Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis
Abstract
1. Introduction
2. Materials and Methods
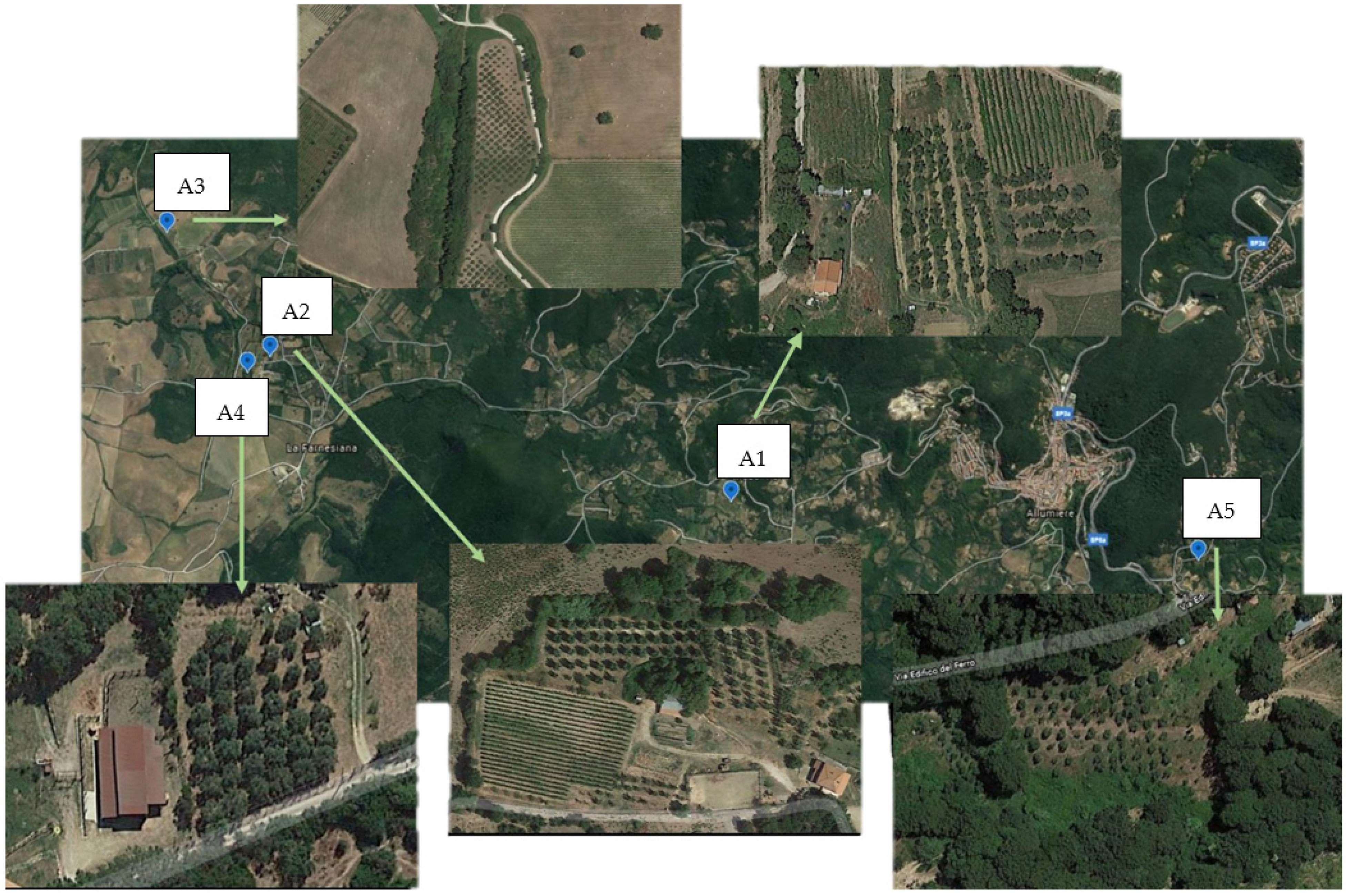
2.1. Sampling and Sampling Sites
2.2. Sample Pre-Treatment
2.3. Determination of Toxic and Potentially Toxic Elements in Olive Drupes and Leaves
2.3.1. Sample Dissolution
2.3.2. ICP-MS Analysis of Drupes
2.3.3. ICP-AES Analysis of Olive Leaves
2.4. Reagents and Glassware
2.5. LPAS Analysis
- minimum wavelength, maximum wavelength; and wavelength step;
- number of measurements at a given wavelength (each measurement lasts 1 s);
- and simply start the instrument from the PC that records the microphone signal and laser power per measurement. Usually, each point of the LPAS spectrum (photoacoustic signal) is given by the ratio of the average of microphone signals and the average of the laser powers.
