Effect of Different Watering Regimes on Olive Oil Quality and Composition of Coratina Cultivar Olives Grown on Karst Soil in Croatia
Abstract
1. Introduction
2. Materials and Methods
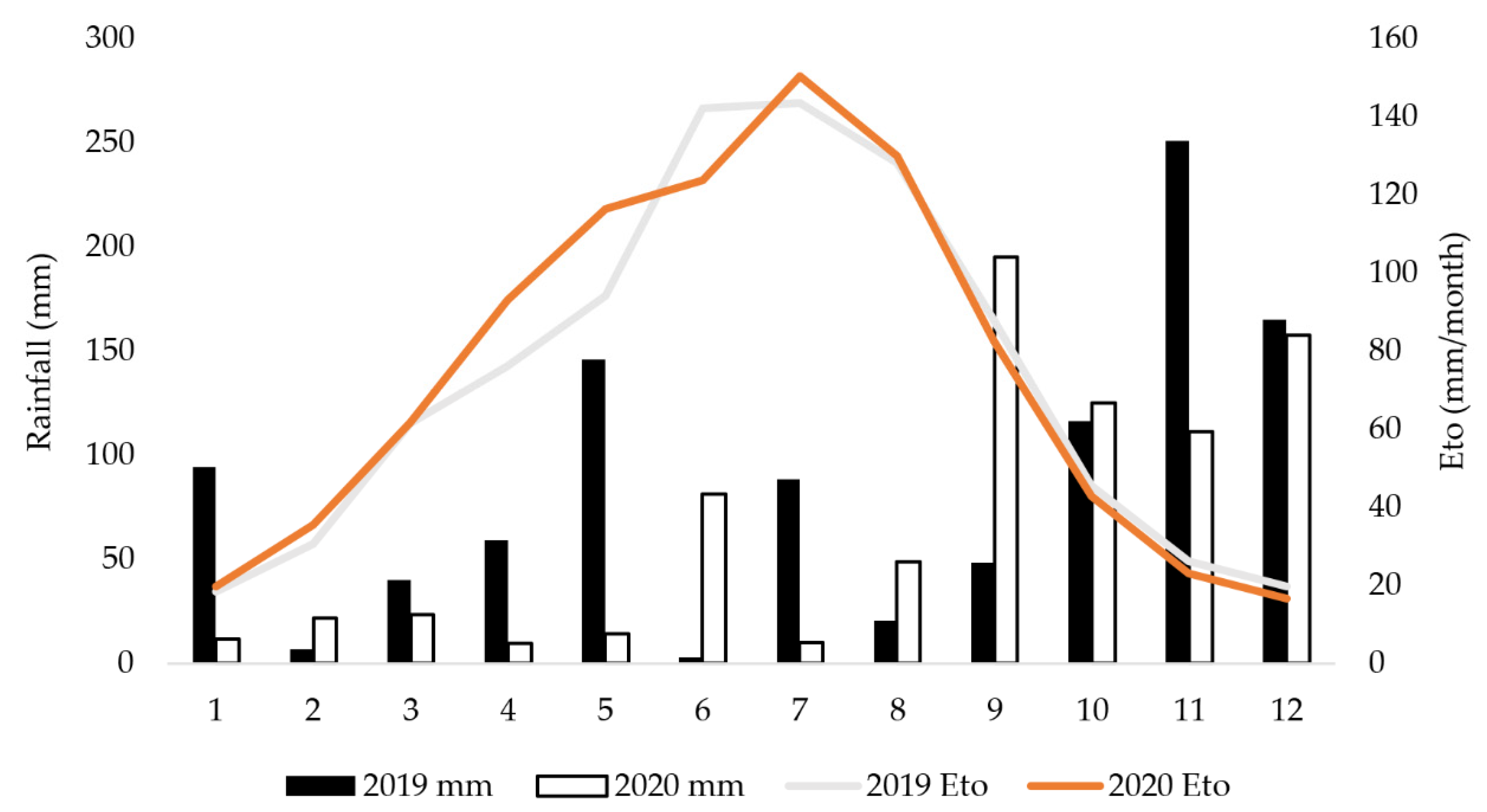
2.1. Plant Material and Growing Area Characteristics
2.2. Watering Regimes
2.3. Harvest of Olive Fruits and Virgin Olive Oil Production
2.4. Virgin Olive Oil Analyses
2.4.1. Oil Content and Oil Yield
2.4.2. Quality parameters of Virgin Olive Oils
2.4.3. Analysis of Pigments in Virgin Olive Oils
2.4.4. Analysis of Fatty Acid Methyl Esters (FAME)
2.4.5. Analysis of Volatile Compounds
2.4.6. Analysis of Phenolic Compounds
2.4.7. Radical-Scavenging Activity Determination
