Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties
Abstract
1. Introduction
2. Chemistry and Biochemistry of Anthocyanins
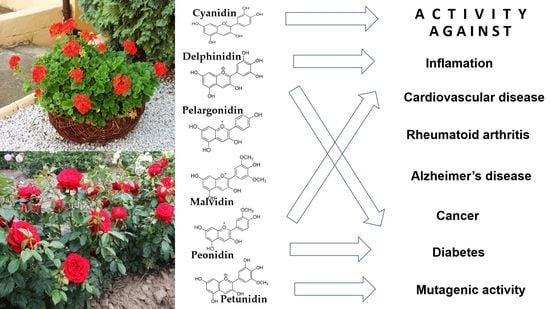
3. Antioxidant Capacity and Anti-Inflammatory Property of Anthocyanins
3.1. Anioxidant Activity
3.2. Anti-Inflamtory Activity and Protection against Chronic Diseases
4. Factors Influencing Anthocyanin Content in Ornamental Plants
5. Anthocyanin Content of Domestic Grown Edible Flowers
| Species | Glycosileted and Acyleted Anthocyanins | Source |
|---|---|---|
| Begonia | Cyanidin 3-O-giucoside, pelargonidin 3-O-diglucoside, cyanidin 3-O-xylosylglucoside, cyanidin 3-O-rhamnosyldiglucoside Malvidin 3,5-diglucoside | [54,55] |
| Bellis | Cyanidin 3-malonylglucuronylglucoside Cyanidin 3-malonylglucoside, Pelargonidin 3-malonylglucoside Delphinidin 3-O-glucoside | [56,111] |
| Campanula | Pelargonidin 3-rutinoside-7-glucoside, Delphinidin 3-O-glucoside Cyanidin 3-glucoside, Cyanidin 3-rutinoside, | [59,112] |
| Dahlia | Cyanidin-rutinoside Pelargonidin 3,5-di-O-glucoside | [62] |
| Dianthus | Cyanidin 3-malylglucoside, Delphinidin 3-malylglucoside pelargonidin 3-malylglucoside | [63] |
| Dendranthema | Cyanidin 3-malonylglucoside Pelargonidin 3-O-glucoside, Delphinidin 3-O-glucoside) | [56] [65] |
| Glechoma | Cyanidin 3-(6”-maionylglucoside)~5-glucoside Delphinidin 3-(6”-pcoumarylgIucoside)-5-glucoside | [68] |
| Hibiscus | Cyanidin-3-sambubioside, Delphinidin-3-sambubioside. | [73] |
| Impatiens | Malvinidin glucoside, Pelargonidin glucoside Paeonidin glucoside | [75] |
| Lobularia maritima | Acylated pelargonidin 3-samububioside 5-glucoside cyanidin 3-sambubioside-5-glucosides | [76] [77] |
| Pelargonium spp. | Cyanidin 3,5-di-O-glucoside, Delphinidin 5-O-glucoside Pelargonidin 5-O-glucoside, Malvidin 3,5-di-O-glucoside Peonidin 3,5-di-O-glucoside, Petunidin 3,5-di-O-glucoside | [78] |
| Petunia | Cyanidin 3-O-rutinoside, Delphinidin 3-O-glucoside Pelargonidin 3-caffeoylrutinoside-5 rutinoside, Malvidin 3-caffeoylrutinoside, Peonidin 3-caffeoylrutinoside 3,5-di-O-glucoside, Petunidin 3 caumrylorutinoside 5-glucoside | [80] |
| Rosa | Cyanidin, Cyanidin-3-O-glucoside | [62] |
| Tagetes erecta | Cyanidin-di-hexoside, Delphinidin-3-O-hexoside | [84] |
| Tagetes patula | Cyanidin-3-galloylsophoroside, cyanidin-3-glucoside, cyanidin-3-sophoroside | [85] |
| Tropaeolum majus | Pelargonidin-3-O-sophoroside, delphinidin3-O-3-dihexosides; cyanidin3-O-sophoroside | [84,88] |
| Torenia sp. | Cyanidin 3,5-di-O-glucoside, Malvidin-3-O-β-D-glucoside,-Peonidin-3-glucoside-5-(p-coumaroyl)-glucoside | [86,87] |
| Tulipa sp. | Cyanidin 3-rutinoside, Delphinidin 3-rutinoside, Pelargonidin 3-rutinoside,, | [89] |
| Viola cornuta | Petunidin-O-deoxyhexoside- hexoside, Cyanidin 3-glucoside, | [93] |
| Viola witrockiana | Cyanidin-rhamnosyl-glucoside, Delphinidin-rhamnosyl-glucoside, yanidin-3-(coumaroyl)-methylpentosyl-hexosyl-5-hexoside | [94] |
6. Pharmacy in the Neighborhood (Balconies, Roofs, Terraces)
7. Preservation and Simple Processing of Edible Flowers
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 May 2021).
