 5) (Intra-Day) | % Recovery of Reference Pesticides (n = 5) (Inter-Day) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 (µg/g) | 0.25 (µg/g) | 0.50 (µg/g) | 0.05 (µg/g) | 0.25 (µg/g) | ||||||
| Mean (%) | RSDr (%) | Mean (%) | RSDr (%) | Mean (%) | RSDr (%) | Mean (%) | RSDR (%) | Mean (%) | RSDR (%) | |
| Lindane | 95.85 | 9.66 | 94.33 | 6.01 | 90.96 | 11.45 | 90.05 | 2.40 | 89.46 | 2.63 |
| Heptachlor | 94.80 | 5.61 | 90.99 | 6.90 | 94.30 | 4.70 | 93.47 | 2.98 | 92.85 | 2.52 |
| Aldrin | 96.52 | 7.90 | 96.68 | 13.83 | 96.10 | 11.58 | 94.52 | 3.78 | 93.08 | 4.96 |
| Endosulfan | 97.44 | 5.13 | 97.41 | 11.62 | 96.20 | 10.38 | 94.63 | 4.77 | 96.61 | 5.10 |
| Dieldrin | 98.78 | 5.16 | 93.97 | 5.17 | 95.23 | 10.98 | 95.78 | 2.70 | 93.17 | 3.74 |
| DDT | 97.75 | 4.50 | 91.30 | 5.13 | 97.18 | 3.36 | 95.46 | 2.03 | 95.30 | 2.20 |
| Endrin | 97.87 | 4.60 | 93.04 | 8.43 | 96.83 | 11.95 | 96.27 | 2.79 | 92.03 | 2.97 |
| Methoxychlor | 97.24 | 5.46 | 93.73 | 8.21 | 98.60 | 10.01 | 96.63 | 4.11 | 93.32 | 4.75 |
| Cypermethrin | 98.19 | 8.80 | 97.05 | 8.29 | 93.65 | 10.44 | 94.19 | 3.12 | 94.84 | 4.22 |
| Reference Pesticides | RT | LOD (µg/g) | LOQ (µg/g) | R2 (Calibration Curve) |
|---|---|---|---|---|
| Lindane | 5.97 | 0.012 | 0.05 | 0.9977 |
| Heptachlor | 7.05 | 0.007 | 0.05 | 0.9962 |
| Aldrin | 7.69 | 0.006 | 0.05 | 0.9971 |
| Endosulfan | 9.24 | 0.009 | 0.05 | 0.9969 |
| Dieldrin | 9.87 | 0.004 | 0.05 | 0.9973 |
| DDT | 10.65 | 0.005 | 0.05 | 0.9959 |
| Endrin | 10.39 | 0.006 | 0.05 | 0.9938 |
| Methoxychlor | 13.13 | 0.010 | 0.05 | 0.9940 |
| Cypermethrin | 15.90 | 0.010 | 0.05 | 0.9982 |
| Pesticides | Measured Residue (µg/g) in Untreated Control (n = 3) | Residue Reduction (%) after Each Treatment (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Lindane | 0.479 ± 0.001 | 15–18 | 11–13 | 47–49 | 51–53 | 47–49 | 71–72 |
| Heptachlor | 0.484 ± 0.001 | 16–18 | 12–14 | 49–50 | 52–53 | 49–50 | 72–74 |
| Aldrin | 0.483 ± 0.016 | 15–17 | 10–14 | 49–50 | 54–55 | 49–50 | 74–75 |
| Endosulfan | 0.488 ± 0.014 | 16–20 | 11–15 | 50–51 | 53–55 | 49–50 | 74–76 |
| Dieldrin | 0.495 ± 0.004 | 16–18 | 13–16 | 49–51 | 55–56 | 50–51 | 78–79 |
| Endrin | 0.491 ± 0.006 | 14–16 | 11–17 | 51–52 | 55–56 | 51–52 | 77–79 |
| DDT | 0.489 ± 0.010 | 15–18 | 12–15 | 49–50 | 53–55 | 49–50 | 76–77 |
| Methoxychlor | 0.488 ± 0.0017 | 16–17 | 15–16 | 48–49 | 53–54 | 48–50 | 76–77 |
| Cypermethrin | 0.492 ± 0.002 | 16–19 | 16–20 | 50–51 | 54–56 | 50–51 | 78–79 |
| Pesticides | Measured Residue (µg/g) in Untreated Control (n = 3) | Residue Reduction (%) after Each Treatment (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Lindane | 0.487 ± 0.002 | 24–30 | 13–15 | 37–38 | 55–56 | 58–60 | 78–80 |
| Heptachlor | 0.484 ± 0.004 | 26–33 | 16–18 | 37–41 | 55–58 | 48–51 | 76–79 |
| Aldrin | 0.491 ± 0.001 | 28–31 | 22–23 | 41–43 | 58–60 | 55–59 | 77–79 |
| Endosulfan | 0.493 ± 0.003 | 33–35 | 18–20 | 44–45 | 58–59 | 59–61 | 76–78 |
| Dieldrin | 0.492 ± 0.003 | 35–37 | 15–18 | 45–46 | 58–60 | 50–53 | 77–78 |
| Endrin | 0.494 ± 0.002 | 28–33 | 12–16 | 51–55 | 59–60 | 55–57 | 76–78 |
