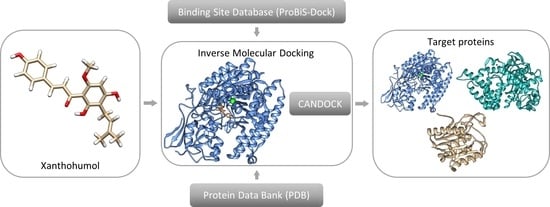
Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites
Abstract
1. Introduction
2. Materials and Methods
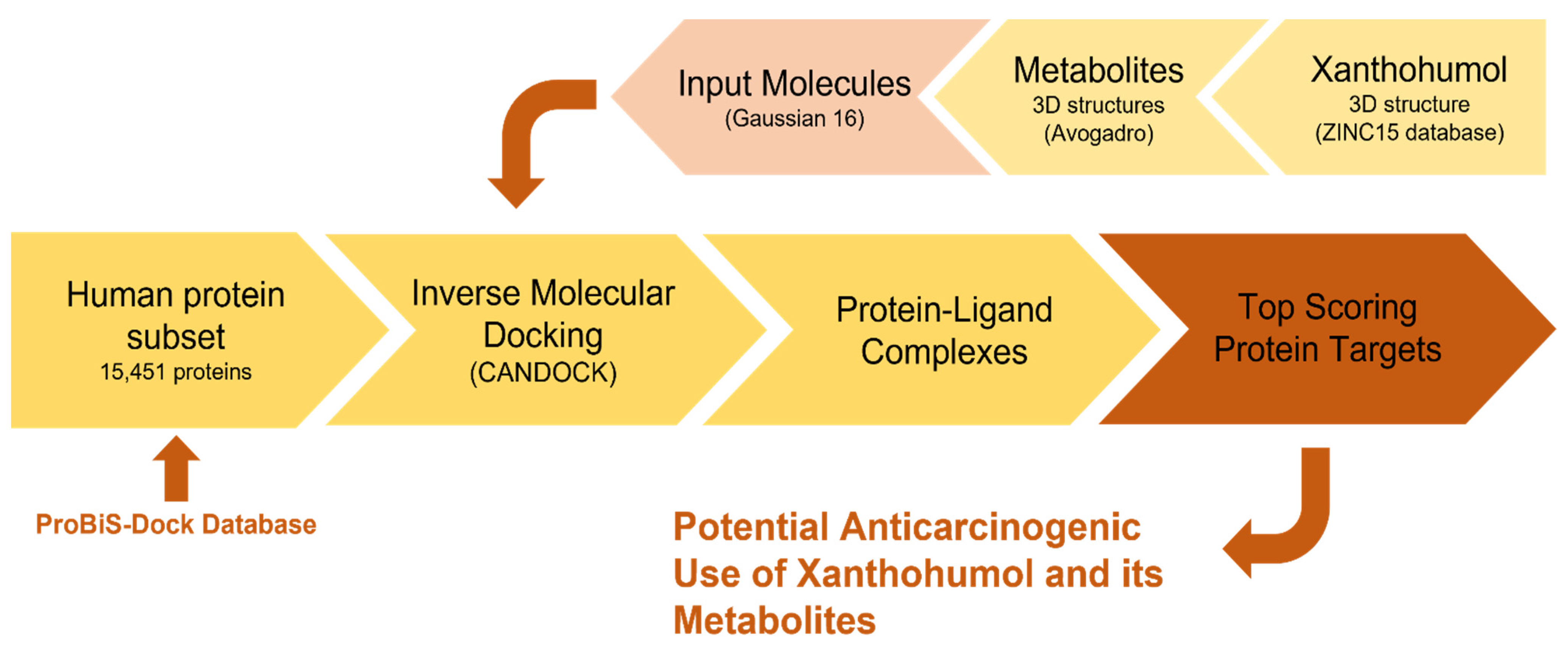
2.1. Inverse Molecular Docking
2.2. Preparation and Execution of Inverse Docking Procedure Using CANDOCK Algorithm
2.3. Method Validation Using Retrospective Metrics
3. Results and Discussion
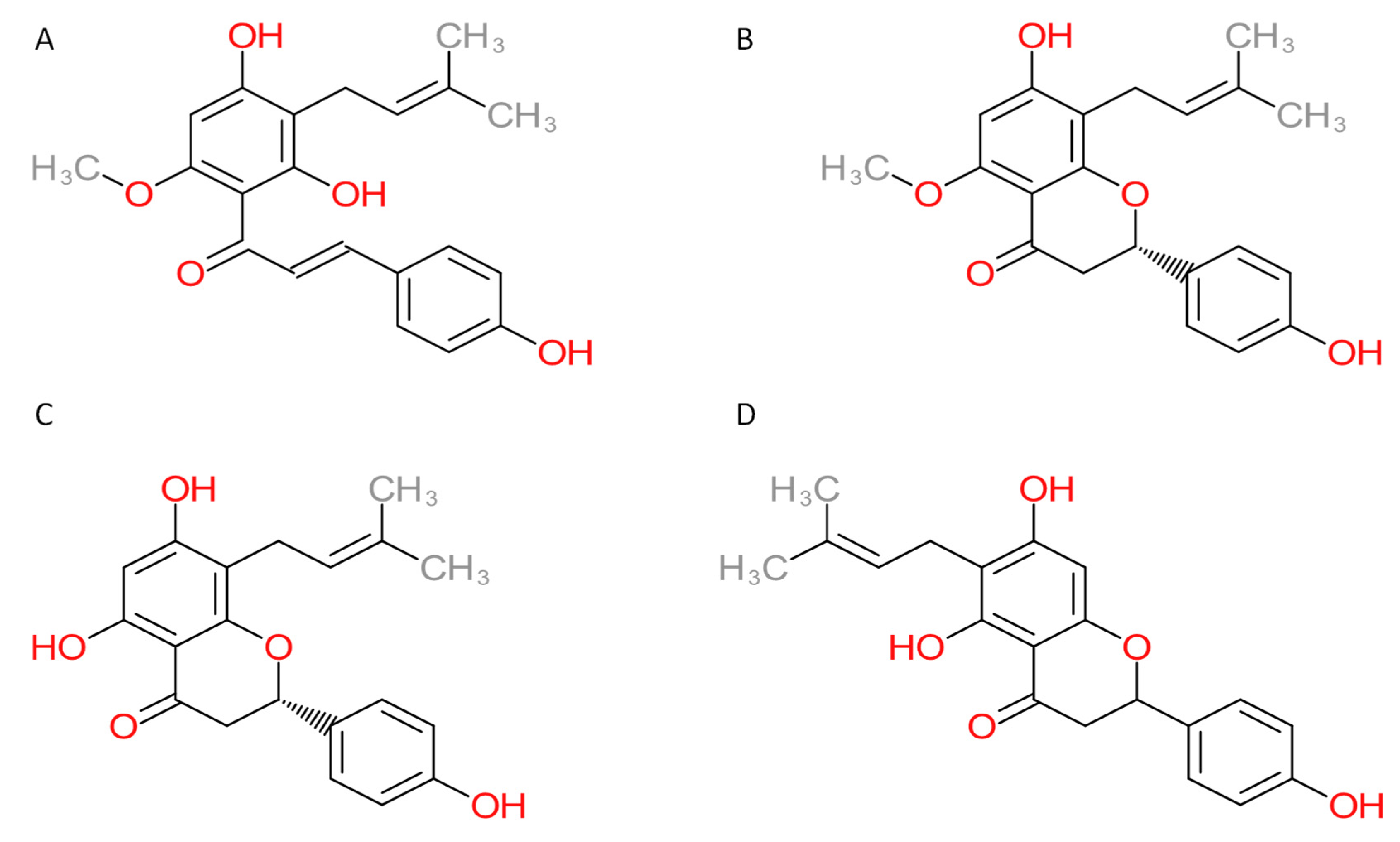
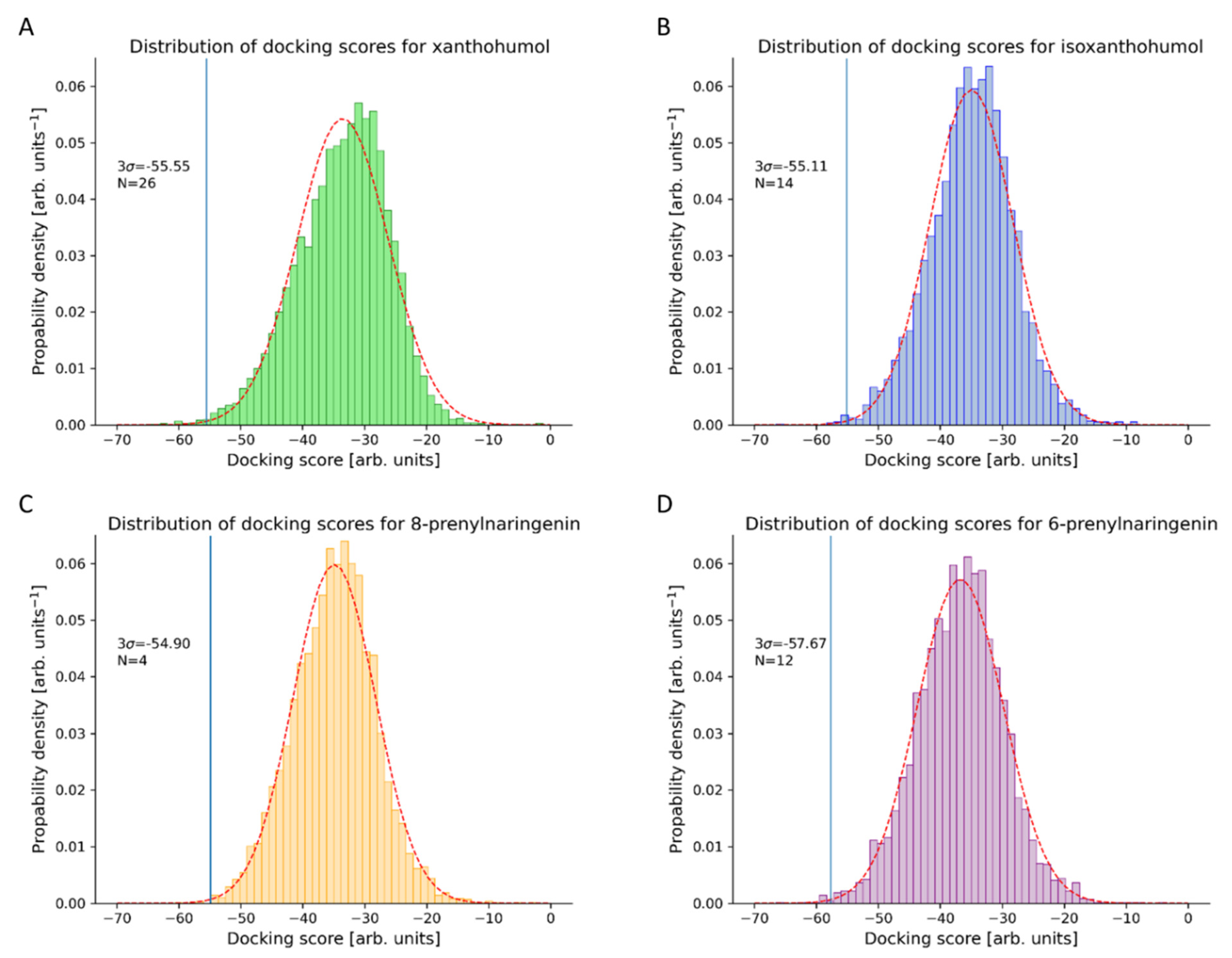
3.1. Novel Human Protein Targets of Xanthohumol
3.2. Novel Human Protein Targets of Isoxanthohumol, 8-Prenylnaringenin, and 6-Prenylnaringenin
3.3. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, K.; Matsuo, Y.; Imafuji, H.; Okubo, T.; Maeda, Y.; Sato, T.; Shamoto, T.; Tsuboi, K.; Morimoto, M.; Takahashi, H.; et al. Xanthohumol Inhibits Angiogenesis by Suppressing Nuclear Factor-ΚB Activation in Pancreatic Cancer. Cancer Sci. 2018, 109, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth Inhibitory and Apoptosis-Inducing Effects of Xanthohumol, a Prenylated Chalone Present in Hops, in Human Prostate Cancer Cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar]
- Štern, A.; Furlan, V.; Novak, M.; Štampar, M.; Kolenc, Z.; Kores, K.; Filipič, M.; Bren, U.; Žegura, B. Chemoprotective Effects of Xanthohumol against the Carcinogenic Mycotoxin Aflatoxin B1. Foods 2021, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of Xanthohumol in the Brewing Process. Mol. Nutr. Food Res. 2005, 49, 874–881. [Google Scholar] [CrossRef]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of Xanthohumol and Metabolites in Rats after Oral and Intravenous Administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Komatsu, M.; Tashiro, E.; Imoto, M.; Osada, H.; Miyoshi, Y.; Honda, J.; Sasa, M.; Katagiri, T. Xanthohumol Suppresses Oestrogen-Signalling in Breast Cancer through the Inhibition of BIG3-PHB2 Interactions. Sci. Rep. 2014, 4, 7355. [Google Scholar] [CrossRef]
- Saenz-Méndez, P.; Eriksson, M.; Eriksson, L.A. Ligand Selectivity between the ADP-Ribosylating Toxins: An Inverse-Docking Study for Multitarget Drug Discovery. ACS Omega 2017, 2, 1710–1719. [Google Scholar] [CrossRef]
- Kharkar, P.S.; Warrier, S.; Gaud, R.S. Reverse Docking: A Powerful Tool for Drug Repositioning and Drug Rescue. Future Med. Chem. 2014, 6, 333–342. [Google Scholar] [CrossRef]
- Wang, F.; Wu, F.-X.; Li, C.-Z.; Jia, C.-Y.; Su, S.-W.; Hao, G.-F.; Yang, G.-F. ACID: A Free Tool for Drug Repurposing Using Consensus Inverse Docking Strategy. J. Cheminform. 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J. A Potential Target of Tanshinone IIA for Acute Promyelocytic Leukemia Revealed by Inverse Docking and Drug Repurposing. Asian Pac. J. Cancer Prev. 2014, 15, 4301–4305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Ung, C.Y. Prediction of Potential Toxicity and Side Effect Protein Targets of a Small Molecule by a Ligand–Protein Inverse Docking Approach. J. Mol. Graph. Model. 2001, 20, 199–218. [Google Scholar] [CrossRef]
