Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electronic Tongue Analysis
2.3. Extraction of Metabolites
2.4. Metabolomic Analysis Using UHPLC-QE-MS/MS
2.5. Identification and Quantification of Free Amino Acids
2.6. Metabolic Pathway Enrichment Analysis
2.7. Data Processing and Statistical Analysis
3. Results and Discussion
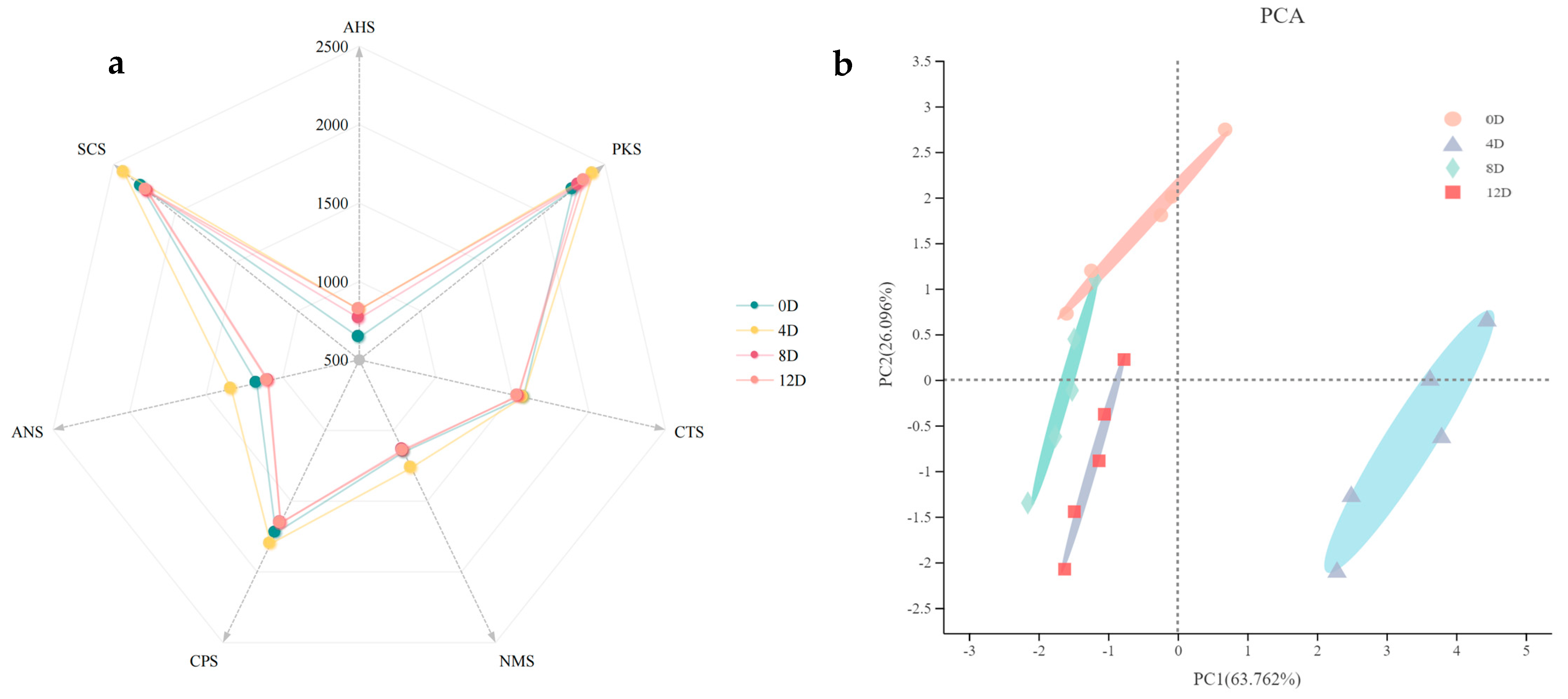
3.1. Analysis of the Taste Profiles during the Fermentation of Mandarin Fish
3.2. Multidimensional Statistical Analysis of the Metabolite Profiles during the Fermentation of Mandarin Fish
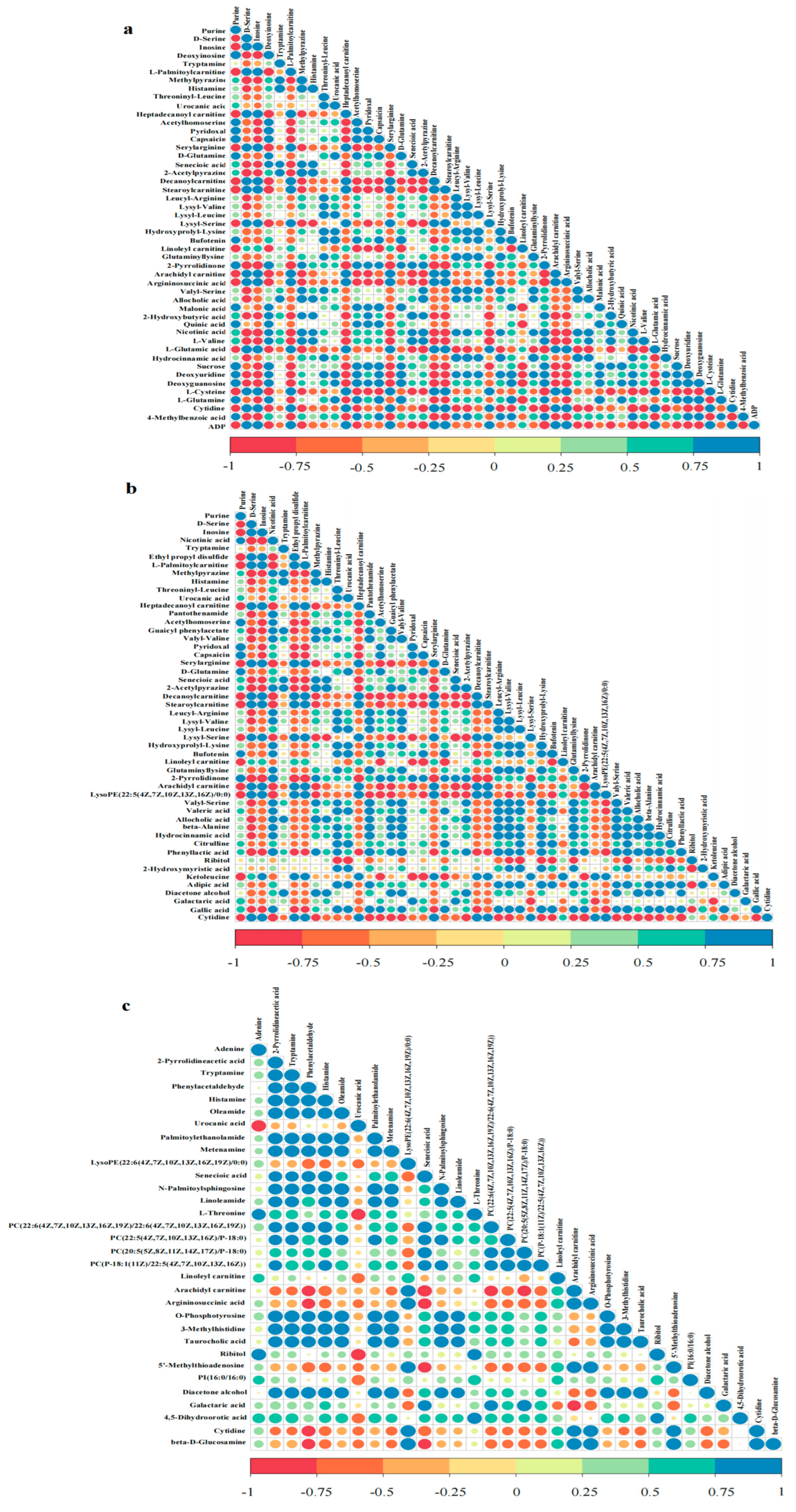
3.3. Screening for Differential Metabolites
3.4. Cluster Analysis of Differential Metabolites
3.4.1. Amino Acids
3.4.2. Small Peptides
3.4.3. Fatty Acid Class
3.4.4. Other Compounds
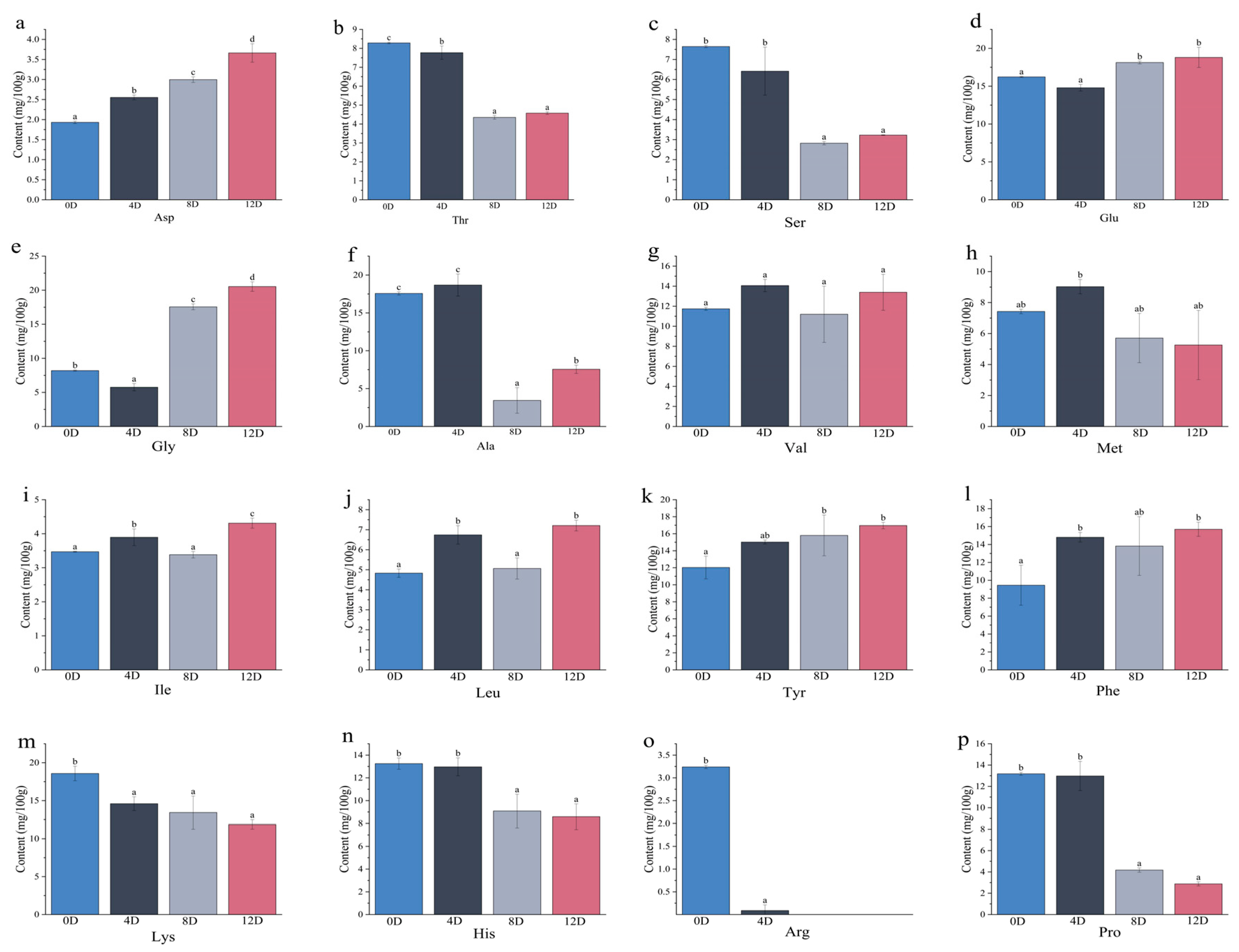
3.5. Quantitative Analysis of Free Amino Acids during Different Stages in the Fermentation of Mandarin Fish
3.6. Pathway Analysis of Differential Metabolites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Wang, Y.; Wu, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Zhao, Y.; Cen, J.; Yang, S.; et al. Investigation of fermentation-induced changes in the volatile compounds of Trachinotus ovatus (meixiangyu) based on molecular sensory and interpretable machine-learning techniques: Comparison of different fermentation stages. Food Res. Int. 2021, 150, 110739. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Niu, Y.; Ye, X.; Dong, J.; Hu, W.; Zeng, Q.; Chen, Z.; Tian, Y.; Zhang, J.; Lu, M. Construction of a high-density linkage map and mapping of sex determination and growth-related loci in the mandarin fish (Siniperca chuatsi). BMC Genom. 