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant. Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Veget. Crops Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Tian, J.; Chen, M.; Zhang, J.; Lee, K.; Song, T.; Zhang, H.; Yao, Y. Characteristics of dihydroflavonol 4-reductase gene pro-moters from different leaf colored Malus crabapple cultivars. Hortic. Res. 2017, 4, 17070. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Ballistreri, G.; Fabroni, S.; Romeo, F.V.; Timpanaro, N.; Amenta, M.; Rapisarda, P. Anthocyanins and other polyphenols in citrus genus: Biosynthesis, chemical profile, and biological activity. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 191–2012. [Google Scholar]
- Nelson, D.L.; Cox, M.; Lehninger, M. Principles of Biochemistry, 3rd ed.; Worth Publishing: New York, NY, USA, 2000; pp. 834–882. [Google Scholar]
- Yahia, E.M. Fruit and Vegetable Phytochemicals (Chemistry and Human Health, 2nd Edition)||Tannins in Fruits and Vegetables: Chemistry and Biological Functions; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 221–268. [Google Scholar]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Eidenberger, T. The routine analysis of anthocyanins from berries and berry products. In Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits; Motohashi, N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; Volume 3, pp. 113–178. [Google Scholar]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Sandhu, A.; Edirisinghe, I. Anthocyanins. In Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Academic: San Diego, CA, USA, 2016; pp. 489–500. [Google Scholar]
- Guo, H.; Xia, M. Anthocyanins and Diabetes Regulation. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2014; Volume 1, pp. 83–93. [Google Scholar]
- Bakowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Pol. J. Food Nutr. Sci. 2005, 14, 107–116. [Google Scholar]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 01, 7–15. [Google Scholar]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inibhition of lipid peroxidation and the active oxygen radical scavengingeffect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A—Studies in platelets, monocytes, and human endothelial cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar]
- Harlen, W.C.; Jati, I.R.A.P. Antioxidant Activity of Anthocyanins in Common Legume Grains. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK, 2018; Volume 1, pp. 87–99. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Robles-Sanchez, M.; Gorinstein, S.; Martin-Belloso, O.; Astiazaran-Garcia, H.; Gonzalez-Aguilar, G.A.; Cruz-Valenzuela, R. Minimal processing of tropical fruits: Antioxidant potential and its impact on human health. Intersciencia 2007, 32, 227–232. [Google Scholar]
- Filho, J.G.D.O.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.J.; Holub, B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [CrossRef]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant antho-cyanins in humans and Watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Kay, C.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Wright, S.D. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 1999, 189, 605–609. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Gut metabolites of anthocyanins, gallic acid, 3-O-Methylgallic acid, and 2,4,6-Trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 5320–5327. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Bedoui, J.E.; Madeira, S.V.; Chalupsky, K.; Schini-Kerth, V.B. Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br. J. Pharmacol. 2006, 3, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. Int. Rev. J. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Ling, W.; Zhu, H.; Wang, Q.; Ma, J.; Hou, M.; Tang, Z.; Li, L.; Ye, Q. Anthocyanin prevents CD40-activated proin-flammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 519–524. [Google Scholar] [CrossRef]