- Kores, K.; Konc, J.; Bren, U. Mechanistic Insights into Side Effects of Troglitazone and Rosiglitazone Using a Novel Inverse Molecular Docking Protocol. Pharmaceutics 2021, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Kores, K.; Lešnik, S.; Bren, U.; Janežič, D.; Konc, J. Discovery of Novel Potential Human Targets of Resveratrol by Inverse Molecular Docking. J. Chem. Inf. Model. 2019, 59, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Furlan, V.; Konc, J.; Bren, U. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 2018, 23, 3351. [Google Scholar] [CrossRef] [PubMed]
- Lešnik, S.; Bren, U. Mechanistic Insights into Biological Activities of Polyphenolic Compounds from Rosemary Obtained by Inverse Molecular Docking. Foods 2022, 11, 67. [Google Scholar] [CrossRef]
- Jukić, M.; Kores, K.; Janežič, D.; Bren, U. Repurposing of Drugs for SARS-CoV-2 Using Inverse Docking Fingerprints. Front. Chem.-Theor. Comput. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Konc, J.; Lešnik, S.; Škrlj, B.; Janežič, D. ProBiS-Dock Database: A Web Server and Interactive Web Repository of Small Ligand–Protein Binding Sites for Drug Design. J. Chem. Inf. Model. 2021, 61, 4097–4107. [Google Scholar] [CrossRef]
- Štular, T.; Lešnik, S.; Rožman, K.; Schink, J.; Zdouc, M.; Ghysels, A.; Liu, F.; Aldrich, C.C.; Haupt, V.J.; Salentin, S.; et al. Discovery of Mycobacterium Tuberculosis InhA Inhibitors by Binding Sites Comparison and Ligands Prediction. J. Med. Chem. 2016, 59, 11069–11078. [Google Scholar] [CrossRef]
- Fine, J.; Konc, J.; Samudrala, R.; Chopra, G. CANDOCK: Chemical Atomic Network-Based Hierarchical Flexible Docking Algorithm Using Generalized Statistical Potentials. J. Chem. Inf. Model. 2020, 60, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Konc, J.; Janežič, D. A Branch and Bound Algorithm for Matching Protein Structures. In Adaptive and Natural Computing Algorithms; Beliczynski, B., Dzielinski, A., Iwanowski, M., Ribeiro, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 399–406. [Google Scholar]
- Sterling, T.; Irwin, J.J. ZINC 15—Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Triballeau, N.; Acher, F.; Brabet, I.; Pin, J.-P.; Bertrand, H.-O. Virtual Screening Workflow Development Guided by the “Receiver Operating Characteristic” Curve Approach. Application to High-Throughput Docking on Metabotropic Glutamate Receptor Subtype 4. J. Med. Chem. 2005, 48, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Truchon, J.-F.; Bayly, C.I. Evaluating Virtual Screening Methods: Good and Bad Metrics for the “Early Recognition” Problem. J. Chem. Inf. Model. 2007, 47, 488–508. [Google Scholar] [CrossRef]
- Empereur-mot, C.; Guillemain, H.; Latouche, A.; Zagury, J.-F.; Viallon, V.; Montes, M. Predictiveness Curves in Virtual Screening. J. Cheminform. 2015, 7, 52. [Google Scholar] [CrossRef]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E.; et al. The ChEMBL Database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Sheridan, R.P.; Singh, S.B.; Fluder, E.M.; Kearsley, S.K. Protocols for Bridging the Peptide to Nonpeptide Gap in Topological Similarity Searches. J. Chem. Inf. Comput. Sci. 2001, 41, 1395–1406. [Google Scholar] [CrossRef]
- Empereur-Mot, C.; Zagury, J.-F.; Montes, M. Screening Explorer–An Interactive Tool for the Analysis of Screening Results. J. Chem. Inf. Model. 2016, 56, 2281–2286. [Google Scholar] [CrossRef]