| DDT | 0.491 ± 0.001 | 29–30 | 15–16 | 51–53 | 60–62 | 54–57 | 76–79 |
| Methoxychlor | 0.488 ± 0.003 | 33–34 | 13–14 | 50–51 | 58–60 | 55–57 | 72–75 |
| Cypermethrin | 0.493 ± 0.003 | 32–33 | 14–15 | 53–55 | 58–60 | 55–57 | 77–78 |
| Pesticides | Measured Residue (µg/g) in Untreated Control (n = 3) | Residue Reduction (%) after each Treatment (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Lindane | 0.482 ± 0.003 | 35–37 | 15–19 | 44–45 | 57–58 | 57–58 | 77–79 |
| Heptachlor | 0.479 ± 0.002 | 35–36 | 17–20 | 42–43 | 56–57 | 55–57 | 75–76 |
| Aldrin | 0.486 ± 0.004 | 34–36 | 16–20 | 44–45 | 58–59 | 55–56 | 74–76 |
| Endosulfan | 0.487 ± 0.003 | 35–36 | 18–19 | 45–47 | 59–60 | 56–59 | 76–78 |
| Dieldrin | 0.488 ± 0.002 | 37–39 | 19–20 | 46–47 | 59–60 | 56–57 | 76–77 |
| Endrin | 0.489 ± 0.003 | 38–41 | 18–19 | 46–48 | 60–61 | 58–59 | 79–80 |
| DDT | 0.486 ± 0.005 | 38–39 | 19–21 | 45–46 | 57–59 | 58–59 | 76–78 |
| Methoxychlor | 0.481 ± 0.005 | 37–38 | 20–22 | 45–47 | 58–59 | 56–58 | 77–78 |
| Cypermethrin | 0.490 ± 0.002 | 38–39 | 19–22 | 46–47 | 58–60 | 58–60 | 79–80 |
| Pesticides | Measured Residue (µg/g) in Untreated Control (n = 3) | Residue Reduction (%) after Each Treatment (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Lindane | 0.490 ± 0.002 | 34–36 | 15–17 | 53–55 | 60–61 | 57–60 | 78–80 |
| Heptachlor | 0.490 ± 0.003 | 35–36 | 17–18 | 54–55 | 62–63 | 58–59 | 79–80 |
| Aldrin | 0.492 ± 0.003 | 35–37 | 16–19 | 56–57 | 61–63 | 59–60 | 78–80 |
| Endosulfan | 0.492 ± 0.002 | 36–38 | 17–19 | 55–57 | 62–64 | 58–60 | 80–81 |
| Dieldrin | 0.493 ± 0.004 | 36–38 | 15–16 | 57–58 | 63–64 | 57–59 | 79–81 |
| Endrin | 0.492 ± 0.004 | 37–38 | 16–18 | 56–58 | 62–63 | 58–60 | 78–79 |
| DDT | 0.493 ± 0.003 | 36–38 | 16–17 | 56–58 | 61–62 | 58–59 | 78–81 |
| Methoxychlor | 0.494 ± 0.002 | 37–38 | 17–18 | 55–56 | 60–62 | 58–60 | 79–81 |
| Cypermethrin | 0.492 ± 0.004 | 36–39 | 18–20 | 56–58 | 60–61 | 59–60 | 78–79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Amin, S.M.N.; Brown, C.L.; Juraimi, A.S.; Uddin, M.K.; Arshad, A. Determination of the Most Efficient Household Technique for the Reduction of Pesticide Residues from Raw Fish Muscles. Foods 2022, 11, 1254. https://doi.org/10.3390/foods11091254
Islam MA, Amin SMN, Brown CL, Juraimi AS, Uddin MK, Arshad A. Determination of the Most Efficient Household Technique for the Reduction of Pesticide Residues from Raw Fish Muscles. Foods. 2022; 11(9):1254. https://doi.org/10.3390/foods11091254
Chicago/Turabian StyleIslam, Md. Ariful, S. M. Nurul Amin, Christopher L. Brown, Abdul Shukor Juraimi, Md. Kamal Uddin, and Aziz Arshad. 2022. "Determination of the Most Efficient Household Technique for the Reduction of Pesticide Residues from Raw Fish Muscles" Foods 11, no. 9: 1254. https://doi.org/10.3390/foods11091254
APA StyleIslam, M. A., Amin, S. M. N., Brown, C. L., Juraimi, A. S., Uddin, M. K., & Arshad, A. (2022). Determination of the Most Efficient Household Technique for the Reduction of Pesticide Residues from Raw Fish Muscles. Foods, 11(9), 1254. https://doi.org/10.3390/foods11091254