2.6. Data Analysis and Statistics
3. Results
3.1. Elements in Drupes
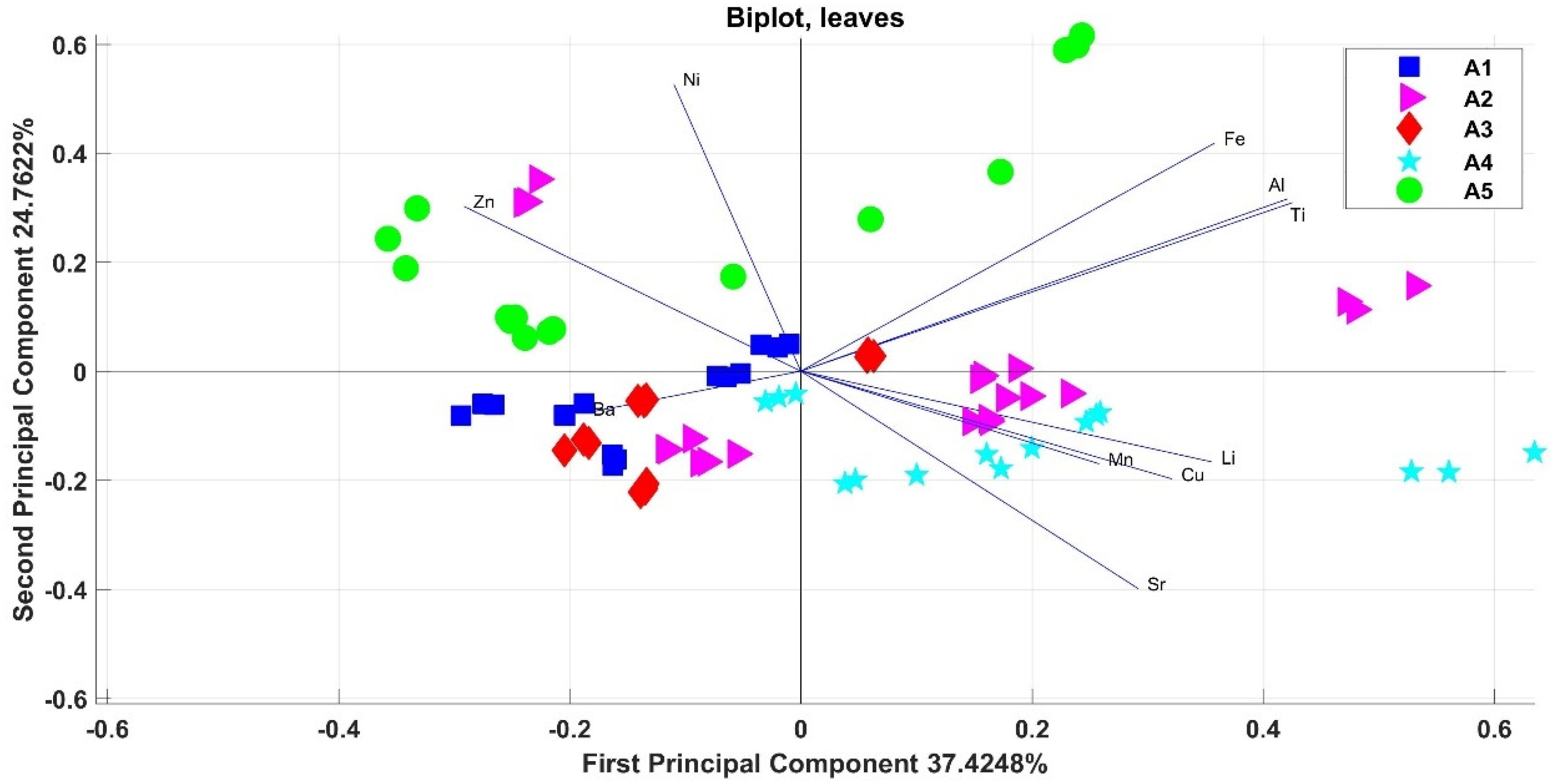
3.2. Elements in Olive Leaves
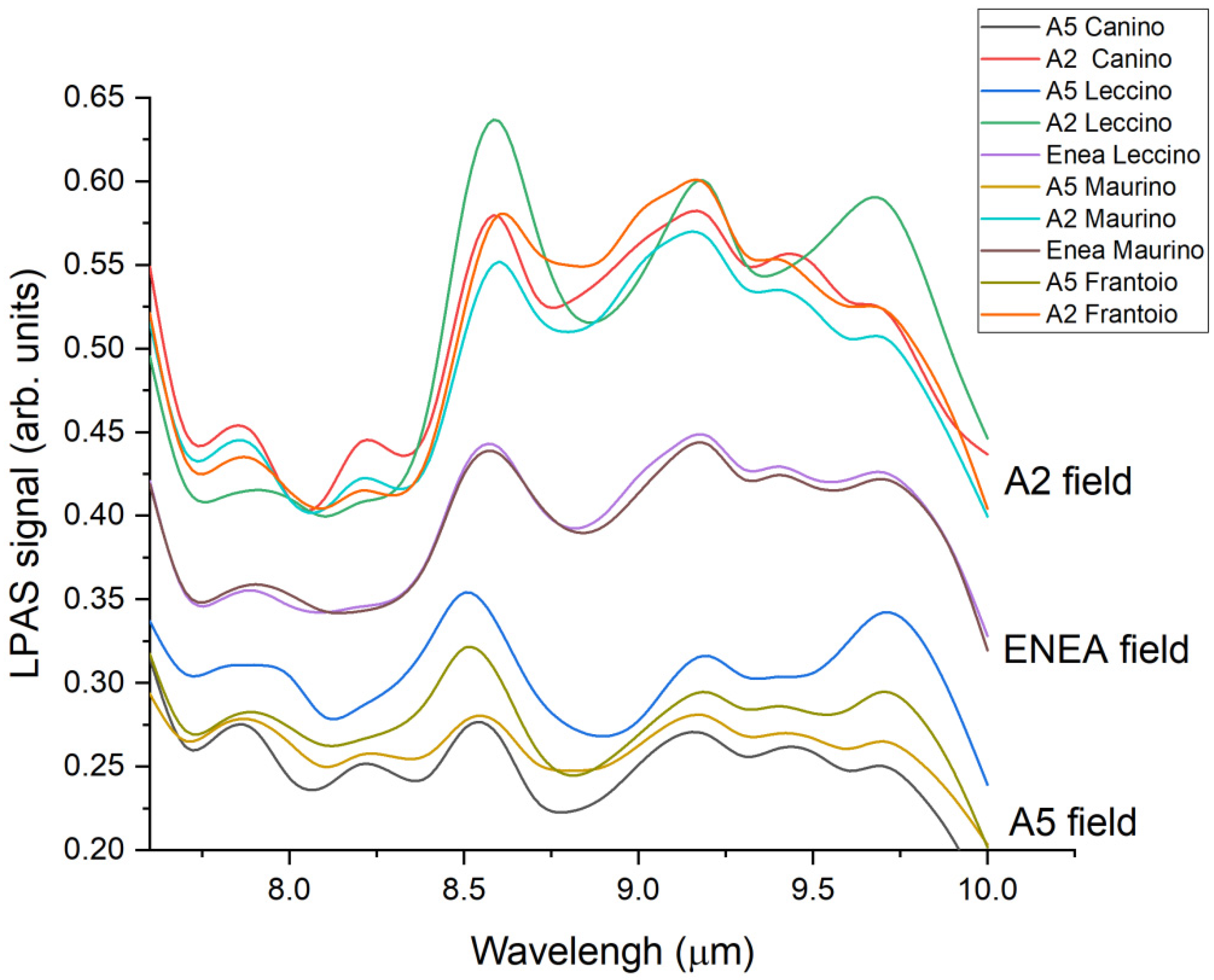
3.3. LPAS Results for Olive Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guerrero Maldonado, N.; López, M.J.; Caudullo, G.; de Rigo, D. Olea europaea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Commission: Luxembourg, 2016; p. 111. [Google Scholar] [CrossRef]
- Ben Ghorbal, A.; Leventdurur, S.; Agirman, B.; Boyaci-Gunduz, C.P.; Kelebek, H.; Carsanba, E.; Darici, M.; Erten, H. Influence of geographic origin on agronomic traits and phenolic content of cv. Gemlik olive fruits. J. Food Compos. Anal. 2018, 74, 1–9. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Agriculture and Rural Development, Study on the Implementation of Conformity Checks in the Olive Oil Sector Throughout the European Union Study on the Implementation of Conformity Checks in the Olive Oil Sector Throughout the European Union, Brussels. 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/606555af-46ff-11ea-b81b-01aa75ed71a1 (accessed on 9 March 2021).
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Lanza, B. Nutritional and Sensory Quality of Table Olives, Olive Germplasm. In The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/chapters/41339 (accessed on 9 March 2021). [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; González, D.G. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Fontanazza, G. Importance of olive-oil production in Italy. In Proceedings of the International Seminar “The Role and Importance of Integrated Soil and Water Management for Orchard Development”, Mosciano S. Angelo, Italy, 9–10 May 2004; Available online: http://www.fao.org/docrep/009/a0007e/a0007e00.htm (accessed on 9 March 2021).
- Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A. Determination of trace element in Italian virgin olive oils and their characterization according to geographical origin by statistical analysis. Anal. Chim. Acta 2007, 585, 366–370. [Google Scholar] [CrossRef]
- Damak, F.; Bougi, M.S.M.; Araoka, D.; Baba, K.; Furuya, M.; Ksibi, M.; Tamura, K. Soil geochemistry, edaphic and climatic characteristics as components of Tunisian olive terroirs: Relationship with the multielemental composition of olive oils for their geographical traceability. Euro-Mediterranean J. Environ. Integr. 2021, 6, 37. [Google Scholar] [CrossRef]
- Damak, F.; Asano, M.; Baba, K.; Ksibi, M.; Tamura, K. Comparison of Sample Preparation Methods for Multielements Analysis of Olive Oil by ICP-MS. Methods Protoc. 2019, 2, 72. [Google Scholar] [CrossRef]
- Cajka, T.; Riddellova, K.; Klimankova, E.; Cerna, M.; Pudil, F.; Hajslova, J. Traceability of olive oil based on volatiles pattern and multivariate analysis. Food Chem. 2010, 121, 282–289. [Google Scholar] [CrossRef]
- Angerosa, F.; Bréas, O.; Contento, S.; Guillou, C.; Reniero, F.; Sada, E. Application of Stable Isotope Ratio Analysis to the Characterization of the Geographical Origin of Olive Oils. J. Agric. Food Chem. 1999, 47, 1013–1017. [Google Scholar] [CrossRef]
- Benabid, H.; Naamoune, H.; Noçairi, H.; Rutledge, D.N. Application of chemometric tools to compare Algerian olive oils produced in different locations. J. Food Agric. Environ. 2008, 6, 43–51. [Google Scholar]
- Di Bella, G.; Maisano, R.; La Pera, L.; Turco, V.L.; Salvo, F.; Dugo, G. Statistical Characterization of Sicilian Olive Oils from the Peloritana and Maghrebian Zones According to the Fatty Acid Profile. J. Agric. Food Chem. 2007, 55, 6568–6574. [Google Scholar] [CrossRef] [PubMed]
- Luykx, D.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Sinelli, N.; Casale, M.; Di Egidio, V.; Oliveri, P.; Bassi, D.; Tura, D.; Casiraghi, E. Varietal discrimination of extra virgin olive oils by near and mid infrared spectroscopy. Food Res. Int. 2010, 43, 2126–2131. [Google Scholar] [CrossRef]
- Zeiner, M.; Steffan, I.; Cindric, I.J. Determination of trace elements in olive oil by ICP-AES and ETA-AAS: A pilot study on the geographical characterization. Microchem. J. 2005, 81, 171–176. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.; Sacco, A. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Cindrić, I.J.; Zeiner, M.; Steffan, I. Comparison of methods for inorganic trace element analysis in croatian olive oils. Acta Agron. Hung. 2008, 56, 33–40. [Google Scholar] [CrossRef]
- Hidalgo, M.J.; Pozzi, M.T.; Furlong, O.J.; Marchevsky, E.J.; Pellerano, R.G. Classification of organic olives based on chemometric analysis of elemental data. Microchem. J. 2018, 142, 30–35. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Benincasa, C.; Gharsallaoui, M.; Perri, E.; Bati, C.B.; Ayadi, M.; Khlif, M.; Gabsi, S. Quality and Trace Element Profile of Tunisian Olive Oils Obtained from Plants Irrigated with Treated Wastewater. Sci. World J. 2012, 2012, 535781. [Google Scholar] [CrossRef][Green Version]
- Lai, A.; Almaviva, S.; Spizzichino, V.; Addari, L.; Giubileo, G.; Dipoppa, G.; Palucci, A. Innovative devices for biohazards and food contaminants. EAI Spec. Issue ENEA Technol. Secur. 2015, 56–63. [Google Scholar]