- Huang, J.; Perez-Burgos, L.; Placek, B.J.; Sengupta, R.; Richter, M.; Dorsey, J.A.; Kubicek, S.; Opravil, S.; Jenuwein, T.; Berger, S.L. Repression of P53 Activity by Smyd2-Mediated Methylation. Nature 2006, 444, 629–632. [Google Scholar] [CrossRef]
- Saddic, L.A.; West, L.E.; Aslanian, A.; Yates, J.R.; Rubin, S.M.; Gozani, O.; Sage, J. Methylation of the Retinoblastoma Tumor Suppressor by SMYD2. J. Biol. Chem. 2010, 285, 37733–37740. [Google Scholar] [CrossRef] [PubMed]
- Vujic, I.; Sanlorenzo, M.; Esteve-Puig, R.; Vujic, M.; Kwong, A.; Tsumura, A.; Murphy, R.; Moy, A.; Posch, C.; Monshi, B.; et al. Acyl Protein Thioesterase 1 and 2 (APT-1, APT-2) Inhibitors Palmostatin B, ML348 and ML349 Have Different Effects on NRAS Mutant Melanoma Cells. Oncotarget 2016, 7, 7297–7306. [Google Scholar] [CrossRef] [PubMed]
- Hlouchova, K.; Barinka, C.; Konvalinka, J.; Lubkowski, J. Structural Insight into the Evolutionary and Pharmacologic Homology of Glutamate Carboxypeptidases II and III. FEBS J. 2009, 276, 4448–4462. [Google Scholar] [CrossRef] [PubMed]
- Hlouchová, K.; Barinka, C.; Klusák, V.; Sácha, P.; Mlcochová, P.; Majer, P.; Rulísek, L.; Konvalinka, J. Biochemical Characterization of Human Glutamate Carboxypeptidase III. J. Neurochem. 2007, 101, 682–696. [Google Scholar] [CrossRef]
- Bonnefond, L.; Stojko, J.; Mailliot, J.; Troffer-Charlier, N.; Cura, V.; Wurtz, J.-M.; Cianférani, S.; Cavarelli, J. Functional Insights from High Resolution Structures of Mouse Protein Arginine Methyltransferase 6. J. Struct. Biol. 2015, 191, 175–183. [Google Scholar] [CrossRef]
- Lim, Y.; Yu, S.; Yun, J.-A.; Do, I.-G.; Cho, L.; Kim, Y.H.; Kim, H.C. The Prognostic Significance of Protein Arginine Methyltransferase 6 Expression in Colon Cancer. Oncotarget 2018, 9, 9010–9020. [Google Scholar] [CrossRef]
- Obianyo, O.; Thompson, P.R. Kinetic Mechanism of Protein Arginine Methyltransferase 6 (PRMT6). J. Biol. Chem. 2012, 287, 6062–6071. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Kim, J.-A.; Kang, Y.-R.; Thapa, D.; Lee, J.-S.; Park, M.-A.; Lee, K.-H.; Lyoo, W.; Lee, Y.-R. Anti-Invasive and Anti-Angiogenic Effects of Xanthohumol and Its Synthetic Derivatives. Biomol. Ther. 2009, 17, 422–429. [Google Scholar] [CrossRef]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the Mitogen-Activated Protein Kinase Cascade to Treat Cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef]
- Mi, X.; Wang, C.; Sun, C.; Chen, X.; Huo, X.; Zhang, Y.; Li, G.; Xu, B.; Zhang, J.; Xie, J.; et al. Xanthohumol Induces Paraptosis of Leukemia Cells through P38 Mitogen Activated Protein Kinase Signaling Pathway. Oncotarget 2017, 8, 31297–31304. [Google Scholar] [CrossRef] [PubMed]
- Herceg, Z.; Wang, Z.Q. Functions of Poly(ADP-Ribose) Polymerase (PARP) in DNA Repair, Genomic Integrity and Cell Death. Mutat. Res. Mol. Mech. Mutagen. 2001, 477, 97–110. [Google Scholar] [CrossRef]