2.4.8. Sensory Analysis
2.4.9. Data Elaboration
3. Results and Discussion
3.1. Influence on Oil Content and Oil Yield
3.2. Influence on VOOs Quality and Composition
3.2.1. Quality Parameters
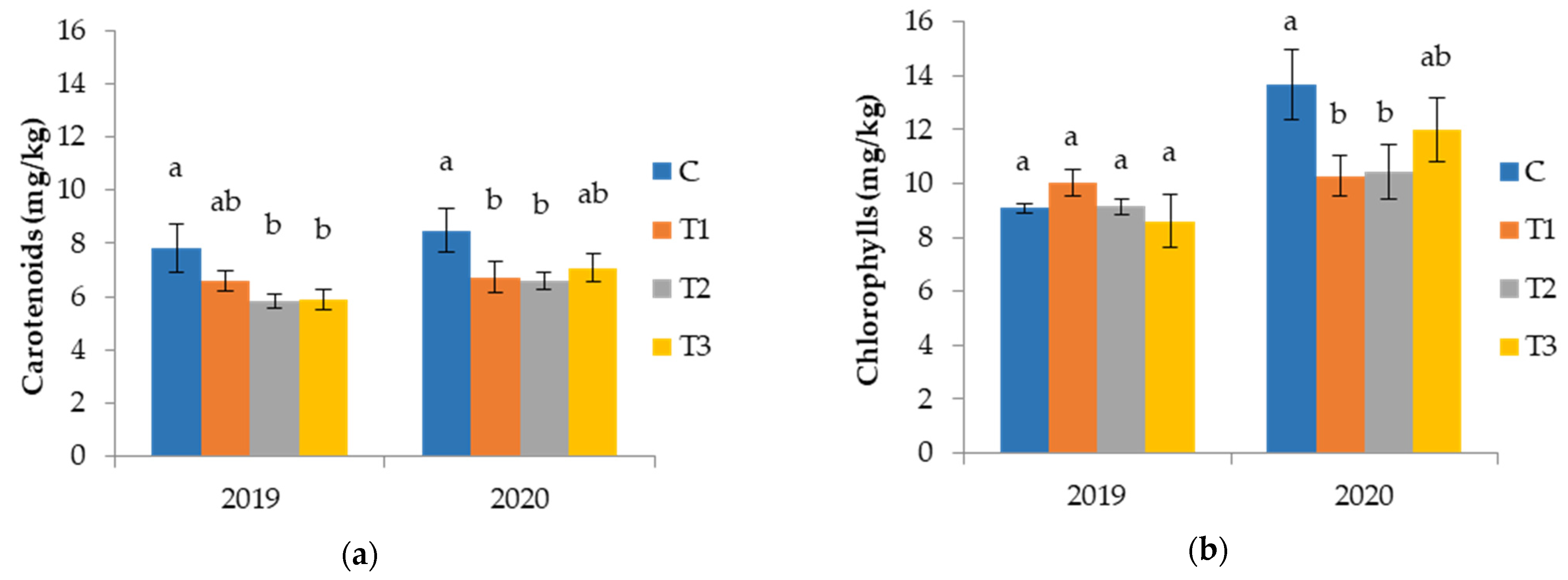
3.2.2. Pigments
3.2.3. Fatty Acid Methyl Esters
3.2.4. Volatile Compounds
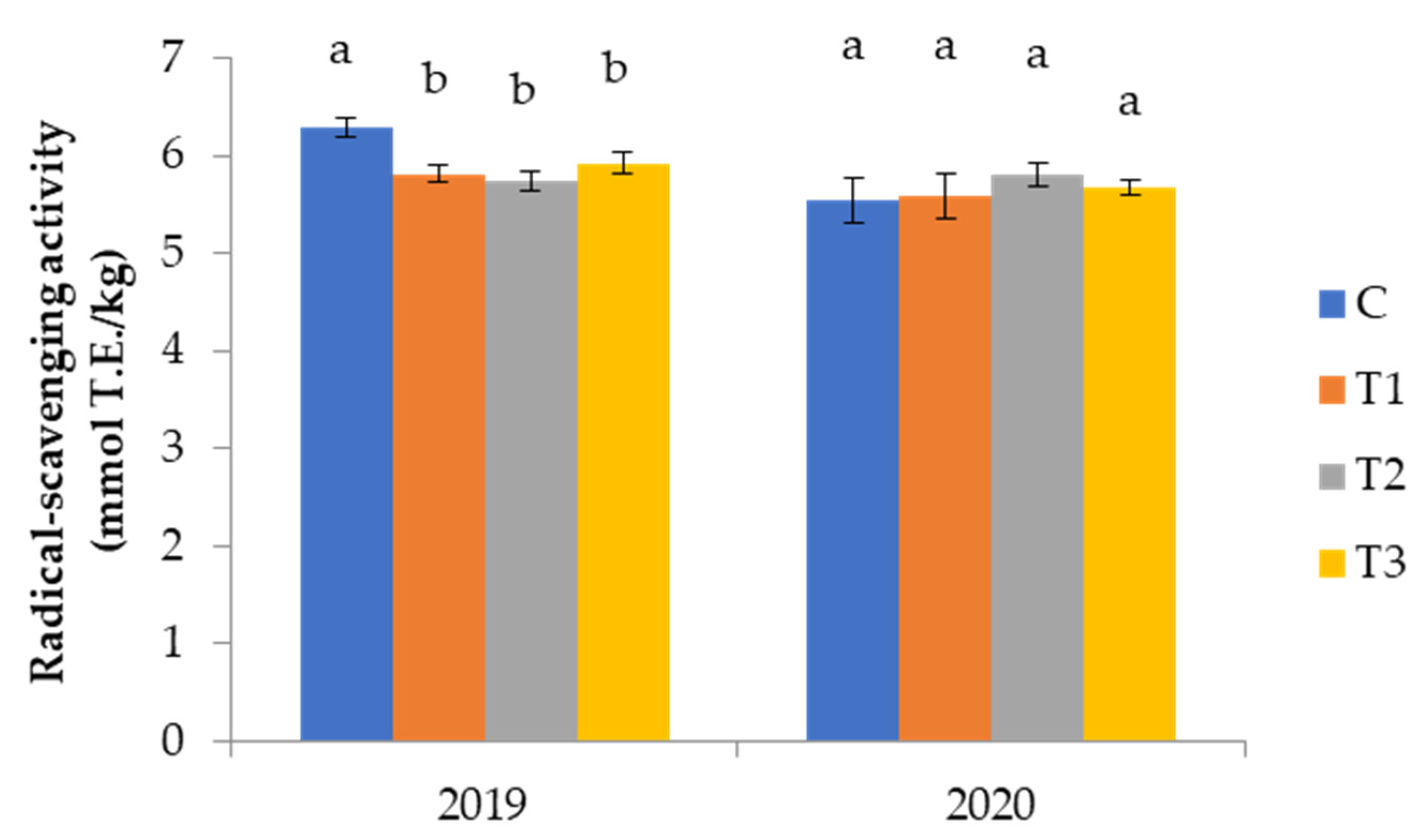
3.2.5. Phenolic Compounds and Antioxidant Capacity
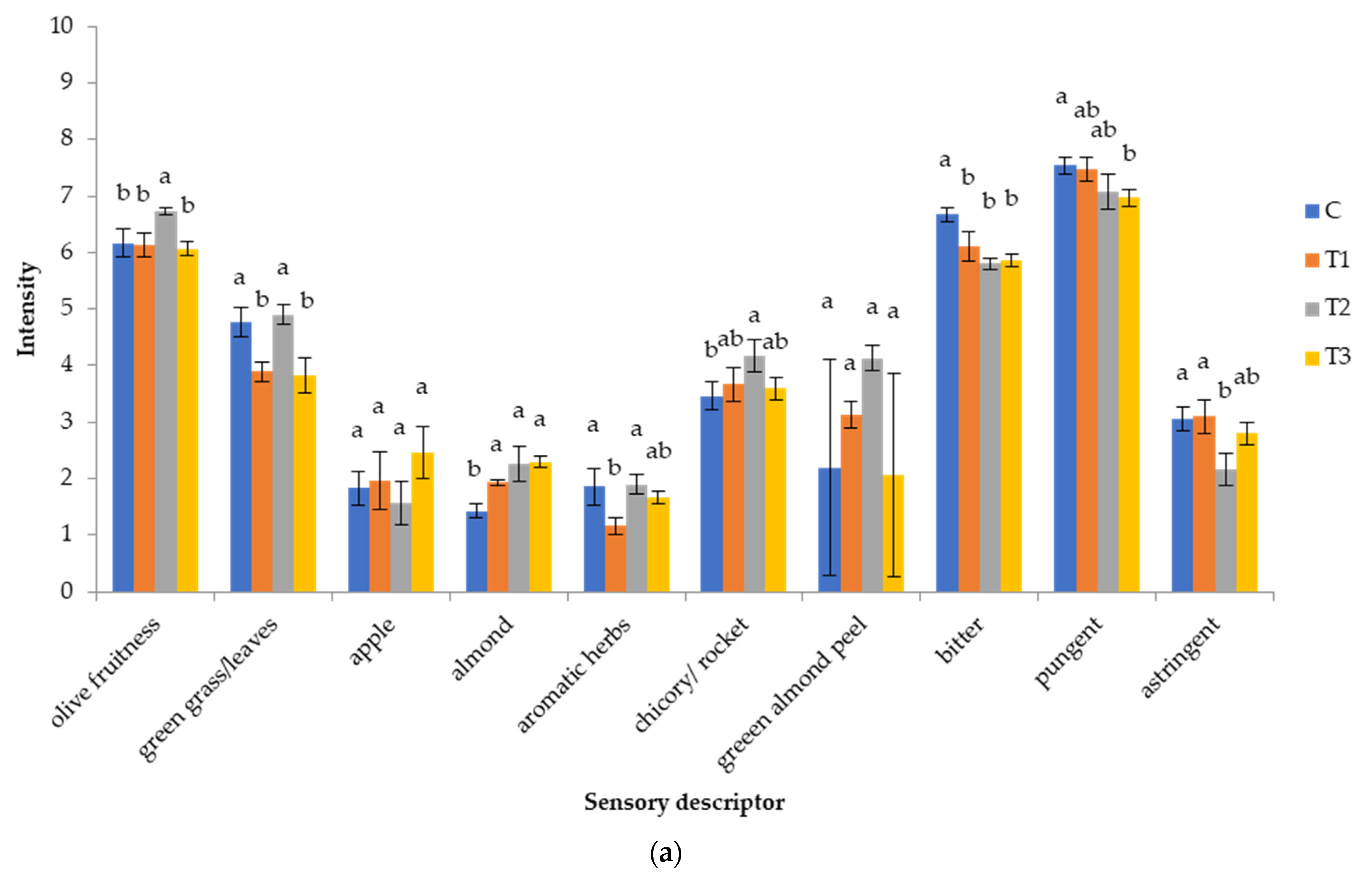
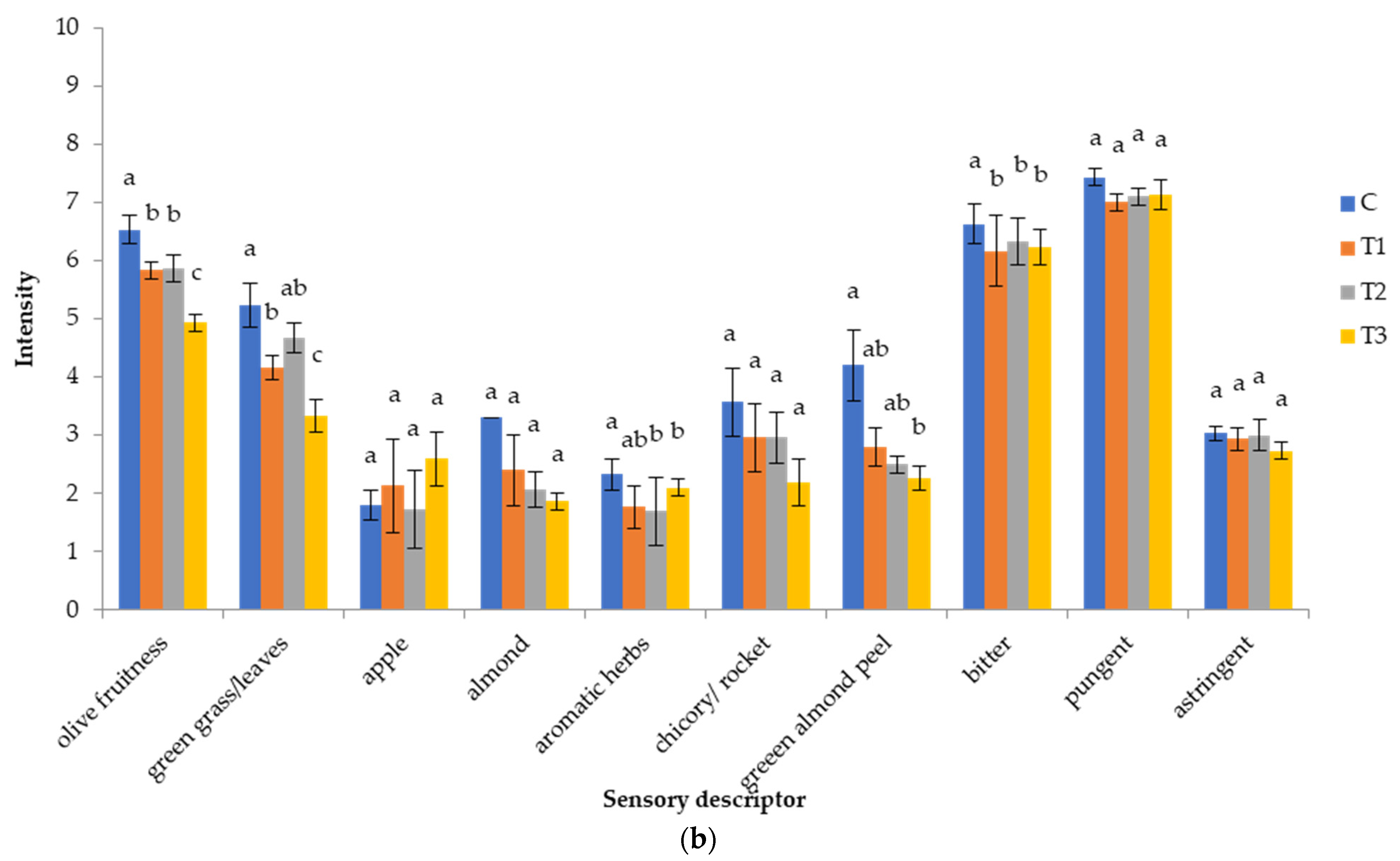
3.2.6. Sensory Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rapoport, H.F.; Fabbri, A.; Sebastiani, L. Olive Biology. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 13–25. [Google Scholar]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. In Horticultural Reviews; Janick, L., Ed.; John Wiley & Sons, Inc.: West Lafayette, IN, USA, 2004; pp. 155–229. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistical Dataset; FAO: Rome, Italy, 2018. [Google Scholar]
- Croatian Bureau of Statistics. Available online: https://web.dzs.hr/PX-Web.asp?url=“Hrv/Archive/stat_databases.htm (accessed on 15 March 2022).
- Strikić, F.; Gugić, J.; Klepo, T. Status overview of the olive growing in Croatia. Glas. Biljn. Zaštite 2012, 4, 271–276. Available online: https://hrcak.srce.hr/168826 (accessed on 22 February 2022). (In Croatian).