- Giubileo, G.; Lai, A.; Piccinelli, D.; Puiu, A. Laser diagnostic technology for early detection of pathogen infestation in orange fruits. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 623, 778–781. [Google Scholar] [CrossRef]
- Puiu, A.; Giubileo, G.; Lai, A. Investigation of Plant—Pathogen Interaction by Laser-Based Photoacoustic Spectroscopy. Int. J. Thermophys. 2014, 35, 2237–2245. [Google Scholar] [CrossRef]
- Puiu, A.; Fiorani, L.; Giubileo, G.; Lai, A.; Mannori, S.; Saleh, W. QCL-based photoacoustic spectroscopy applied to rice flour analysis. Food Sci. Eng. 2021, 2, 65–76. [Google Scholar] [CrossRef]
- Fiorani, L.; Artuso, F.; Giardina, I.; Lai, A.; Mannori, S.; Puiu, A. Photoacoustic Laser System for Food Fraud Detection. Sensors 2021, 21, 4178. [Google Scholar] [CrossRef] [PubMed]
- Shlens, J. A Tutorial on Principal Component Analysis. arXiv 2014, arXiv:1404.1100. [Google Scholar]
- Pearson, K. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Miranda, A.A.; Le Borgne, Y.-A.; Bontempi, G. New Routes from Minimal Approximation Error to Principal Components. Neural Process. Lett. 2008, 27, 197–207. [Google Scholar] [CrossRef]
- Rutan, S.C.; de Juan, A.; Tauler, R. Introduction to Multivariate Curve Resolution. In Comprehensive Chemometrics; Elsevier: Amsterdam, The Netherlands, 2009; pp. 249–259. ISBN 9780444527011. [Google Scholar]
- Davis, J.C. Statistics and Data Analysis in Geology, 3rd ed.; Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–638. [Google Scholar] [CrossRef]
- Wagner, P.J.; Harper, D.A.T. Numerical Palaeobiology: Computer-Based Modelling and Analysis of Fossils and Their Distributions. PALAIOS 2000, 15, 364–366. [Google Scholar] [CrossRef]
- Diana, G.; Tommasi, C. Cross-validation methods in principal component analysis: A comparison. Stat. Methods Appl. 2002, 11, 71–82. [Google Scholar] [CrossRef]
- Damak, F.; Asano, M.; Baba, K.; Suda, A.; Araoka, D.; Wali, A.; Isoda, H.; Nakajima, M.; Ksibi, M.; Tamura, K. Interregional traceability of Tunisian olive oils to the provenance soil by multielemental fingerprinting and chemometrics. Food Chem. 2019, 283, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Sahan, Y. Chapter 32—Some Metals in Table Olives. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 299–306. ISBN 9780123744203. [Google Scholar] [CrossRef]
- López-López, A.; López, R.; Madrid, F.; Garrido-Fernández, A. Heavy Metals and Mineral Elements Not Included on the Nutritional Labels in Table Olives. J. Agric. Food Chem. 2008, 56, 9475–9483. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Azizi, M.; Rezaee, R.; Giesy, J.P.; Karimi, G. Polycyclic aromatic hydrocarbons, pesticides, and metals in olive: Analysis and probabilistic risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 39723–39741. [Google Scholar] [CrossRef]
- Yücel, Y.; Kılıçoğlu, A.L. Determination of heavy metals in olive fruits as an indicator of environmental pollution. Int. J. Environ. Anal. Chem. 2020, 100, 922–934. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]





| Time (min) | E (W) | T1 (°C) | T2 (°C) | P (bar) | |
|---|---|---|---|---|---|
| 1° step | 15:00 | 1500 | 200 | 70 | 120 |
| 2° step | 15:00 | 1500 | 200 | 70 | 120 |