- Donawho, C.K.; Luo, Y.; Luo, Y.; Penning, T.D.; Bauch, J.L.; Bouska, J.J.; Bontcheva-Diaz, V.D.; Cox, B.F.; DeWeese, T.L.; Dillehay, L.E.; et al. ABT-888, an Orally Active Poly(ADP-Ribose) Polymerase Inhibitor That Potentiates DNA-Damaging Agents in Preclinical Tumor Models. Clin. Cancer Res. 2007, 13, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Becker, H.; Gerhäuser, C. Xanthohumol Induces Apoptosis in Cultured 40-16 Human Colon Cancer Cells by Activation of the Death Receptor- and Mitochondrial Pathway. Mol. Nutr. Food Res. 2005, 49, 837–843. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Della-Fera, M.A.; Rayalam, S.; Baile, C.A. Enhanced Effects of Xanthohumol plus Honokiol on Apoptosis in 3T3-L1 Adipocytes. Obesity 2008, 16, 1232–1238. [Google Scholar] [CrossRef]
- Wanschers, B.F.J.; van de Vorstenbosch, R.; Schlager, M.A.; Splinter, D.; Akhmanova, A.; Hoogenraad, C.C.; Wieringa, B.; Fransen, J.A.M. A Role for the Rab6B Bicaudal-D1 Interaction in Retrograde Transport in Neuronal Cells. Exp. Cell Res. 2007, 313, 3408–3420. [Google Scholar] [CrossRef]
- Yoo, B.C.; Yeo, S.-G. Identification of CEA-Interacting Proteins in Colon Cancer Cells and Their Changes in Expression after Irradiation. Radiat. Oncol. J. 2017, 35, 281–288. [Google Scholar] [CrossRef][Green Version]
- Huang, L.; Xu, A.-M. SET and MYND Domain Containing Protein 3 in Cancer. Am. J. Transl. Res. 2017, 9, 1–14. [Google Scholar]
- Devy, L.; Huang, L.; Naa, L.; Yanamandra, N.; Pieters, H.; Frans, N.; Chang, E.; Tao, Q.; Vanhove, M.; Lejeune, A.; et al. Selective Inhibition of Matrix Metalloproteinase-14 Blocks Tumor Growth, Invasion, and Angiogenesis. Cancer Res. 2009, 69, 1517–1526. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Maranchuk, R.; Kucharski, C.; Mahdipoor, P.; Hugh, J.; Uludağ, H. Effective Response of Doxorubicin-Sensitive and -Resistant Breast Cancer Cells to Combinational SiRNA Therapy. J. Control. Release 2013, 172, 219–228. [Google Scholar] [CrossRef]
- Godahewa, G.I.; Perera, N.C.N.; Bathige, S.D.N.K.; Nam, B.-H.; Noh, J.K.; Lee, J. Complement Factor D Homolog Involved in the Alternative Complement Pathway of Rock Bream (Oplegnathus Fasciatus): Molecular and Functional Characterization and Immune Responsive MRNA Expression Analysis. Fish Shellfish Immunol. 2016, 55, 423–433. [Google Scholar] [CrossRef]
- Matsumoto, R.; Tsuda, M.; Yoshida, K.; Tanino, M.; Kimura, T.; Nishihara, H.; Abe, T.; Shinohara, N.; Nonomura, K.; Tanaka, S. Aldo-Keto Reductase 1C1 Induced by Interleukin-1β Mediates the Invasive Potential and Drug Resistance of Metastatic Bladder Cancer Cells. Sci. Rep. 2016, 6, 34625. [Google Scholar] [CrossRef] [PubMed]
- Grimsby, J.; Chen, K.; Wang, L.J.; Lan, N.C.; Shih, J.C. Human Monoamine Oxidase A and B Genes Exhibit Identical Exon-Intron Organization. Proc. Natl. Acad. Sci. USA 1991, 88, 3637–3641. [Google Scholar] [CrossRef]
- Liu, G.; Peng, X.; Cai, Y.; Cheng, A.; Zha, L.; Wang, Z. DIMT1 Overexpression Correlates with Progression and Prognosis in Gastric Carcinoma. Hum. Pathol. 2017, 70, 35–42. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Tang, Q.; Mo, C.; Guo, J.; Chen, T.; Lin, H.; Tang, J.; Guo, L.; Huang, L.; et al. Functional Expression of Two NADPH-Cytochrome P450 Reductases from Siraitia Grosvenorii. Int. J. Biol. Macromol. 2018, 120, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Loughney, K.; Hill, T.R.; Florio, V.A.; Uher, L.; Rosman, G.J.; Wolda, S.L.; Jones, B.A.; Howard, M.L.; McAllister-Lucas, L.M.; Sonnenburg, W.K.; et al. Isolation and Characterization of CDNAs Encoding PDE5A, a Human CGMP-Binding, CGMP-Specific 3′,5′-Cyclic Nucleotide Phosphodiesterase. Gene 1998, 216, 139–147. [Google Scholar] [CrossRef]