- Fimognari, C.; Berti, F.; Nusse, M.; Cantelli-Forti, G.; Hrelia, P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-beta-glucopyranoside. Biochem. Pharmacol. 2004, 67, 2047–2056. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vareed, S.K.; Olson, A.L.K.; Nair†, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2004, 53, 28–31. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, J.-M.; Lin, W.-L.; Chu, C.-Y.; Chou, F.-P.; Tseng, T.-H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Werlein, H.-D.; Kütemeyer, C.; Schatton, G.; Hubbermann, E.; Schwarz, K. Influence of elderberry and blackcurrant concentrates on the growth of microorganisms. Food Control 2005, 16, 729–733. [Google Scholar] [CrossRef]
- Knox, Y.M.; Suzutani, T.; Yosida, I.; Azuma, M. Anti-influenza virus activity of crude extract of Ribes nigrum L. Phytotherapy Res. 2003, 17, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D.; Denisova, N.A. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer Disease Model. Nutr. Neurosci. 2003, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Rasool, M. Cyanidin attenuates IL-17A cytokine signaling mediated monocyte migration and differentiation into mature osteoclasts in rheumatoid arthritis. Cytokine 2021, 142, 155502. [Google Scholar] [CrossRef] [PubMed]
- Maya-Cano, D.A.; Arango-Varela, S.; Santa-Gonzalez, G.A. Phenolic compounds of blueberries (Vaccinium spp.) as a protective strategy against skin cell damage induced by ROS: A review of antioxidant potential and antiproliferative capacity. Heliyon 2021, 7, e06297. [Google Scholar] [CrossRef]
- Chirol, N.; Jay, M. Acylated anthocyanins from flowers of Begonia. Phytochemistry 1995, 40, 275–277. [Google Scholar] [CrossRef]
- de Morais, J.S.; Sant’Ana, A.S.; Dantas, A.M.; Silva, B.S.; Lima, M.S.; Borges, G.C.; Magnani, M. Antioxidant activity and bioaccessibility of phenolic compounds in white, red, blue, purple, yellow and orange edible flowers through a simulated intestinal barrier. Food Res. Int. 2020, 131, 109046. [Google Scholar] [CrossRef]
- Saito, N.; Toki, K.; Honda, T.; Kawase, K. Cyanidin 3-malonylglucuronylglucoside in Bellis and cyanidin 3-malonylglucoside in Dendranthema. Phytochemistry 1988, 27, 2963–2966. [Google Scholar] [CrossRef]
- Brandt, K.; Kondo, T.; Aoki, H.; Goto, T. Structure and biosynthesis of anthocyanins in flowers of Campanula. Phytochemistry 1993, 33, 209–212. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Asen, S.; Stewart, R.N.; Norris, K.H. Pelargonidin 3-di (p-hydroxybenzoyl) rutinoside-7-glucoside from flowers of Campanula. Phytochemistry 1979, 18, 1251–1252. [Google Scholar] [CrossRef]
- Janarny, G.; Ranaweera, K.K.D.S.; Gunathilake, K.D.P.P. Antioxidant activities of hydro-methanolic extracts of Sri Lankan edible flowers Biocatal. Agric. Biotechnol. 2021, 35, 102081. [Google Scholar] [CrossRef]
- Lara-Cortes, E.; Martin-Belloso, O.; Osorio-Diaz, P.; Barrera-Necha, L.L.; Sanchez-Lopez, J.A.; Bautista-Banos, S. Actividad antioxidante, composicion nutrimental y funcional de flores comestibles de dalia. Rev. Chapingo Ser. Hortic. 2014, 20, 101–116. [Google Scholar]
- Pires, T.C.; Dias, M.I.; Barros, L.; Barreira, J.C.; Santos-Buelga, C.; Ferreira, I.C. Incorporation of natural colorants obtained from edible flowers in yogurts. LWT 2018, 97, 668–675. [Google Scholar] [CrossRef]
- Abe, Y.; Tera, M.; Sasaki, N.; Okamura, M.; Umemoto, N.; Momose, M.; Kawahara, N.; Kamakura, H.; Goda, Y.; Nagasawa, K.; et al. Detection of 1-O-malylglucose: Pelargonidin 3-O-glucose-6′′-O-malyltransferase activity in carnation (Dianthus caryophyllus). Biochem. Biophys. Res. Commun. 2008, 373, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2015, 57, 1729–1741. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakayama, T.; Yamaguchi, M.-A.; Nishino, T. cDNA cloning and characterization of two Dendranthema×morifolium anthocyanin malonyltransferases with different functional activities. Plant. Sci. 2004, 166, 89–96. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xiao, Y.; Fu, N.-L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Crowden, R.K.; Wright, J.; Harborne, J.B. Anthocyanins of Fuchsia (onagraceae) Isorhamnetin 3,7-disulphate from flaveria bidentis. Phytochemistry. 1977, 16, 40. [Google Scholar]