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liang, X.-F.; Fang, J. The optimal stocking density for hybrid of Siniperca chuatsi (♀) × Siniperca scherzeri (♂) mandarin fish fed minced prey fish. Aquac. Res. 2017, 48, 1342–1345. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Wang, Y.; Li, L.; Li, C.; Zhao, Y.; Yang, S. Contribution of autochthonous microbiota succession to flavor formation during Chinese fermented mandarin fish (Siniperca chuatsi). Food Chem. 2021, 348, 129107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, R.; Wei, X.; Zhang, Z.; Wang, W.; Liu, A.; Yang, J.; Ji, C.; Liang, H.; Zhang, S.; et al. Moderate fermentation contributes to the formation of typical aroma and good organoleptic properties: A study based on different brands of Chouguiyu. LWT 2021, 152, 112325. [Google Scholar] [CrossRef]
- Petrova, I.; Aasen, I.M.; Rustad, T.; Eikevik, T.M. Manufacture of dry-cured ham: A review. Part 1. Biochemical changes during the technological process. Eur. Food Res. Technol. 2015, 241, 587–599. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Duncan, A.; Bah, C.S.F.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Amin, H.F. Impact of fermentation conditions on the physicochemical properties, fatty acid and cholesterol contents in salted-fermented hoki roe. Food Chem. 2018, 264, 73–80. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.J.; Chao, Y.Z.; Wu, Z.Q.; Zhou, M.X.; Xiao, S.T.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef]