| Year | Cultivar | Sr | Cu | Rb | Ti | Cr | Ni | V | As | Sn | Zr | Co | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | Cipresso | 18.3 | 12.54 | 4.89 | 1.094 | 0.532 | 0.352 | 0.111 | 0.033 | 0.033 | 0.021 | 0.016 | 0.013 |
| 2019 | Pendolino | 12.4 | 12.45 | 4.67 | 0.575 | 0.191 | 0.228 | 0.076 | 0.025 | 0.002 | 0.018 | 0.024 | 0.009 |
| 2019 | Itrana | 8.9 | 11.42 | 4.48 | 0.800 | 0.314 | 0.328 | 0.110 | 0.042 | 0.010 | 0.013 | 0.010 | 0.005 |
| 2019 | Frantoio | 12.9 | 13.85 | 5.69 | 0.581 | 0.394 | 0.366 | 0.122 | 0.019 | 0.033 | 0.024 | 0.012 | 0.039 |
| 2019 | Uovo di piccione | 15.0 | 13.70 | 5.85 | 0.675 | 0.589 | 0.644 | 0.126 | 0.029 | 0.036 | 0.019 | 0.019 | 0.031 |
| 2019 | Moraiolo | 11.5 | 9.20 | 3.36 | 0.538 | 0.430 | 0.120 | 0.124 | 0.090 | 0.013 | 0.019 | 0.027 | 0.050 |
| 2019 | Maurino | 12.4 | 22.52 | 6.59 | 1.148 | 0.420 | 0.517 | 0.118 | 0.028 | 0.031 | 0.016 | 0.037 | 0.037 |
| 2019 | Ortice | 11.6 | 15.38 | 1.95 | 5.770 | 0.325 | 0.104 | 0.111 | 0.045 | 0.008 | 0.022 | 0.032 | 0.017 |
| 2019 | Leccino | 15.6 | 9.06 | 3.33 | 1.020 | 0.226 | 0.242 | 0.080 | 0.034 | 0.015 | 0.017 | 0.027 | 0.040 |
| 2019 | Canino | 15.7 | 10.34 | 4.29 | 0.645 | 0.213 | 0.228 | 0.101 | 0.056 | 0.002 | 0.021 | 0.012 | 0.020 |
| 2019 | Ascolana | 8.8 | 17.37 | 5.33 | 0.516 | 0.358 | 0.238 | 0.134 | 0.017 | 0.002 | 0.008 | 0.011 | 0.052 |
| Mean | 13.0 | 13.44 | 4.58 | 1.215 | 0.363 | 0.306 | 0.110 | 0.038 | 0.017 | 0.018 | 0.021 | 0.028 | |
| Std.dev. | 2.9 | 3.93 | 1.33 | 1.528 | 0.128 | 0.162 | 0.018 | 0.021 | 0.014 | 0.005 | 0.009 | 0.016 | |
| Median | 12.4 | 12.54 | 4.67 | 0.675 | 0.358 | 0.242 | 0.111 | 0.033 | 0.013 | 0.019 | 0.019 | 0.031 | |
| Min. | 8.8 | 9.06 | 1.95 | 0.516 | 0.191 | 0.104 | 0.076 | 0.017 | 0.002 | 0.008 | 0.010 | 0.005 | |
| Max. | 18.3 | 22.52 | 6.59 | 5.770 | 0.589 | 0.644 | 0.134 | 0.090 | 0.036 | 0.024 | 0.037 | 0.052 | |
| D.L. (mg/kg d.w.) | 0.0003 | 0.002 | 0.001 | 0.002 | 0.002 | 0.0001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.001 | 0.002 | |
| Limit value—Reg. (CE) N.1881/2006 s.m.i.—mg/kg | . | 0.1 | |||||||||||
| Fields | Cultivar | Rb | Cu | Sr | Ti | Cr | Ni | V | Co | As | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Frantoio | 13.78 | 9.06 | 5.72 | 0.400 | 0.332 | 0.204 | 0.066 | 0.022 | 0.031 | 0.003 |
| A1 | Uovo di piccione | 9.16 | 15.15 | 3.40 | 0.243 | 0.193 | 0.179 | 0.046 | 0.024 | 0.014 | <0.002 |
| A1 | Leccino | 13.73 | 14.77 | 4.67 | 0.254 | 0.310 | 0.337 | 0.079 | 0.028 | 0.002 | 0.004 |
| A1 | Pendolino | 8.78 | 12.68 | 3.94 | 0.319 | 0.177 | 0.406 | 0.049 | 0.028 | 0.023 | 0.013 |
| A1 | Canino | 10.22 | 7.09 | 4.42 | 0.434 | 0.199 | 0.194 | 0.071 | 0.022 | 0.014 | 0.003 |
| A2 | Frantoio | 14.77 | 12.31 | 6.65 | 0.093 | 0.442 | 0.261 | 0.124 | 0.038 | 0.002 | <0.002 |
| A2 | Uovo di piccione | 11.02 | 14.11 | 4.03 | 0.139 | 0.353 | 0.333 | 0.133 | 0.016 | 0.014 | 0.018 |
| A2 | Leccino | 9.89 | 12.68 | 5.67 | 0.119 | 0.334 | 0.335 | 0.104 | 0.051 | 0.016 | 0.003 |
| A2 | Pendolino | 9.95 | 11.62 | 5.56 | 0.137 | 0.192 | 0.336 | 0.053 | 0.070 | 0.007 | 0.007 |
| A2 | Maurino | 13.63 | 8.38 | 7.26 | 0.149 | 0.128 | 0.127 | 0.036 | 0.018 | 0.002 | <0.002 |
| A2 | Ascolana | 11.34 | 17.13 | 4.37 | 0.188 | 0.265 | 0.173 | 0.069 | 0.030 | 0.012 | 0.018 |
| A2 | Canino | 11.34 | 10.25 | 5.11 | 0.111 | 0.191 | 0.146 | 0.049 | 0.039 | 0.008 | <0.002 |