- Saito, N.; Harborne, J.B. Correlations between anthocyanin type, pollinator and flower colour in the labiatae. Phytochemistry 1992, 31, 3009–3015. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A comparative exploration of the phytochemical profiles and bio-pharmaceutical potential of Helichrysum stoechas subsp. barrelieri extracts obtained via five extraction techniques. Process. Biochem. 2019, 91, 113–125. [Google Scholar] [CrossRef]
- Kao, F.J.; Chiang, W.D.; Liu, H.M. Inhibitory effect of daylily buds at various stages of maturity on nitric oxide production and the involved phenolic compounds. LWT 2015, 61, 130–137. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; De Freitas, V.; Salas, E. Isolation and Characterization of Anthocyanins from Hibiscus sabdariffa Flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hidaka, M.; Masakia, H.; Seto, H.; Uozumi, T. Major anthocyanin of the flowers of hibiscus (Hibiscus rosasinensis L.). Agric. Biol. Chem. 1990, 54, 3345–3346. [Google Scholar]
- Lu, B.; Li, M.; Yin, R. Phytochemical content, health benefits, and toxicology of common edible flowers: A review (2000–2015). Crit. Rev. Food Sci. Nutr. 2015, 56 (Suppl. 1), S130–S148. [Google Scholar] [CrossRef]
- Klozová, E.; Rokosavá, K. Anthocyanins of Impatiens holstii. Biol. Plantarum 1961, 3, 291–296. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Usuki, R.; Toki, K.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Acylated pelargonidin 3-samububioside-5-glucosides from the red-purple flowers of Lobularia maritima. J. Jpn. Soc. Hortic. Sci. 2010, 79, 84–90. [Google Scholar] [CrossRef][Green Version]
- Tatsuzawa, F.; Toki, K.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Four acylated cyanidin 3-sambubioside-5-glucosides from the purple-violet flowers of Lobularia maritima. Heterocycles 2007, 71, 1117–1125. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Markham, K.R.; Boase, M.R. Pigment chemistry and colour of Pelargonium flowers. Phytochemistry 1998, 47, 355–361. [Google Scholar] [CrossRef]
- Griesbach, R.; Asen, S.; Leonnarat, B. Petunia hybrida anthocyanins acylated with caffeic acid. Phytochemistry 1991, 30, 1729–1731. [Google Scholar] [CrossRef]
- Berardi, A.E.; Esfeld, K.; Jäggi, L.; Mandel, T.; Cannarozzi, G.M.; Kuhlemeier, C. Complex evolution of novel red floral color in Petunia. Plant. Cell 2021, 33, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.-J.; Choung, M.-G. Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Ścibisz, I.; Mitek, M.; Kieliszek, M.; Kolniak-Ostek, J. Profile of the phenolic compounds of Rosa rugosa petals. J. Food Qual. 2017, 2, 7941347. [Google Scholar]
- Friedman, O.; Agami, Y.; Vinokur, S.; Droby, L.; Cohen, G.; Refaeli, N.; Resnick, N.; Umiel, N. Characterization of yield: Sensitivity to Botrytis cinerea and antioxidant content of several rose species suitable for edible flowers. Sci. Hortic. 2010, 123, 395–401. [Google Scholar] [CrossRef]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2014, 16, 805–822. [Google Scholar] [CrossRef]
- Deineka, V.; Kulchenko, Y.; Blinova, I.; Deineka, L.; Chulkov, A. Anthocyanins of Tagetes patula flower petals. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2986–2993. [Google Scholar]
- Fukuzaki, E.; Kawasaki, K.; Kajiyama, S.; An, C.; Suzuki, K.; Tanaka, Y.; Kobayashi, A. Flower color modifications of Torenia hybrida by down-regulation of chalcone synthase genes with RNA interference. J. Biotechnol. 2004, 111, 229–240. [Google Scholar] [CrossRef]