- Liu, J.; Lin, C.; Zhang, W.; Yang, Q.; Meng, J.; He, L.; Deng, L.; Zeng, X. Exploring the bacterial community for starters in traditional high-salt fermented Chinese fish (Suanyu). Food Chem. 2021, 358, 129863. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Zhao, Y.; Li, L.; Yang, X.; Cen, J.; Chen, S.; Li, C.; Wang, Y. Application of UHPLC-Q-TOF-MS/MS metabolomics approach to investigate the taste and nutrition changes in tilapia fillets treated with different thermal processing methods. Food Chem. 2021, 356, 129737. [Google Scholar] [CrossRef]
- Seo, S.-H.; Kim, E.-J.; Park, S.-E.; Park, D.-H.; Park, K.M.; Na, C.-S.; Son, H.-S. GC/MS-based metabolomics study to investigate differential metabolites between ale and lager beers. Food Biosci. 2020, 36, 100671. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Lau, H.; Lim, S.Y.; Li, S.F.Y.; Liu, S.-Q. Untargeted LC-QTOF-MS/MS based metabolomics approach for revealing bioactive components in probiotic fermented coffee brews. Food Res. Int. 2021, 149, 110656. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z.; Zhang, L. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Zhao, Y.; Wang, J.; Wei, Y.; Yang, D. Application of UHPLC-Q/TOF-MS-based metabolomics in the evaluation of metabolites and taste quality of Chinese fish sauce (Yu-lu) during fermentation. Food Chem. 2019, 296, 132–141. [Google Scholar] [CrossRef]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT 2020, 118, 108773. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Zheng, Y.; Tao, N.-P.; Wu, X.; Wang, X. Short-term rearing in brackish water regulates the taste-related metabolites of abdomen muscle for adult male Eriocheir sinensis. LWT 2021, 142, 110898. [Google Scholar] [CrossRef]