- Mook, O.R.F.; Frederiks, W.M.; Van Noorden, C.J.F. The Role of Gelatinases in Colorectal Cancer Progression and Metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, I.-S.; Moon, A. 2-Hydroxychalcone and Xanthohumol Inhibit Invasion of Triple Negative Breast Cancer Cells. Chem. Biol. Interact. 2013, 203, 565–572. [Google Scholar] [CrossRef]
- Xu, X.; Gammon, M.D.; Wetmur, J.G.; Rao, M.; Gaudet, M.M.; Teitelbaum, S.L.; Britton, J.A.; Neugut, A.I.; Santella, R.M.; Chen, J. A Functional 19-Base Pair Deletion Polymorphism of Dihydrofolate Reductase (DHFR) and Risk of Breast Cancer in Multivitamin Users. Am. J. Clin. Nutr. 2007, 85, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Peters, G.J.; Jansen, G.; Martel, F.; Calhau, C. Modulation of Folate Uptake in Cultured Human Colon Adenocarcinoma Caco-2 Cells by Dietary Compounds. Eur. J. Nutr. 2007, 46, 329–336. [Google Scholar] [CrossRef]
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of Peroxisome Proliferator-Activated Receptor Delta on Placentation, Adiposity, and Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Tan, J.; Krause, W.F.; Geraci, M.W.; Willson, T.M.; Dey, S.K.; DuBois, R.N. Prostacyclin-Mediated Activation of Peroxisome Proliferator-Activated Receptor Delta in Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 13275–13280. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sies, C.W.; Pike, L.S. Analytical and Clinical Validation of an LC-MS/MS Method to Measure Thiopurine S-Methyltransferase Activity by Quantifying D3-6-MMP. Clin. Biochem. 2018, 54, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Baekkeskov, S.; Kanaani, J. Palmitoylation Cycles and Regulation of Protein Function (Review). Mol. Membr. Biol. 2009, 26, 42–54. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK Signalling Pathways and Colorectal Cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Kalogris, C.; Garulli, C.; Pietrella, L.; Gambini, V.; Pucciarelli, S.; Lucci, C.; Tilio, M.; Zabaleta, M.E.; Bartolacci, C.; Andreani, C.; et al. Sanguinarine Suppresses Basal-like Breast Cancer Growth through Dihydrofolate Reductase Inhibition. Biochem. Pharmacol. 2014, 90, 226–234. [Google Scholar] [CrossRef]
- RCSB PDB: Homepage. Available online: http://www.rcsb.org/ (accessed on 5 February 2018).
- Tachibana, K.; Yamasaki, D.; Ishimoto, K.; Doi, T. The Role of PPARs in Cancer. PPAR Res. 2008, 1–15. [Google Scholar] [CrossRef]
- Tie, Y.; Zheng, H.; He, Z.-Y.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X.; et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Fisher, D.A.; Smith, J.F.; Pillar, J.S.; Denis, S.H.S.; Cheng, J.B. Isolation and Characterization of PDE9A, a Novel Human cGMP-specific Phosphodiesterase. J. Biol. Chem. 1998, 273, 15559–15564. [Google Scholar] [CrossRef]