- Suzuki, K.I.; Xue, H.M.; Tanaka, Y.; Fukui, Y.; Fukuchi-Mizutani, M.; Murakami, Y.; Katsumoto, Y.; Tsuda, S.; Kusumi, T. Flower color modifications of Torenia hybrida by co-suppression of anthocyanin biosynthesis genes. Mol. Breed. 2000, 6, 239–246. [Google Scholar] [CrossRef]
- Garzón, A.G.; Manns, D.C.; Riedl, K.; Schwartz, S.J.; Padilla-Zakour, O. Identification of Phenolic Compounds in Petals of Nasturtium Flowers (Tropaeolum majus) by High-Performance Liquid Chromtography Coupled to Mass Spectrometry and Determination of Oxygen Radical Absorbance Capacity (ORAC) J. Agric. Food Chem. 2015, 18, 1803–1811. [Google Scholar] [CrossRef]
- Nakayama, M.; Okada, M.; Taya-Kizu, M.; Urashima, O.; Kan, Y.; Fukui, Y.; Koshioka, M. Coloration and Anthocyanin Profile in Tulip Flowers. Jpn. Agric. Res. Q. JARQ 2004, 38, 185–190. [Google Scholar] [CrossRef][Green Version]
- Yuan, Y.; Ma, X.; Tang, D.; Shi, Y. Comparison of anthocyanin components, expression of anthocyanin biosynthetic structural genes, and TfF3′H1 sequences between Tulipa fosteriana “Albert heijn” and its reddish sport. Sci. Hort. 2014, 175, 16–26. [Google Scholar] [CrossRef]
- Fawzi Mahomoodally, M.; Zengin, G.; Ibrahime Sinan, K.; Yıldıztugay, E.; Lobine, D.; Ouelbani, R.; Bensari, S.; Yilmaz, M.A.; Montesano, D. A comprehensive evaluation of the chemical profiles and biological properties of six geophytes from Turkey: Sources of bioactive compounds for novel nutraceuticals. Food Res. Int. 2021, 140, 110068. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska, A.; Gąsecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules 2020, 25, 5627. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; Reigada, I.; Ferreira, I.C.; López, V.; Langa, E.; Rincón, C.G. Viola cornuta and Viola × wittrockiana: Phenolic compounds, antioxidant and neuroprotective activities on Caenorhabditis elegans. J. Food Drug Anal. 2019, 27, 849–859. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Skowyra, M.; Calvo, M.I.; Gallego, M.G.; Azman, N.A.M.; Almajano, M.P. Characterization of phytochemicals in petals of different colours from Viola × wittrockiana gams. And their correlation with antioxidant activity. J. Agric. Sci. 2014, 6, 9. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant. Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Passeri, V.; Koes, R.; Quattrocchio, F.M. New Challenges for the design of high value plant products: Stabilization of anthocyanins in plant vacuoles. Front. Plant. Sci. 2016, 7, 153. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant. Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Liu, C.-H.; Liu, Y. Fruit quality and differentially expressed genes of winter-harvested pineapple in response to elevated tem-perature over a short postharvest period. Postharvest Biol. Technol. 2017, 130, 21–27. [Google Scholar] [CrossRef]
- Ban, T.; Ishimaru, M.; Kobayashi, S.; Shiozaki, S.; Goto-Yamamoto, N.; Horiuchi, S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J. Hortic. Sci. Biotechnol. 2003, 78, 586–589. [Google Scholar] [CrossRef]
- Xi, X.; Zha, Q.; Jiang, A.; Tian, Y. Impact of cluster thinning on transcriptional regulation of anthocyanin biosynthesis-related genes in ‘Summer Black’ grapes. Plant. Physiol. Biochem. 2016, 104, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, J.; Miller, M.H. Purpling in maize hybrids as influenced by temperature and soil phosphorus. Agron. J. 1987, 79, 576–582. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Melatonin and its relationship to plant hormones. Ann. Bot. 2017, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fang, H.; Wang, J.; Yue, X.; Su, M.; Mao, Z.; Chen, X. Ultraviolet B-induced MdWRKY72 expression promotes antho-cyanin synthesis in apple. Plant Sci. 2020, 292, 110377. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J.; Bradley, J.M. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2007, 7, 281–299. [Google Scholar] [CrossRef]