- Benlloch-González, M.; Sanchez-Lucas, R.; Benlloch, M.; Fernández-Escobar, R. An approach to global warming effects on flowering and fruit set of olive trees growing under field conditions. Sci. Hortic. 2018, 240, 405–410. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High temperature environment reduces olive oil yield and quality. PLoS ONE 2020, 4, e0231956. [Google Scholar] [CrossRef] [PubMed]
- Faghim, J.; Ben Mohamed, M.; Bagues, M.; Guasmi, F.; Triki, T.; Nagaz, K. Irrigation effects on phenolic profile and extra virgin olive oil quality of “Chemlali” variety grown in South Tunisia. S. Afr. J. Bot. 2021, 141, 322–329. [Google Scholar] [CrossRef]
- Batelja-Lodeta, K.; Čorluka, V.; Gugić, J.; Očić, V.; Šakić-Bobić, B.; Kereša, S.; Bolarić, S.; Perčin, A.; Pohajda, I.; Gadže, J. Climate change and olive growing. Glas. Zaštite Bilja 2021, 44, 62–72. (In Croatian) [Google Scholar] [CrossRef]
- Šikić, Z.; Pernar, N.; Yerkovich, B.B.; Rogošić, J.; Širac., S. Influence of water levels of Vrana Lake and the Adriatic Sea to the water chemistry of Vrana lake. Acta Adriatica Int. J. Marine Sci. 2013, 54, 199–212. [Google Scholar]
- Croatian Meteorological and Hydrological Service (CMHS), in Croatian. Available online: www.dhmz.hr (accessed on 12 November 2019).
- Ponti, L.; Gutierrez, A.P.; Ruti, P.M.; Dell´Aquila, A. Fine-scale ecological and economic assessment of climate change on olive in the Mediterranean Basin reveals winners and losers. Proc. Nalt. Acad. Sci. USA 2014, 111, 5598–5603. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef]
- Fregapane, G.; Gómez-Rico, A.; Salvador, M.D. Influence of irrigation management and ripening on virgin olive oil quality and composition. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: La Mancha, Spain, 2010; pp. 51–58. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of different irrigation volumes during fruit development on quality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Dabbou, S.; Brahmi, F.; Selvaggini, R.; Chehab, H.; Dabbou, S.; Taticchi, A.; Servili, M.; Hammami, M. Contribution of irrigation and cultivars to volatile profile and sensory attributes of selected virgin olive oils produced in Tunisia. Int. J. Food Sci. Technol. 2011, 46, 1964–1976. [Google Scholar] [CrossRef]
- Fernández, J.E.; Diaz-Espejo, A.; Romero, R.; Hernandez-Santana, V.; García, J.M.; Padilla-Díaz, C.M.; Cuevas, M.V. Precision irrigation in Olive (Olea europaea L.) tree orchards. In Water Scarcity and Sustainable Agriculture in Semiarid Environment. Tools, Strategies, and Challenges for Woody Crops; García Tejero, I.F., Durán Zuazo, V.H., Eds.; Academic Press: Cordoba, Spain, 2018; pp. 179–217. [Google Scholar] [CrossRef]
- Saiz-Rubio, V.; Rovira-Más, F. From Smart Farming towards Agriculture 5.0: A Review on Crop Data Management. Agronomy 2020, 10, 207. [Google Scholar] [CrossRef]
- Smart Agriculture Network. Available online: https://epl.hr/san (accessed on 11 May 2022).
- Džaja, K. Geomorphological characteristics of Dugi Otok island, Croatia. Geoadria 2003, 8, 5–44. [Google Scholar] [CrossRef][Green Version]
- Šegota, T.; Filipčić, A. Köppen’s classification of climates and the problem of corresponding Croatian terminology. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef]
- PinovaMeteo Agriculture Weather Station. Available online: https://pinova-meteo.com (accessed on 12 November 2021).
- Stuhne, G. Analytical Report for Water Quality no 19/otp/23125; Euroinspect Croatiakontrola: Zagreb, Croatia, 2019. [Google Scholar]
- Stuhne, G. Analytical Report for Water Quality no 20/otp/21119; Euroinspect Croatiakontrola: Zagreb, Croatia, 2020. [Google Scholar]
- Husnjak, S.; Magdić, I.; Balog, N. Characteristics of Anthropogenic Soils of Olive Groves in the Area of Novigrad in Flat Districts and Žman on Dugi Otok; Elaborate; University of Zagreb, Faculty of Agriculture: Zagreb, Croatia, 2019; pp. 1–14. [Google Scholar]
- Barranco, D.; Cimato, A.; Fiorino, P.; Rallo, L.; Touzani, A.; Castaneda, C.; Serafini, F.; Trujillo, I. World Catalogue of Olive Varieties; International Olive Oil Council (IOC): Madrid, Spain, 2000; p. 360. [Google Scholar]
- Caponio, F.; Gomes, T.; Pasqualone, A. Phenolic compounds in virgin olive oils: Influence of the degree of olive ripeness on organoleptic characteristics and shelf-life. Eur. Food Res. Technol. 2001, 212, 329–333. [Google Scholar] [CrossRef]
- Večernik, N. Olives: Economic Importance, Origin, Botanical Affiliation, Ecology and Properties of Olives, Cultivation, Care, Protection and Processing of Olives; Mimica, R., Večernik, N., Eds.; Adria Book d.o.o.: Split, Croatia, 1994; pp. 41–97. [Google Scholar]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological growth stages of olive trees (Olea europea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- d´Andria, R.; Lavini, A.; Tombesi, A.; Tombesi, S. Irrigation. In Production Techniques in Olive Growing; Artegraf, S.A., Ed.; International Olive Council: Madrid, Spain, 2007; pp. 169–209. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Beltrán, G.; Del Río, C.; Sánchez, S.; Martínez, L. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric. 2004, 84, 1783–1790. [Google Scholar] [CrossRef]
- Brkić, K.; Radulović, M.; Sladonja, B.; Lukić, I.; Šetić, E. Application of Soxtec apparatus for oil content determination in olive fruit. Riv. Ital. Sostanze Grasse 2006, 83, 115–119. [Google Scholar]
- Koprivnjak, O.; Brkić Bubola, K.; Kosić, U. Sodium chloride compared to talc as processing aid has similar impact on volatile compounds but more favorable on ortho-diphenols in virgin olive oil. Eur. J. Lipid Sci. Technol. 2016, 118, 318–324. [Google Scholar] [CrossRef]
- EEC. Characteristics of olive oil and olive-residue oil and the relevant methods of analysis. Regulation EEC/2568/91 and later modifications. Off. J. Eur. Community 1991, L24, 1–83. Available online: http://data.europa.eu/eli/reg/1991/2568/oj (accessed on 12 March 2022).
- Mínguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sánchez-Gòmez, A.H.; Garrido-Fernandez, J. Color—Pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Lukić, I.; Koprivnjak, O. Effect of different clarification methods on volatile aroma compound composition of virgin olive oil. Food Technol. Biotechnol. 2019, 57, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Škevin, D.; Valić, S.; Majetić, V.; Petričević, S.; Ljubenkov, I. The antioxidant capacity and oxidative stability of virgin olive oil enriched with phospholipids. Food Chem. 2008, 111, 121–126. [Google Scholar] [CrossRef]
- International Olive Council. Sensory Analysis of Olive Oil: Method for the Organoleptic Assessment of Virgin Olive Oil; COI/T.20/Doc. No 15/Rev.10; International Olive Council: Madrid, Spain, 2018; Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (accessed on 14 March 2022).