- Souza, M.D.F.D.D.; Filho, A.F.D.S.; Albuquerque, A.P.D.B.; Quirino, M.W.L.; Albuquerque, M.S.D.S.; Cordeiro, M.F.; Martins, M.R.; Pitta, I.D.R.; Lucena-Araujo, A.R.; Pitta, M.G.D.R.; et al. Overexpression of UDP-Glucose 4-Epimerase Is Associated with Differentiation Grade of Gastric Cancer. Dis. Markers 2019, 1–5. [Google Scholar] [CrossRef]
- Coughtrie, M. Sulfation through the looking glass—recent advances in sulfotransferase research for the curious. Pharmacogenom. J. 2002, 2, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990, 9, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Haagenson, K.K.; Wu, G.S. Mitogen activated protein kinase phosphatases and cancer. Cancer Biol. Ther. 2010, 9, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Yoshimura, K.; Kurabe, N.; Kahyo, T.; Kawase, A.; Tanahashi, M.; Ogawa, H.; Inui, N.; Funai, K.; Shinmura, K.; et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 2017, 8, 8738–8751. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Takahashi, M.; Morishita, T.; Noguchi, T.; Matsuzawa, A. Post-Translational Modifications of the TAK1-TAB Complex. Int. J. Mol. Sci. 2017, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl. Oncol. 2015, 8, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.K.; Kang, S.; Elsliger, M.-A.; Gymnopoulos, M. Cancer-specific mutations in phosphatidylinositol 3-kinase. Trends Biochem. Sci. 2007, 32, 342–349. [Google Scholar] [CrossRef]
- Pollock, J.; Larrea, M.D.; Jasper, J.S.; McDonnell, D.P.; McCafferty, D.G. Lysine-Specific Histone Demethylase 1 Inhibitors Control Breast Cancer Proliferation in ERα-Dependent and -Independent Manners. ACS Chem. Biol. 2012, 7, 1221–1231. [Google Scholar] [CrossRef]
- Oh, S.; You, E.; Ko, P.; Jeong, J.; Keum, S.; Rhee, S. Genetic disruption of tubulin acetyltransferase, αTAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017, 482, 8–14. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Sugita, S.; Enokida, H.; Yoshino, H.; Miyamoto, K.; Yonemori, M.; Sakaguchi, T.; Osako, Y.; Nakagawa, M. HRAS as a potential therapeutic target of salirasib RAS inhibitor in bladder cancer. Int. J. Oncol. 2018, 53, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, B.; Zhu, F. Molecular characterization of glutaminyl-peptide cyclotransferase(QPCT)in Scylla paramamosain and its role in Vibrio alginolyticus and white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2018, 78, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Matsuda, S.; Liu, D.; Corey, E. Molecular-Cloning of the Human Gene Encoding Lanosterol Synthase from a Liver cDNA Library. Biochem. Biophys. Res. Commun. 1995, 213, 154–160. [Google Scholar] [CrossRef]
- Yang, H.; Xiang, S.; Kazi, A.; Sebti, S.M. The GTPase KRAS suppresses the p53 tumor suppressor by activating the NRF2-regulated antioxidant defense system in cancer cells. J. Biol. Chem. 2020, 295, 3055–3063. [Google Scholar] [CrossRef]
- Graff, J.R.; Konicek, B.W.; Carter, J.H.; Marcusson, E.G. Targeting the Eukaryotic Translation Initiation Factor 4E for Cancer Therapy: Figure 1. Cancer Res. 2008, 68, 631–634. [Google Scholar] [CrossRef]