- Weiss, D.; van Blokland, R.; Kooter, J.M.; Mol, J.N.M.; van Tunen, A.J. Gibberellic acid regulates chalcone synthase gene transcription in the corolla of Petunia hybrida. Plant. Physiol. 1992, 98, 191–197. [Google Scholar] [CrossRef]
- Ben-Nissan, G.; Weiss, D. The petunia homologue of tomato gast1: Transcript accumulation coincides with gibberellin-induced corolla cell elongation. Plant Mol. Biol. 1996, 32, 1067–1074. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Nørbæk, R.; Nodland, E.; Øvstedal, D.O.; Andersen, O.M. Anthocyanin content of Tulipa species and cultivars and its impact on tepal colours. Biochem. Syst. Ecol. 2005, 33, 499–510. [Google Scholar] [CrossRef]
- Krzymińska, A.; Frąszczak, B.; Gąsecka, M.; Magdziak, Z.; Kleiber, T. The content of phenolic compounds and organic acids in two Tagetes patula cultivars flowers and its dependence on light colour and substrate. Molecules 2022, 27, 527. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Suzuki, H.; Ichimaida, F.; Yamaguchi, M.-A.; Iwashita, T.; Fukui, Y.; Hemmi, H.; Nishino, T.; Nakayama, T. UDP-glucuronic Acid: Anthocyanin Glucuronosyltransferase from Red Daisy (Bellis perennis) Flowers. J. Biol. Chem. 2005, 280, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Matsuura, E.; Terahara, N.; Ishimaru, K. Secondary metabolites in transformed root cultures of Campanula glomerata. J. Plant. Physiol. 1999, 155, 251–254. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Cabrera, J.L.; Juliani, H. R Isorhamnetin 3,7-disulphate from Flaveria bidentis. Phytochemistry 1977, 16, 4000–4003. [Google Scholar] [CrossRef]
- Lovell, S.; Johnston, D. Designing landscapes for performance based on emerging principles in landscape ecology. Ecol. Soc. 2009, 14, 44. [Google Scholar] [CrossRef]
- Palmer, I. The Balcony Gardener: Creative Ideas for Small Spaces; Ryland Peters & Small; CICO Books: London, UK, 2012. [Google Scholar]
- Krzymińska, A.; Bocianowski, J.; Mądrachowska, K. The use of plants on balconies in the city. Hortic. Sci. 2020, 47, 180–187. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2015, 64, 2467–2474. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; González Aguilar, G.A. High oxygen treatment increases antioxidant capacity and post-harvest life of strawberry fruit. Food Technol. Biotechnol. 2007, 45, 2. [Google Scholar]
- Leja, M.; Mareczek, A.; Ben, J. Antioxidant properties of two apple cultivars during long-term storage. Food Chem. 2003, 80, 303–307. [Google Scholar] [CrossRef]
- Purohit, S.R.; Rana, S.R.; Idrishi, R.; Sharma, V.; Ghosh, P. A review on nutritional, bioactive, toxicological properties and preservation of edible flowers. Future Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Kelley, K.M.; Cameron, A.C.; Biernbaum, J.A.; Poff, K.L. Effect of storage temperature on the quality of edible flowers. Post-Harvest. Biol. Technol. 2003, 27, 341–344. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Łysiak, G. Physicochemical properties and antioxidant activity of fruit of selected plum cultivars. Ecol. Chem. Eng. 2010, 17, 1197–1202. [Google Scholar]
- Shantamma, S.; Vasikaran, E.M.; Waghmare, R.; Nimbkar, S.; Moses, J.M.; Anandharamakrishnan, C. Emerging techniques for the processing and preservation of edible flowers. Future Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Łysiak, G.; Rutkowski, K.; Walkowiak-Tomczak, D. Effect of storage conditions on storability and antioxidant potential of pears cv. ‘conference’. Agriculture 2021, 11, 545. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- Łysiak, G.; Florkowski, W.; Prussia, S. Postharvest Calcium Chloride Application and Moisture Barrier Influence on Peach Fruit Quality. HortTechnology 2008, 18, 100–105. [Google Scholar] [CrossRef]