- Hernández, M.L.; Velázquez-Palmero, D.; Sicardo, M.D.; Fernández, J.E.; Diaz-Espejo, A.; Martínez-Rivas, J.M. Effect of a regulated deficit irrigation strategy in a hedgerow ‘Arbequina’ olive orchard on the mesocarp fatty acid composition and desaturase gene expression with respect to olive oil quality. Agric. Water Manag. 2018, 204, 100–106. [Google Scholar] [CrossRef]
- Pierantozzi, P.; Torres, M.; Tivani, M.; Contreras, C.; Gentili, L.; Parera, C.; Maestri, D. Spring deficit irrigation in olive (cv. Genovesa) growing under arid continental climate: Effects on vegetative growth and productive parameters. Agric. Water Manag. 2020, 238, 106212. [Google Scholar] [CrossRef]
- Moriana, A.; Pérez López, D.; Gómez-Rico, A.; Salvador, M.; Olmedilla, N.; Ribas, F.; Fregapane, G. Irrigation scheduling for traditional, low-density olive orchards: Water relations and influence on oil characteristics. Agric. Water Manag. 2007, 87, 171–179. [Google Scholar] [CrossRef]
- Tovar, M.J.; Romero, M.P.; Alegre, S.; Girona, J.; Motilva, M.J. Composition and organoleptic characteristics of oil from Arbequina olive (Olea europaea L.) trees under deficit irrigation. J. Sci. Food Agric. 2002, 82, 1755–1763. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Sifola, M.I.; Selvaggini, R.; Urbani, S.; Esposto, S. Irrigation and fruit canopy position modify oil quality of olive trees (cv. Frantoio). J. Sci. Food Agric. 2017, 97, 3530–3539. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. Fruit growth, yield and oil quality changes induced by deficit irrigation at different stages of olive fruit development. Agric. Water Manag. 2019, 212, 88–98. [Google Scholar] [CrossRef]
- Sdiri, W.; Dabbou, S.; Chehab, H.; Selvaggini, R.; Servili, M.; Di Bella, G.; Ben Mansour, H. Quality characteristics and chemical evaluation of Chemlali olive oil produced under dairy wastewater irrigation. Agric. Water Manag. 2020, 236, 106124. [Google Scholar] [CrossRef]
- Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Sikorska, E.; Gomes, T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol. 2005, 221, 92–98. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Vivaldi, G.A.; Nicolás, E.N.; Paduano, A.; Salcedo, F.P.; Camposeo, S. Ripening indices, olive yield and oil quality in response to irrigation with saline reclaimed water and deficit strategies. Front. Plant Sci. 2019, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, M.; El Hassan, S.; Boussakouran, A.; Rharrabti, Y. Influence of ripening index and water regime on the yield and quality of “Moroccan Picholine” virgin olive oil. OCL 2020, 27, 11. [Google Scholar] [CrossRef]
- García, J.M.; Hueso, A.; Gómez-del-Campo, M. Deficit irrigation during the oil synthesis period affects olive oil quality in high-density orchards (cv. Arbequina). Agric. Water Manag. 2020, 230, 105858. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.A.; Falco, V.; Correia, C.M.; Villalobos, F.J. Sensory analysis and volatile compounds of olive oil (cv. Cobrançosa) from different irrigation regimes. Grasas Aceites 2013, 64, 59–67. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Desamparados, M.S.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
| Treatment * | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Amount (l) | Rate Number | Saved Water (l) | ||||
| C | 0 | 0 | 0 | 0 | 800 | 1800 |
| T1 | 448 | 1393 | 5 | 11 | 352 | 407 |
| T2 | 560 | 1261 | 8 | 19 | 240 | 539 |
| T3 | 800 | 1800 | 8 | 19 | 0 | 0 |
| Phenological Stages | BBCH * [30] | 2019 | 2020 |
|---|---|---|---|
| Flowering | 61–68 | 3/5–15/5 | 2/5–12/5 |
| Fruit set | 69 | 16/5–20/5 | 13/5–19/5 |
| Pit hardening | 75 | 10/7–23/7 | 11/7–20/7 |
| Oil accumulation | 79–89 | 10/8–10/9 | 10/8–10/9 |
| Parameter | 2019 | 2020 | ||||||
|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | |
| Oil yield (%) | 5.55 ± 0.76 b | 8.83 ± 0.50 a | 9.09 ± 0.20 a | 9.31 ± 0.95 a | 5.58 ± 0.32 c | 8.69 ± 0.21 b | 9.44 ± 0.55 ab | 10.11 ± 0.50 a |
| Dry matter (%) | 44.79 ± 1.87 a | 47.14 ± 0.75 a | 47.16 ± 0.45 a | 46.96 ± 2.12 a | 42.91 ± 1.00 a | 43.96 ± 1.11 a | 45.00 ± 0.40 a | 44.66 ± 0.63 a |
| Moisture (%) | 55.21 ± 1.87 a | 52.86 ± 0.75 a | 52.84 ± 0.45 a | 53.04 ± 2.12 a | 57.09 ± 1.00 a | 56.04 ± 1.11 a | 55.00 ± 0.40 a | 55.34 ± 0.63 a |
| Oil on dry weight basis (%) | 21.29 ± 0.74 b | 28.04 ± 0.95 a | 29.69 ± 2.01 a | 30.98 ± 0.93 a | 27.16 ± 1.87 b | 37.54 ± 1.44 a | 36.65 ± 1.79 a | 38.87 ± 0.70 a |