- Taylor, K.M.; Ray, D.W.; Sommer, P. Glucocorticoid receptors in lung cancer: New perspectives. J. Endocrinol. 2016, 229, R17–R28. [Google Scholar] [CrossRef]
- Tian, D.; Tian, M.; Han, G.; Li, J.-L. Increased glucocorticoid receptor activity and proliferation in metastatic colon cancer. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Yaghoobi, H.; Azizi, H.; Banitalebi-Dehkordi, M.; Rezaei, F.M.; Arsang-Jnag, S.; Taheri, M.; Ghafouri-Fard, S. Beta-Secretase 1 (BACE1) Is Down-Regulated in Invasive Ductal Carcinoma of Breast. Rep. Biochem. Mol. Biol. 2019, 8, 200–207. [Google Scholar]
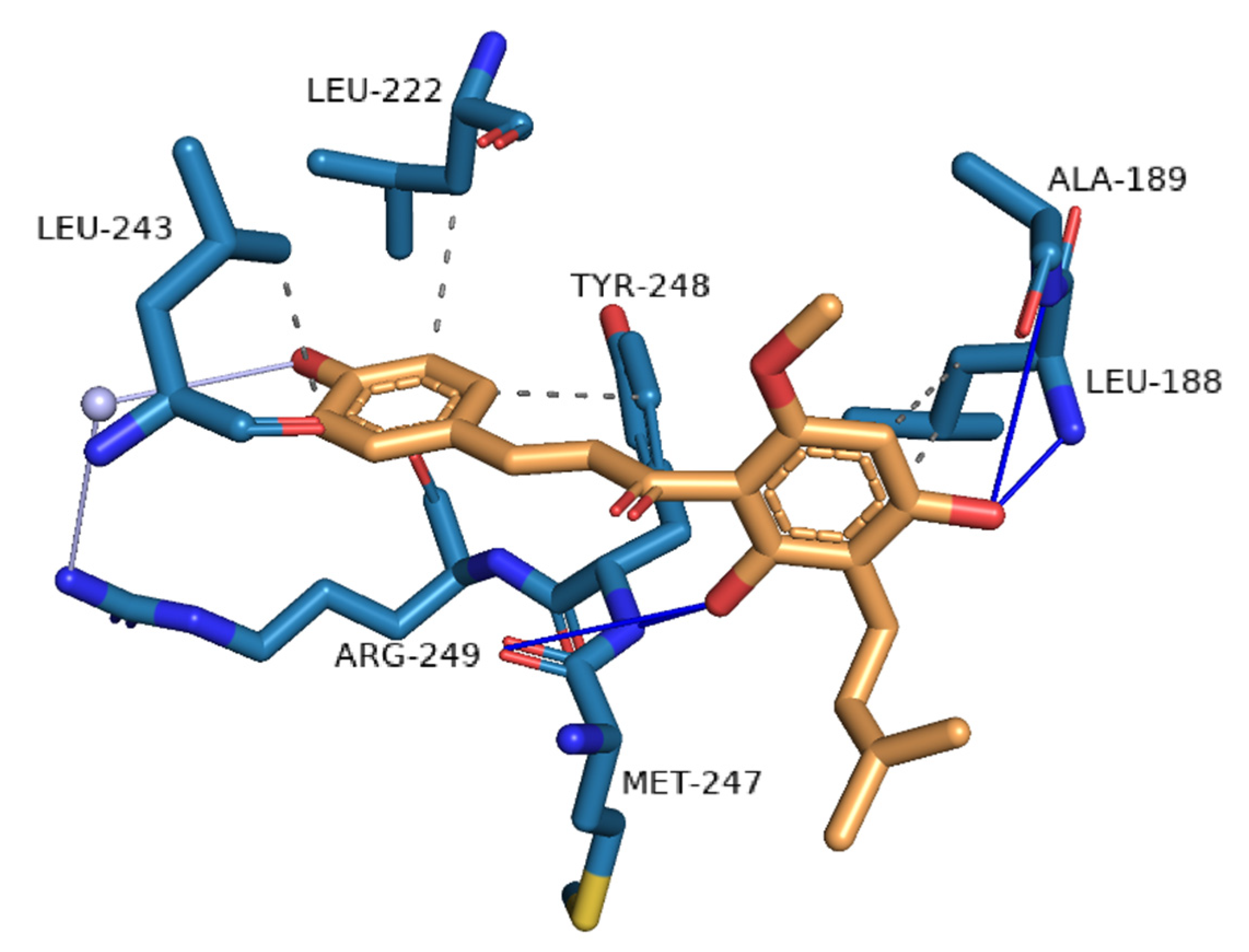
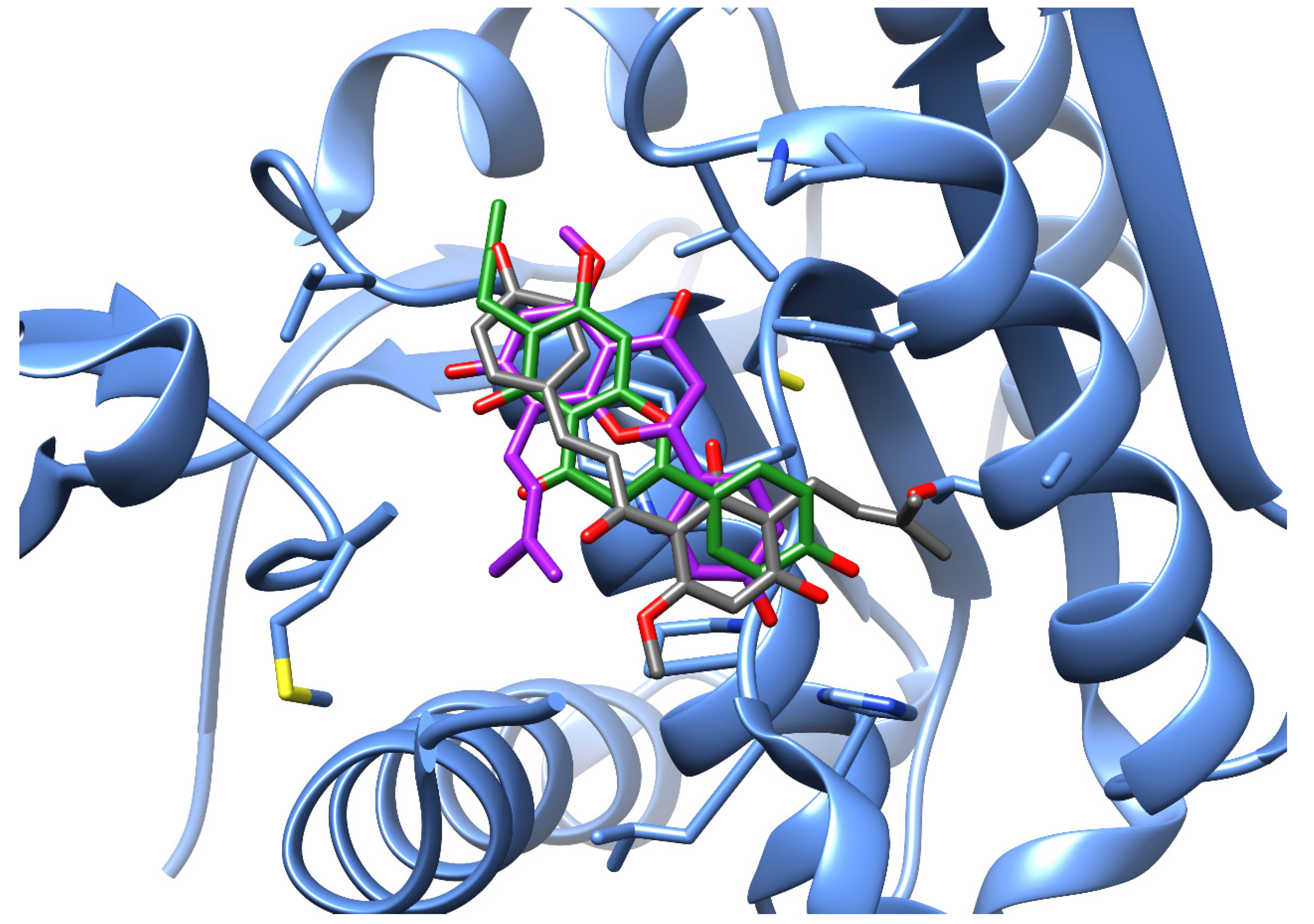
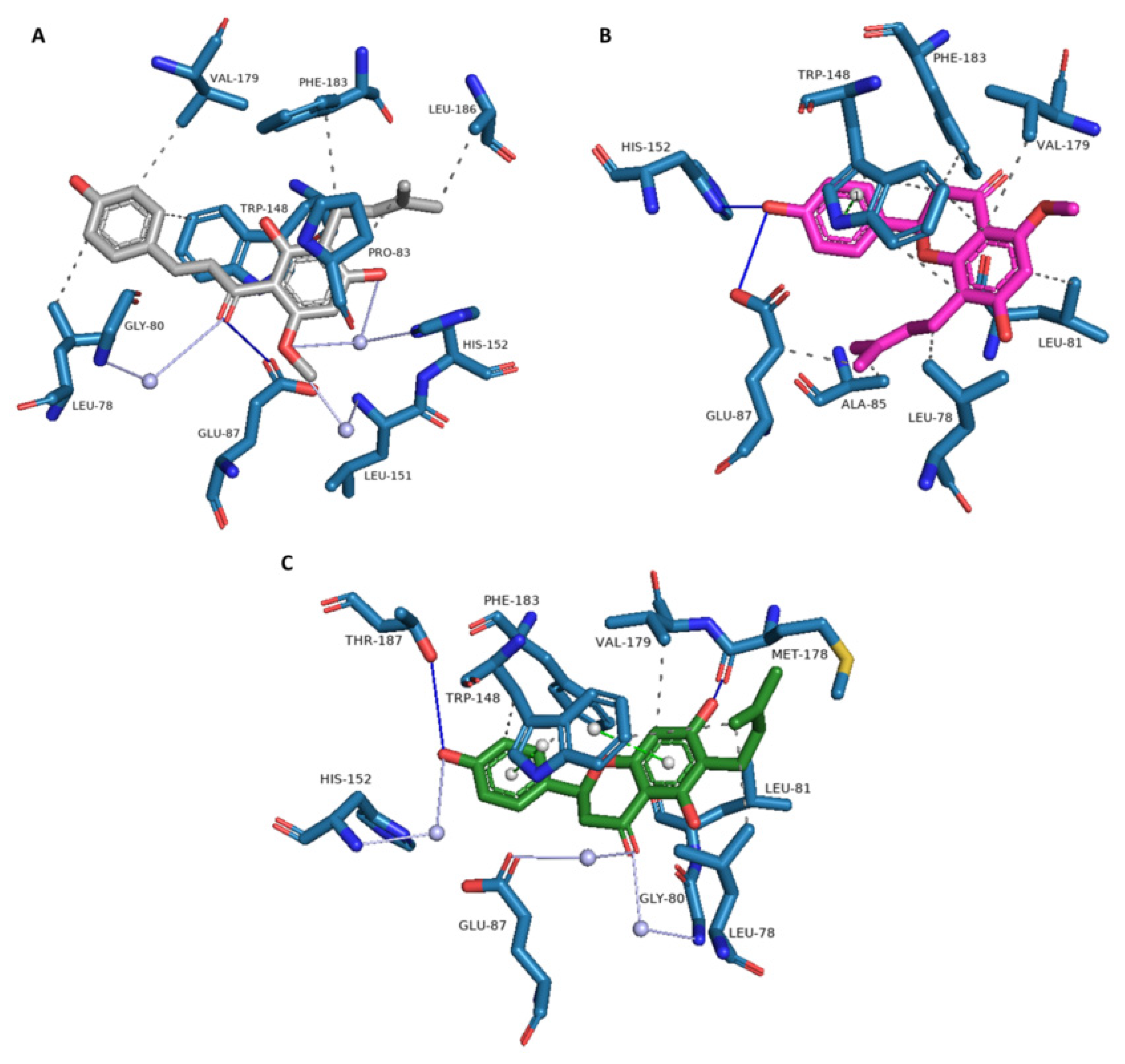
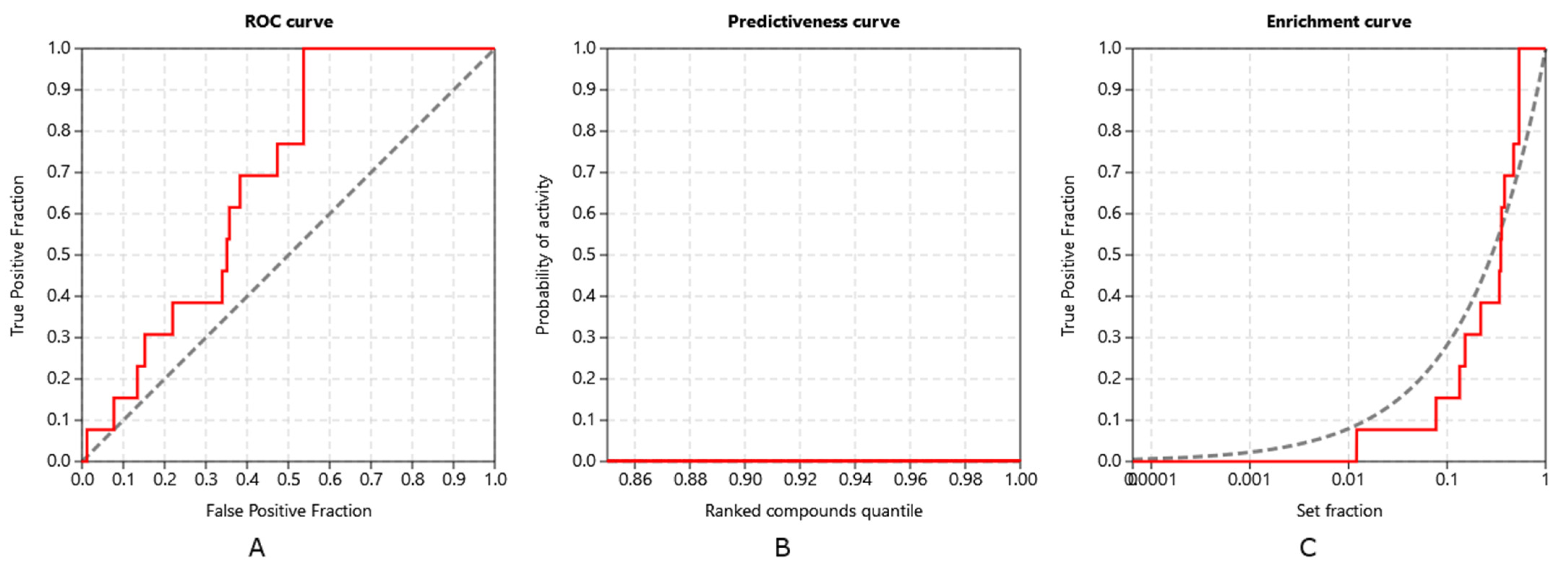
| PDB ID with Chain | Protein Name | Predicted Docking Score (arb. Units) * | Protein Function | Anticarcinogenic Function ** | Correlation with Xanthohumol *** |
|---|---|---|---|---|---|
| 5kjkA | N-lysine methyltransferase SMYD2 | −64.79 | Suppresses cell proliferation and directly regulates p53 function [32,33]. | Yes [32] | No |
| 5synA | Acyl-protein thioesterase 2 | −64.13 | Involved in depalmitoylation [34]. | Yes [34] | No |
| 3fedA | Glutamate carboxypeptidase III | −62.50 | Involved in a variety of neuropathologies and malignancies such as glutamatergic neurotoxicity and prostate cancer [35]. | Yes [36] | No |
| 4y30A | Arginine N-methyltransferase 6 | −62.00 | Involved in the regulation of transcription process, signal transduction, human immunodeficiency virus pathogenesis, DNA damage response, and cell cycle progression [37,38,39]. | Yes [38] | No |
| 4jijA | Matrix metalloproteinase 9 | −59.85 | The main function of MMP-9 is proteolytic activity in the extracellular environment [40,41]. | Yes [40] | Yes [41] |
| 3e7oA | Mitogen-activated protein kinase 9 | −59.84 | The mitogen-activated protein kinase pathway controls the growth and survival of a broad spectrum of human tumors [42]. | Yes [42] | Yes [43] |
| 4zzxA | Poly [ADP-ribose] polymerase 2 | −59.83 | Involved in a number of cellular processes such as DNA repair, genomic stability, programmed cell death [44]. | Yes [45] | Yes [46,47] |
| 2ffqA | Ras-related protein Rab-6B | −59.78 | Protein has a regulatory role in the retrograde transport of cargo in neutral cells [48,49]. | Yes [49] | No |
| 4lhwA | Ras-related protein Rab-8A | −59.77 | Overactivity of Ras signaling can lead to cancer, and it was found in human tumors [49]. | No | No |
| 3ru0A | SET and MYND domain-containing protein 3 | −59.53 | Regulates chromatin during the development of myocardial and skeletal muscles [50]. | Yes [50] | No |
| 3ma2D | Matrix metalloproteinase-14 | −59.24 | Plays a critical role in conferring cells with the ability to remodel and penetrate the extracellular matrix [51]. | Yes [51] | No |
| 3lawA | Ras-related protein Rab-7a | −58.93 | Ras inhibitors have been studied as a treatment for cancer and other diseases with Ras overexpression [49]. | No | No |
| 1zd9A | ADP-ribosylation factor-like 10B | −57.56 | Physiological function of this protein is not known. | No | No |