- Kou, L.; Turner, E.R.; Luo, Y.G. Extending the shelf Life of edible flowers with controlled release of 1-methylcyclopropene and modified atmosphere packaging. J. Food Sci. 2012, 77, 188–193. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of modified (MAP) and controlled atmosphere (CA) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Morachis-Valdez, A.G.; Gómez-Oliván, L.M.; García-Argueta, I.; Hernández-Navarro, M.D.; Díaz-Bandera, D.; Dublán-García, O. Effect of Chitosan Edible Coating on the Biochemical and Physical Characteristics of Carp Fillet (Cyprinus carpio) Stored at −18 °C. Int. J. Food Sci. 2017, 2017, 2812483. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Pereira, E.L.; Saraiva, J.; Ramalhosa, E. Effect of alginate coating on the physico-chemical and microbial quality of pansies (Viola × wittrockiana) during storage. Food Sci. Biotechnol. 2018, 27, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.D. Optimization of coating formulation for vegetable night-fragrant flower fresh keeping. North. Hortic. 2015, 12, 127–131. [Google Scholar]
| Flower Species | Anthocyanins * | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cyanidin | Delphinidin | Pelargonidin | Malvidin | Peonidin | Petunidin | Total | ORAC/ FRAP 1 (TE/100 g) A (mmol FeSO4 /100 g) B | ||
| Ageratum houstonianum | 27.85 8 | 2.99 A,8 | [5], | ||||||
| Argyranthemum houstonianum | 2.99 8 | 27.85 B | [5] | ||||||
| Begonia sp. | p D | p D | 759.1 | 5.09 8 | 21.18 B | [5,54,55] | |||
| Bellis perennis | p D | p D | no data | [56] | |||||
| Campanula sp. | p D | p D | [57,58] | ||||||
| Calendula officinalis | 22.1 7 | 3.68 A 58.05 B | [5,59,60] | ||||||
| Dahlia sp. | 121.2 | 2.65 | 17.6–257.5 7,9 | 17–24 5,9 | [61,62] | ||||
| Dianthus | 52.4 | p D | p D | 0.73–13.35 8,9 | 5.4–10.2 A,9 | [5,63,64] | |||
| Dendranthema | p D | p D | p D | 168–182 B | [56,65,66] | ||||
| Phaseolus coccineus | no data | ||||||||
| Fuchsia sp. | p D | p D | 7.58 8,9 | 47.52 B | [5,67] | ||||
| Glechoma hederacea | p D | p D | no data | [68] | |||||
| Heliotropium oxalis | no data | ||||||||
| Helichrysum | 419.8 B | [69] | |||||||
| Hemerocallis | 21.0–29.0 A | [70] | |||||||
| Hibiscus sp. | 2080 | 5650 | 155–206 8 | 83.1 5 | [71,72,73,74] | ||||
| Impatiens | p D | p D | p D | no data | [75] | ||||
| Lavandula | 277.60 B | [68] | |||||||
| Lobelia | no data | ||||||||
| Lobularia maritima | p D | p D | no data | [76,77] | |||||
| Myosotis | 171.60 A | [68] | |||||||
| Pelargonium spp. | p D | p D | p D | p D | p D | p D | 12.52 8 | 34.78 B,9 | [5,78] |
| Petunia | 53.2 2 | 31.3 2 | 49.0 4 | 2.6 4 | 87.1 3 | 8.5 3 | 28–114 9 | 5.4–10.22 B,9 | [5,79,80] |
| Rosa | 357.0 | 31.2 | 140.4–153.1 10 | 2.3–7.0 8 | 71.4–397.4 A,9 | [58,62,81,82,83] | |||
| Tagetes erecta | 33 | 3.8 | 0.75 8 | 70.42 B 266.11 A | [5,84] | ||||
| Tagetes patula | 0.25 6,9 | p D | p D | p D | 0.076–0.433 6,9 | [60,85] | |||
| Torenia sp. | 0.9–41.0 9 | 210.96 | 4.2–134.9 9 | 5.0–152.7 9 | 8907.50 A | [55,86,87], | |||
| Tropaeolum majus | 4.77 8 | 32.208 8 | 32.06 8 | – | – | – | 68.12 8 | 7111–18,719 A,9 | [88] |
| Tulipa sp. | p D | p D | p D | 3.8–4.0 9 | 29.23 B,11 | [89,90,91,92] | |||
| Viola cornuta | 70.0 7 | 1350 7 | 25.0 B | [60,93] | |||||
| Viola wittrockiana | 1.9–16.7 9 | 8.6–21.8 9 | 8.8–14.2 9 | 1.2–15. 9 | 0.35–13.6 9 | 0.82–36.55 B,9 | [5,94,95] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysiak, G.P. Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods 2022, 11, 948. https://doi.org/10.3390/foods11070948
Łysiak GP. Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods. 2022; 11(7):948. https://doi.org/10.3390/foods11070948
Chicago/Turabian StyleŁysiak, Grzegorz P. 2022. "Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties" Foods 11, no. 7: 948. https://doi.org/10.3390/foods11070948
APA StyleŁysiak, G. P. (2022). Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods, 11(7), 948. https://doi.org/10.3390/foods11070948