| Oil on fresh weight basis (%) | 11.76 ± 0.74 b | 14.83 ± 0.62 a | 15.69 ± 1.17 a | 16.42 ± 0.52 a | 15.51 ± 1.18 b | 21.04 ± 1.12 a | 20.16 ± 1.04 a | 21.51 ± 0.22 a |
| 2019 | 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | EVOO * | |
| FFA % (oleic acid) | 0.20 ± 0.00 a | 0.19 ± 0.01 ab | 0.18 ± 0.01 ab | 0.17 ± 0.01 b | 0.19 ± 0.00 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.18 ± 0.01 a | ≤2.50 |
| PV (meq O2/kg) | 2.34 ± 0.05 a | 1.89 ± 0.02 b | 1.63 ± 0.05 c | 1.68 ± 0.08 c | 2.21 ± 0.06 a | 1.56 ± 0.03 b | 1.19 ± 0.11 c | 1.06 ± 0.06 c | ≤20.0 |
| K232 | 1.94 ± 0.07 a | 1.93 ± 0.12 a | 1.82 ± 0.10 a | 1.97 ± 0.07 a | 1.91 ± 0.06 a | 2.02 ± 0.05 a | 2.04 ± 0.02 a | 2.02 ± 0.07 a | ≤0.22 |
| K270 | 0.17 ± 0.01 a | 0.15 ± 0.02 b | 0.15 ± 0.00 ab | 0.15 ± 0.01 ab | 0.17 ± 0.00 b | 0.19 ± 0.01 ab | 0.20 ± 0.00 a | 0.19 ± 0.01 ab | ≤0.01 |
| ∆K | 0.00 ± 0.01 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.01 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| 2019 | 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | EVOO * | |
| Myristic (C 14:0) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | ≤0.03 |
| Palmitic (C 16:0) | 13.72 ± 0.30 a | 12.32 ± 0.11 b | 12.10 ± 0.23 b | 12.39 ± 0.13 b | 10.30 ± 0.35 c | 12.28 ± 0.18 b | 12.77 ± 0.23 b | 13.62 ± 0.29 a | 7.50–20.00 |
| Palmitoleic (C 16:1) | 0.95 ± 0.09 a | 0.60 ± 0.02 b | 0.62 ± 0.03 b | 0.65 ± 0.04 b | 0.85 ± 0.03 a | 0.64 ± 0.04 b | 0.60 ± 0.02 b | 0.58 ± 0.02 b | 0.30–3.50 |
| Heptadecanoic (C 17:0) | 0.08 ± 0.01 a | 0.04 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.01 b | 0.05 ± 0.00 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | ≤0.40 |
| Heptadecenoic (C 17:1) | 0.07 ± 0.01 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.01 a | 0.07 ± 0.00 a | 0.07 ± 0.00 b | ≤0.60 |
| Stearic (C 18:0) | 2.52 ± 0.04 a | 2.61 ± 0.11 a | 2.60 ± 0.02 a | 2.53 ± 0.14 a | 2.39 ± 0.05 a | 2.43 ± 0.02 a | 2.48 ± 0.01 a | 2.40 ± 0.15 a | 0.50–5.00 |
| Oleic (C 18:1) | 73.40 ± 0.30 b | 76.02 ± 0.19 a | 75.76 ± 0.14 a | 75.55 ± 0.21 a | 77.48 ± 0.30 a | 76.71 ± 0.30 b | 76.18 ± 0.16 b | 75.56 ± 0.11 c | 55.00–83.00 |
| Linoleic (C 18:2) | 7.30 ± 0.12 a | 6.55 ± 0.20 b | 7.06 ± 0.10 a | 7.06 ± 0.07 a | 6.84 ± 0.10 a | 6.14 ± 0.04 b | 6.18 ± 0.04 b | 6.13 ± 0.04 b | 2.50–21.00 |
| Linolenic (C18:3) | 1.00 ± 0.03 a | 0.81 ± 0.02 b | 0.77 ± 0.01 b | 0.78 ± 0.02 b | 0.95 ± 0.03 a | 0.71 ± 0.03 b | 0.68 ± 0.02 b | 0.65 ± 0.05 b | ≤1.00 |
| Arachidic (C 20:0) | 0.41 ± 0.02 a | 0.43 ± 0.01 a | 0.43 ± 0.00 a | 0.41 ± 0.03 a | 0.43 ± 0.01 a | 0.41 ± 0.00 a | 0.42 ± 0.00 a | 0.40 ± 0.03 a | ≤0.60 |
| Eicosenoic (C 20:1) | 0.36 ± 0.01 a | 0.38 ± 0.01 a | 0.38 ± 0.01 a | 0.37 ± 0.01 a | 0.44 ± 0.02 a | 0.39 ± 0.01 ab | 0.40 ± 0.00 ab | 0.38 ± 0.04 b | ≤0.50 |
| Behenic (C 22:0) | 0.11 ± 0.03 a | 0.11 ± 0.01 a | 0.11 ± 0.00 a | 0.10 ± 0.01 a | 0.12 ± 0.00 a | 0.11 ± 0.00 b | 0.11 ± 0.00 b | 0.10 ± 0.01 b | ≤0.20 |
| Eicosenoic acid (C 22:1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Lignoceric (C 24:0) | 0.05 ± 0.01 a | 0.06 ± 0.00 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | ≤0.20 |
| 18:2t + 18:3t | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ≤0.05 |
| ∑ SFA | 16.90 ± 0.28 a | 15.57 ± 0.05 b | 15.32 ± 0.23 b | 15.53 ± 0.15 b | 13.35 ± 0.31 c | 15.33 ± 0.20 b | 15.89 ± 0.23 b | 16.63 ± 0.12 a | |
| ∑ MUFA | 74.79 ± 0.39 b | 77.07 ± 0.20 a | 76.84 ± 0.13 a | 76.63 ± 0.20 a | 78.85 ± 0.28 a | 77.82 ± 0.25 b | 77.25 ± 0.18 b | 76.58 ± 0.10 b | |
| ∑ PUFA | 8.31 ± 0.12 a | 7.35 ± 0.20 c | 7.84 ± 0.10 b | 7.84 ± 0.08 b | 7.79 ± 0.12 a | 6.85 ± 0.06 b | 6.86 ± 0.06 b | 6.78 ± 0.09 b | |
| Oleic/linoleic ratio (C18:1/C18:2) | 10.05 ± 0.20 c | 11.62 ± 0.38 a | 10.73 ± 0.13 b | 10.70 ± 0.13 b | 11.33 ± 0.17 b | 12.49 ± 0.12 a | 12.33 ± 0.06 a | 12.32 ± 0.10 a | |
| Volatile Compounds (mg/kg) | 2019 | 2020 | ||||||
|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | |
| 3-methylbutanal | 0.95 ± 0.06 a | 0.63 ± 0.07 b | 0.65 ± 0.01 b | 0.73 ± 0.04 b | 0.04 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a |
| 3-pentanone | 0.07 ± 0.00 b | 0.08 ± 0.00 ab | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.09 ± 0.01 a | 0.09 ± 0.02 a | 0.07 ± 0.00 b | 0.05 ± 0.00 b |
| 1-penten-3-one | 0.80 ± 0.04 a | 0.84 ± 0.03 a | 0.84 ± 0.02 a | 0.84 ± 0.04 a | 3.22 ± 0.46 a | 2.94 ± 0.30 a | 2.10 ± 0.11 b | 1.78 ± 0.13 b |