| A3 | Frantoio | 19.94 | 12.04 | 18.93 | 0.224 | 0.159 | 0.097 | 0.059 | 0.038 | 0.007 | 0.017 |
| A3 | Pendolino | 17.73 | 9.02 | 8.96 | 0.184 | 0.096 | 0.047 | 0.044 | 0.015 | 0.026 | <0.002 |
| A3 | Maurino | 18.10 | 8.76 | 32.18 | 0.138 | 0.260 | 0.068 | 0.092 | 0.052 | 0.011 | <0.002 |
| A3 | Canino | 18.28 | 9.04 | 9.37 | 0.147 | 0.089 | 0.044 | 0.040 | 0.022 | 0.018 | <0.002 |
| A4 | Frantoio | 17.18 | 10.71 | 7.75 | 0.124 | 0.188 | 0.088 | 0.083 | 0.060 | 0.006 | 0.003 |
| A4 | Leccino | 10.93 | 10.95 | 4.28 | 0.139 | 0.143 | 0.054 | 0.062 | 0.051 | 0.002 | 0.006 |
| A4 | Pendolino | 11.00 | 11.59 | 4.70 | 0.172 | 0.225 | 0.112 | 0.089 | 0.060 | 0.002 | 0.005 |
| A4 | Ascolana | 15.33 | 10.10 | 5.56 | 0.092 | 0.260 | 0.063 | 0.085 | 0.113 | 0.008 | <0.002 |
| A4 | Moraiolo | 14.70 | 10.64 | 6.53 | 0.099 | 0.171 | 0.076 | 0.075 | 0.054 | 0.002 | <0.002 |
| A5 | Frantoio | 8.65 | 10.10 | 2.60 | 0.121 | 0.302 | 0.270 | 0.079 | 0.001 | 0.020 | 0.003 |
| A5 | Cipresso | 14.88 | 13.82 | 4.83 | 0.129 | 0.314 | 0.613 | 0.114 | 0.016 | 0.020 | 0.004 |
| A5 | leccino | 8.72 | 12.68 | 2.88 | 0.102 | 0.573 | 0.119 | 0.156 | 0.014 | 0.010 | 0.032 |
| A5 | Maurino | 10.84 | 8.79 | 3.29 | 0.114 | 0.290 | 0.301 | 0.107 | 0.002 | 0.013 | <0.002 |
| A5 | Ascolana | 10.50 | 11.76 | 2.47 | 0.125 | 0.238 | 0.598 | 0.099 | <0.001 | 0.027 | 0.007 |
| Mean | 12.86 | 11.36 | 6.74 | 0.173 | 0.247 | 0.215 | 0.079 | 0.035 | 0.012 | 0.009 | |
| Std.dev. | 3.37 | 2.38 | 6.11 | 0.090 | 0.108 | 0.158 | 0.031 | 0.025 | 0.008 | 0.008 | |
| Median | 11.34 | 11.27 | 4.97 | 0.139 | 0.232 | 0.176 | 0.077 | 0.028 | 0.011 | 0.006 | |
| Min. | 8.65 | 7.09 | 2.47 | 0.092 | 0.089 | 0.044 | 0.036 | 0.001 | 0.002 | 0.003 | |
| Max. | 19.94 | 17.13 | 32.18 | 0.434 | 0.573 | 0.613 | 0.156 | 0.113 | 0.031 | 0.032 | |
| D.L. (mg/kg d.w.) | 0.001 | 0.002 | 0.0003 | 0.002 | 0.002 | 0.0001 | 0.001 | 0.001 | 0.002 | 0.002 | |
| Year | Cultivar | Al | Sr | Fe | Ba | Rb | Mn | Zn | Cu | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | Cipresso | 127 | 225 | 70.7 | 58.5 | 77 | 20.13 | 13.8 | 6.9 | 3.1 |
| 2019 | Pendolino | 89 | 157 | 60.4 | 188.8 | 117 | 17.08 | 12.5 | 5.3 | 2.2 |
| 2019 | Itrana | 81 | 117 | 57.4 | 92.5 | 183 | 16.71 | 9.6 | 5.7 | 2.0 |
| 2019 | Frantoio | 69 | 189 | 46.3 | 284.3 | 168 | 19.18 | 15.8 | 4.7 | 1.5 |
| 2019 | Uovo di piccione | 116 | 203 | 67.1 | 250.7 | 65 | 19.14 | 9.8 | 4.7 | 2.6 |
| 2019 | Moraiolo | 170 | 232 | 93.3 | 43.3 | 75 | 22.13 | 13.3 | 4.9 | 4.4 |
| 2019 | Maurino | 108 | 166 | 64.3 | 169.6 | 160 | 18.30 | 11.2 | 5.8 | 2.5 |
| 2019 | Ortice | 124 | 284 | 77.9 | 98.6 | 110 | 25.81 | 9.9 | 4.9 | 2.9 |
| 2019 | Leccino | 88 | 261 | 61.4 | 117.6 | 164 | 22.76 | 9.5 | 4.2 | 2.2 |
| 2019 | Canino | 93 | 167 | 55.7 | 194.6 | 151 | 17.77 | 11.9 | 5.3 | 2.3 |
| 2019 | Ascolana | 100 | 160 | 59.5 | 96.1 | 117 | 21.76 | 12.2 | 5.1 | 2.5 |
| Mean | 106 | 196 | 64.9 | 145.0 | 126.1 | 20.07 | 11.8 | 5.2 | 2.6 | |
| Std.dev. | 28 | 50 | 12.5 | 78.4 | 41.7 | 2.78 | 2.0 | 0.7 | 0.7 | |
| Median | 100 | 189 | 61.4 | 117.6 | 117.1 | 19.18 | 11.9 | 5.1 | 2.5 | |
| Min. | 69 | 117 | 46.3 | 43.3 | 64.6 | 16.71 | 9.5 | 4.2 | 1.5 | |
| Max. | 170 | 284 | 93.3 | 284.3 | 182.7 | 25.81 | 15.8 | 6.9 | 4.4 | |