| 1vzoA | Ribosomal protein S6 kinase alpha 5 | −57.32 | Involved in several pathways such as MAPK signaling pathway, adrenergic signaling in cardiomyocytes, TNF signaling pathway, and possesses several biochemical functions such as ATP binding, histone kinase activity (H3-S10 specific), magnesium ion binding [52]. | Yes [52] | No |
| 5fbeA | Complement factor D | −57.27 | The complement system plays an important role in the innate defense against common invading pathogens [53]. | No | No |
| 1mrqA | Aldo-keto reductase family 1 member C1 | −56.90 | Involved in maintaining steroid hormone homeostasis, prostaglandin metabolism, and metabolic activation of polycyclic aromatic hydrocarbons [54]. | Yes [54] | No |
| 2c73A | Amine oxidase (flavin-containing) B | −56.87 | Plays an important role in neuroactive, vasoactive amines and is correlated with the production of neurotoxins in Parkinson’s disease [55]. | No | No |
| 1zq9A | Probable dimethyladenosine transferase | −56.86 | Protein is involved in the pre-rRNA procedure, which leads to small-subunit rRNA production [56]. | Yes [56] | No |
| 5fa6A | NADPH- -cytochrome P450 reductase | −56.81 | Protein is the redox partner of various P450s involved in primary and secondary metabolism [57]. | No | No |
| 2h44A | cGMP-specific 3′,5′-cyclic phosphodiesterase | −56.65 | The protein catalyzes the hydrolysis of 3′,5′-cyclic nucleotides to their respective nucleoside 5′-monophosphates [58]. | No | No |
| 1ck7A | Gelatinase A | −56.53 | Protein is a member of the gelatin-binding structure group and forms part of the matrix metalloproteinases (MMPs) [59]. | Yes [59] | Yes [60] |
| 1t91A | Ras-related protein Rab-7 | −56.21 | Ras signaling proteins have been found in human tumors [49]. | No | No |
| 3ghvA | Dihydrofolate reductase | −56.01 | Converts dihydrofolate into tetrahydrofolate and plays a crucial role in cell metabolism and cellular growth [61]. | Yes [62] | No |
| 3gz9A | Peroxisome proliferator-activated receptor delta | −55.95 | Protein is involved in differentiation, lipid accumulation, directional sensing, polarization, and migration of keratinocytes [63,64]. | Yes [64] | No |
| 2bzgA | Thiopurine S-methyltransferase | −55.80 | Protein is an enzyme in the cytoplasm that is involved in catalyzing the S-methylation of thiopurine drugs [65]. | No | No |
| 1zc3A | Ras-related protein Ral-A | −55.56 | Because of presence of Ras proteins in tumor, the Ras inhibitors have been studied [49]. | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kores, K.; Kolenc, Z.; Furlan, V.; Bren, U. Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods 2022, 11, 1253. https://doi.org/10.3390/foods11091253
Kores K, Kolenc Z, Furlan V, Bren U. Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods. 2022; 11(9):1253. https://doi.org/10.3390/foods11091253
Chicago/Turabian StyleKores, Katarina, Zala Kolenc, Veronika Furlan, and Urban Bren. 2022. "Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites" Foods 11, no. 9: 1253. https://doi.org/10.3390/foods11091253
APA StyleKores, K., Kolenc, Z., Furlan, V., & Bren, U. (2022). Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods, 11(9), 1253. https://doi.org/10.3390/foods11091253