| Ethyl 2-methylbutanoate | 0.93 ± 0.03 b | 1.23 ± 0.00 a | 1.24 ± 0.02 a | 1.21 ± 0.07 a | n.d. | n.d. | n.d. | n.d. |
| Hexanal | 6.00 ± 0.18 a | 6.23 ± 0.10 a | 6.36 ± 0.34 a | 5.95 ± 0.19 a | 2.10 ± 0.31 a | 1.45 ± 0.10 b | 1.50 ± 0.01 b | 1.10 ± 0.06 b |
| (Z)-2-pentenal * | 0.08 ± 0.01 a | 0.09 ± 0.01 a | 0.09 ± 0.00 a | 0.09 ± 0.01 a | 0.03 ± 0.01 b | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a |
| Isoamyl acetate | 0.10 ± 0.01 b | 0.12 ± 0.01 a | 0.11 ± 0.01 ab | 0.11 ± 0.01 ab | n.d. | n.d. | n.d. | n.d. |
| (E)-2-pentenal | 0.13 ± 0.01 a | 0.13 ± 0.00 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.16 ± 0.01 a | 0.13 ± 0.00 b | 0.09 ± 0.00 c | 0.08 ± 0.00 d |
| (E)-3-hexenal * | 0.87 ± 0.02 ab | 0.98 ± 0.06 a | 0.73 ± 0.08 b | 0.95 ± 0.08 a | 0.25 ± 0.03 b | 0.31 ± 0.01 a | 0.25 ± 0.01 b | 0.19 ± 0.02 c |
| (Z)-3-hexenal * | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.60 ± 0.06 b | 1.63 ± 0.19 a | 1.95 ± 0.26 a | 1.61 ± 0.12 a |
| (Z)-2-hexenal * | 0.22 ± 0.01 a | 0.22 ± 0.01 b | 0.17 ± 0.02 b | 0.19 ± 0.01 ab | 0.98 ± 0.06 a | 0.73 ± 0.03 b | 0.64 ± 0.02 b | 0.68 ± 0.03 b |
| (E)-2-hexenal | 16.48 ± 0.5 a | 12.79 ± 0.09 b | 11.29 ± 0.32 c | 11.47 ± 0.16 c | 85.07 ± 9.42 a | 49.13 ± 8.15 b | 42.71 ± 1.98 b | 36.34 ± 1.85 b |
| Hexyl acetate | 0.03 ± 0.00 ab | 0.03 ± 0.00 a | 0.03 ± 0.00 ab | 0.02 ± 0.00 b | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a |
| Octanal | 0.04 ± 0.00 a | 0.03 ± 0.00 b | 0.03 ± 0.00 ab | 0.04 ± 0.00 c | 0.28 ± 0.01 a | 0.26 ± 0.00 b | 0.28 ± 0.00 a | 0.26 ± 0.00 b |
| (E)-2-penten-1-ol | 0.47 ± 0.03 bc | 0.62 ± 0.07 a | 0.58 ± 0.01 ab | 0.43 ± 0.02 c | 0.16 ± 0.01 a | 0.14 ± 0.01 b | 0.11 ± 0.00 c | 0.09 ± 0.00 d |
| (Z)-2-penten-1-ol + (Z)-3-hexenyl acetate | 0.75 ± 0.02 a | 0.75 ± 0.03 ab | 0.68 ± 0.03 b | 0.69 ± 0.03 ab | 4.69 ± 0.37 a | 4.29 ± 0.3 a | 3.39 ± 0.21 b | 2.58 ± 0.18 c |
| Hexanol | 0.05 ± 0.00 a | 0.04 ± 0.01 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b |
| (E)-3-hexen-1-ol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| (Z)-3-hexen-1-ol | 1.15 ± 0.05 a | 0.77 ± 0.02 b | 0.44 ± 0.03 c | 0.48 ± 0.06 c | 0.79 ± 0.11 a | 0.36 ± 0.01 b | 0.30 ± 0.01 b | 0.25 ± 0.02 b |
| (E)-2-hexen-1-ol | 0.33 ± 0.01 a | 0.29 ± 0.00 ab | 0.23 ± 0.05 b | 0.24 ± 0.02 b | 0.47 ± 0.01 a | 0.29 ± 0.07 b | 0.34 ± 0.01 b | 0.28 ± 0.03 b |
| (Z)-2-hexen-1-ol | 0.25 ± 0.02 a | 0.07 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | n.d. | n.d. | n.d. | n.d. |
| (E)-2-octenal | 1.48 ± 0.06 a | 0.82 ± 0.02 b | 0.83 ± 0.04 b | 0.77 ± 0.03 b | n.d. | n.d. | n.d. | n.d. |
| Total C5 volatiles | 1.75 ± 0.04 a | 1.79 ± 0.02 a | 1.73 ± 0.05 a | 1.72 ± 0.07 a | 3.65 ± 0.48 a | 3.35 ± 0.31 a | 2.43 ± 0.12 b | 2.05 ± 0.13 b |
| Total C6 volatiles | 24.05 ± 0.53 a | 20.14 ± 0.12 b | 18.38 ± 0.47 c | 18.2 ± 0.30 c | 90.36 ± 9.64 a | 54.00 ± 8.22 b | 47.78 ± 1.8 b | 40.54 ± 2.00 b |
| Total aldehydes | 25.05 ± 0.60 a | 20.6 ± 0.08 b | 19.25 ± 0.55 c | 19.03 ± 0.33 c | 89.19 ± 9.56 a | 53.41 ± 8.19 b | 47.17 ± 1.80 b | 40.02 ± 1.98 b |
| Total alcoholes | 2.29 ± 0.07 a | 1.84 ± 0.05 b | 1.38 ± 0.10 c | 1.44 ± 0.03 c | 1.28 ± 0.12 a | 0.67 ± 0.06 b | 0.66 ± 0.01 b | 0.55 ± 0.05 b |
| Total esters | 0.96 ± 0.03 b | 1.26 ± 0.01 a | 1.27 ± 0.02 a | 1.24 ± 0.07 a | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a |
| Total ketones | 0.87 ± 0.05 a | 0.91 ± 0.03 a | 0.93 ± 0.02 a | 0.92 ± 0.04 a | 3.31 ± 0.47 a | 3.02 ± 0.32 a | 2.17 ± 0.11 b | 1.83 ± 0.13 b |
| Total volatile compounds | 31.24 ± 0.54 a | 26.79 ± 0.24 b | 24.61 ± 0.41 c | 24.51 ±0.49 c | 99.01 ± 9.25 a | 61.94 ± 8.28 b | 53.91 ± 2.12 b | 45.46 ± 2.20 b |
| Phenolic Compounds (mg/kg) | 2019 | 2020 | ||||||
|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | |
| Simple phenols | ||||||||
| Tyrosol | 6.0 ± 1.0 c | 8.1 ± 0.4 cb | 9.9 ± 1.2 ab | 12.3 ± 0.8 a | 5.7 ± 0.5 d | 9.0 ± 0.9 c | 11.5 ± 0.9 b | 13.8 ± 1.0 a |
| Hydroxytyrosol | 4.1 ± 0.7 d | 6.0 ± 0.5 c | 8.4 ± 1.0 b | 10.3 ± 0.6 a | 2.4 ± 0.2 c | 4.5 ± 0.8 b | 6.2 ± 0.4 a | 7.7 ± 0.8 a |
| Hydroxytyrosol acetate * | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 c | 0.1 ± 0.0 bc |
| Vanillin | 0.1 ± 0.0 b | 0.2 ± 0.0 a | 0.2 ± 0.0 ab | 0.1 ± 0.0 ab | 0.2 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 b |