| D.L. (mg/kg d.w.) | 5 | 5 | 0.2 | 0.1 | 5 | 0.04 | 0.1 | 0.4 | 0.5 |
| Fields | Cultivar | Al | Sr | Fe | Ba | Rb | Mn | Zn | Cu | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Frantoio | 36 | 34 | 29.3 | 9.8 | 23 | 6.17 | 8.5 | 5.4 | 0.8 |
| A1 | Uovo di piccione | 53 | 32 | 32.1 | 20.0 | 19 | 8.60 | 12.2 | 6.6 | 1.1 |
| A1 | Leccino | 31 | 26 | 27.0 | 8.2 | 23 | 11.20 | 16.6 | 7.2 | 0.6 |
| A1 | Pendolino | 62 | 35 | 41.4 | 13.0 | 43 | 14.27 | 15.4 | 10.4 | 1.2 |
| A1 | Canino | 72 | 35 | 45.0 | 11.8 | 49 | 17.68 | 19.2 | 7.7 | 1.4 |
| A2 | Frantoio | 84 | 43 | 44.7 | 5.8 | <5 | 20.53 | 7.7 | 10.4 | 1.7 |
| A2 | Uovo di piccione | 54 | 13 | 45.3 | 8.0 | <5 | 21.14 | 22.7 | 10.4 | 1.1 |
| A2 | Leccino | 134 | 35 | 64.1 | 5.3 | <5 | 26.59 | 6.2 | 29.3 | 2.4 |
| A2 | Pendolino | 61 | 45 | 48.8 | 7.9 | 94 | 27.04 | 9.6 | 15.2 | 1.4 |
| A2 | Maurino | 78 | 33 | 44.7 | 4.3 | 88 | 12.70 | 8.1 | 14.0 | 1.7 |
| A2 | Ascolana | 34 | 31 | 32.4 | 3.4 | 68 | 14.92 | 9.1 | 9.9 | 0.8 |
| A2 | Canino | 36 | 41 | 33.5 | 6.3 | 80 | 13.32 | 12.4 | 7.2 | 0.9 |
| A3 | Frantoio | 30 | 27 | 26.1 | 5.3 | 72 | 30.08 | 11.4 | 4.8 | 0.7 |
| A3 | Pendolino | 50 | 24 | 33.5 | 6.1 | 62 | 19.24 | 10.3 | 3.9 | 0.9 |
| A3 | Maurino | 27 | 33 | 29.2 | 6.4 | 58 | 39.65 | 9.2 | 4.1 | 0.6 |
| A3 | Canino | 78 | 30 | 45.9 | 7.1 | 70 | 29.27 | 10.4 | 4.1 | 1.4 |
| A4 | Frantoio | 49 | 35 | 32.2 | 6.2 | <5 | 27.97 | 8.9 | 49.9 | 1.0 |
| A4 | Leccino | 92 | 47 | 49.8 | 4.6 | <5 | 36.75 | 7.2 | 74.5 | 1.9 |
| A4 | Pendolino | 49 | 21 | 38.0 | 2.0 | <5 | 32.53 | 13.3 | 48.5 | 1.0 |
| A4 | Ascolana | 72 | 26 | 42.3 | 2.7 | <5 | 47.77 | 7.8 | 77.5 | 1.6 |
| A4 | Moraiolo | 66 | 44 | 38.1 | 7.1 | <5 | 38.67 | 12.6 | 64.7 | 1.3 |
| A5 | Frantoio | 43 | 12 | 34.6 | 7.2 | 18 | 14.92 | 13.8 | 6.8 | 0.8 |
| A5 | Cipresso | 117 | 21 | 82.0 | 3.8 | 10 | 18.01 | 12.8 | 5.0 | 2.3 |
| A5 | Leccino | 88 | 13 | 51.8 | 2.1 | 28 | 15.18 | 13.5 | 8.6 | 2.0 |
| A5 | Maurino | 52 | 17 | 35.4 | 15.0 | 26 | 12.07 | 11.1 | 4.6 | 1.0 |
| A5 | Ascolana | 39 | 8 | 27.2 | 4.0 | 7 | 16.91 | 18.7 | 5.9 | 0.9 |
| Mean | 61 | 29 | 40.5 | 7.1 | 48 | 22.04 | 11.9 | 19.1 | 1.2 | |
| Std.dev. | 27 | 11 | 12.4 | 4.1 | 28 | 10.71 | 4.1 | 23.1 | 0.5 | |
| Median | 53 | 31 | 38.1 | 6.3 | 49 | 18.63 | 11.2 | 8.2 | 1.1 | |
| Min. | 27 | 8 | 26.1 | 2.0 | 7 | 6.17 | 6.2 | 3.9 | 0.6 | |
| Max. | 134 | 47 | 82.0 | 20.0 | 94 | 47.77 | 22.7 | 77.5 | 2.4 | |
| D.L. (mg/kg d.w.) | 5 | 5 | 0.2 | 0.1 | 5 | 0.04 | 0.1 | 0.4 | 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, E.; Palumbo, D.; Puiu, A.; Lai, A.; Fiorani, L.; Zoani, C. Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis. Foods 2022, 11, 1085. https://doi.org/10.3390/foods11081085
Pucci E, Palumbo D, Puiu A, Lai A, Fiorani L, Zoani C. Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis. Foods. 2022; 11(8):1085. https://doi.org/10.3390/foods11081085
Chicago/Turabian StylePucci, Emilia, Domenico Palumbo, Adriana Puiu, Antonia Lai, Luca Fiorani, and Claudia Zoani. 2022. "Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis" Foods 11, no. 8: 1085. https://doi.org/10.3390/foods11081085
APA StylePucci, E., Palumbo, D., Puiu, A., Lai, A., Fiorani, L., & Zoani, C. (2022). Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis. Foods, 11(8), 1085. https://doi.org/10.3390/foods11081085