| Total simple phenols | 10.3 ± 1.8 c | 14.4 ± 0.08 bc | 18.5 ± 2.2 b | 22.8 ± 1.1 a | 8.4 ± 0.3 d | 13.8 ± 1.7 c | 17.9 ± 1.25 b | 21.7 ± 1.7 a |
| Phenolic acids | ||||||||
| Vanillic acid | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.2 ab | 0.1 ± 0.1 b | 2.9 ± 0.8 a | 2.7 ± 0.6 a | 2.6 ± 0.2 a | 2.5 ± 0.4 a |
| p-Coumaric acid | 1.2 ± 0.2 a | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 1.0 ± 0.1 a | 2.2 ± 0.2 a | 1.6 ± 0.1 b | 1.5 ± 0.1 b | 1.5 ± 0.1 b |
| Total phenolic acids | 1.5 ± 0.2 a | 1.4 ± 0.1 ab | 1.34 ± 0.1 ab | 1.17 ± 0.1 b | 5.1 ± 0.9 a | 4.3 ± 0.6 a | 4.0 ± 0.1 a | 4.0 ± 0.4 a |
| Flavonoids | ||||||||
| Luteolin | 0.8 ± 0.2 a | 1.1 ± 0.3 a | 0.9 ± 0.1 a | 1.0 ± 0.2 a | 1.8 ± 0.2 a | 1.5 ± 0.1 ab | 1.2 ± 0.2 b | 1.3 ± 0.2 ab |
| Apigenin | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.3 ± 0.0 a | 0.2 ± 0.0 ab | 0.2 ± 0.0 b | 0.2 ± 0.0 b |
| Total flavonoids | 0.9 ± 0.2 a | 1.2± 0.4 a | 1.0 ± 0.1 a | 1.1 ± 0.2 a | 2.0 ± 0.2 a | 1.7 ± 0.1 ab | 1.4 ± 0.2 b | 1.5 ± 0.2 b |
| Lignans | ||||||||
| Pinoresinol | 3.2 ± 0.1 c | 3.7 ± 0.0 cb | 4.3 ± 0.1 ab | 4.8 ± 0.6 a | 4.5 ± 0.4 a | 3.1 ± 0.3 b | 3.9 ± 0.5 ab | 4.4 ± 0.4 a |
| Acetoxypinoresinol * | 19.0 ± 2.5 a | 19.2 ± 2.3 a | 17.3 ± 0.9 a | 17.9 ± 3.0 a | 20.0 ± 0.4 a | 15.4 ± 0.2 b | 16.0 ± 1.7 b | 16.9 ± 1.6 b |
| Total lignans | 22.1 ± 2.44 a | 22.8 ± 2.3 a | 21.6 ± 1.0 a | 22.8 ± 3.4 a | 24.5 ± 0.3 a | 18.5 ± 0.1 b | 20.0 ± 2.1 b | 21.3 ± 1.9 ab |
| Secoiridoids | ||||||||
| 3,4-DHPEA-EDA * | 228.8 ± 45.1 a | 165.1 ± 7.7 a | 181.9 ± 9.4 a | 185.1 ± 43.0 a | 256.8 ± 34.4 a | 133.4 ± 16.4 b | 163.3 ± 14.4 b | 158.8 ± 26.4 b |
| Oleuropein aglycone (isomer I) * | 449.7 ± 56.4 a | 374.6 ± 50.8 a | 357.6 ± 35.3 a | 348.2 ± 30.1 a | 339.2 ± 48.1 a | 394.7 ± 38.3 a | 382.1 ± 41.2 a | 367.7 ± 30.9 a |
| p-HPEA-EDA * | 195.6 ± 32.2 a | 139.4 ± 17.2 a | 159.0 ± 18.7 a | 155.0 ± 42.5 a | 207.6 ± 18.2 a | 181.1 ± 10.3 a | 211.6 ± 20.1 a | 200.1 ± 9.6 a |
| Oleuropein + ligstroside aglycones I & II * | 258.3 ± 24.9 a | 194.6 ± 19.5 b | 202.7 ± 23.2 ab | 199.6 ± 26.7 ab | 186.8 ± 29.5 b | 298.4 ± 34.8 a | 298.0 ± 29.5 a | 284.1 ± 29.4 a |
| Oleuropein aglycone (isomer II) * | 77.8 ± 4.5 a | 68.4 ± 5.8 a | 75.2 ± 7.8 a | 71.5 ± 9.3 a | 39.5 ± 6.7 a | 41.3 ± 2.7 a | 46.4 ± 2.7 a | 46.5 ± 0.8 a |
| Ligstroside aglycone (isomer III) * | 16.4 ± 0.6 a | 17.8 ± 2.5 a | 18.4 ± 1.0 a | 20.1 ± 3.4 a | 11.3 ± 0.8 b | 12.2 ± 0.1 b | 14.2 ± 1.6 ab | 15.5 ± 1.3 a |
| Oleuropein aglycone (isomer III) * | 50.1 ± 3.5 a | 43.5 ± 8.2 a | 40.5 ± 7.8 a | 40.3 ± 7.7 a | 16.1 ± 2.6 b | 22.0 ± 2.1 b | 30.3 ± 2.9 a | 30.7 ± 3.1 a |
| Total secoiridoids | 1276.8 ± 151.6 a | 1003.3 ± 108.6 a | 1035.3 ± 100.6 a | 1019.8 ± 148.1 a | 1057.4 ± 123.8 a | 1083.2 ± 60.0 a | 1146.1± 108.0 a | 1103.4± 42.7 a |
| Total phenolic content | 1311.8 ± 153.3 a | 1043.3 ± 110.8 a | 1077.9 ± 102.2 a | 1067.7 ± 152.7 a | 1097.5 ±123.7 a | 1121.5 ± 59.9 a | 1189.4 ± 109.6 a | 1152.0 ± 45.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brkić Bubola, K.; Kolega, Š.; Marcelić, Š.; Šikić, Z.; Gašparović Pinto, A.; Zorica, M.; Klisović, D.; Novoselić, A.; Jukić Špika, M.; Kos, T. Effect of Different Watering Regimes on Olive Oil Quality and Composition of Coratina Cultivar Olives Grown on Karst Soil in Croatia. Foods 2022, 11, 1767. https://doi.org/10.3390/foods11121767
Brkić Bubola K, Kolega Š, Marcelić Š, Šikić Z, Gašparović Pinto A, Zorica M, Klisović D, Novoselić A, Jukić Špika M, Kos T. Effect of Different Watering Regimes on Olive Oil Quality and Composition of Coratina Cultivar Olives Grown on Karst Soil in Croatia. Foods. 2022; 11(12):1767. https://doi.org/10.3390/foods11121767
Chicago/Turabian StyleBrkić Bubola, Karolina, Šimun Kolega, Šime Marcelić, Zoran Šikić, Ana Gašparović Pinto, Marko Zorica, Dora Klisović, Anja Novoselić, Maja Jukić Špika, and Tomislav Kos. 2022. "Effect of Different Watering Regimes on Olive Oil Quality and Composition of Coratina Cultivar Olives Grown on Karst Soil in Croatia" Foods 11, no. 12: 1767. https://doi.org/10.3390/foods11121767
APA StyleBrkić Bubola, K., Kolega, Š., Marcelić, Š., Šikić, Z., Gašparović Pinto, A., Zorica, M., Klisović, D., Novoselić, A., Jukić Špika, M., & Kos, T. (2022). Effect of Different Watering Regimes on Olive Oil Quality and Composition of Coratina Cultivar Olives Grown on Karst Soil in Croatia. Foods, 11(12), 1767. https://doi.org/10.3390/foods11121767