- Gao, B.; Hu, X.; Li, R.; Zhao, Y.; Tu, Y.; Zhao, Y. Screening of characteristic umami substances in preserved egg yolk based on the electronic tongue and UHPLC-MS/MS. LWT 2021, 152, 112396. [Google Scholar] [CrossRef]
- Breslin, P.A.S. Interactions among salty, sour and bitter compounds. Trends Food Sci. Tech. 1996, 7, 390–399. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kwak, H.S.; Kim, M.J.; Kim, Y.; Kim, K.-O.; Kim, S.S. Comparison of a descriptive analysis and instrumental measurements (electronic nose and electronic tongue) for the sensory profiling of Korean fermented soybean paste (doenjang). J. Sens. Stud. 2017, 32, e12282. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef]
- Xue, G.; Su, S.; Yan, P.; Shang, J.; Wang, J.; Yan, C.; Li, J.; Wang, Q.; Du, Y.; Cao, L.; et al. Quality control of Zingiberis Rhizoma and its processed products by UHPLC-Q-TOF/MS-based non-targeted metabonomics combining with SIBDV method. Food Res. Int. 2022, 154, 111021. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wu, Y.; Li, C.; Li, L.; Zhao, Y.; Hu, X.; Wei, Y.; Huang, H. Comparison of the microbial community and flavor compounds in fermented mandarin fish (Siniperca chuatsi): Three typical types of Chinese fermented mandarin fish products. Food Res. Int. 2021, 144, 110365. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, M.; Tian, L.; Liu, F.; Wang, Q.; Xu, M.; Rao, Z. Enhancing L-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresour. Technol. 2021, 341, 125799. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M.; Yu, D.; Yang, F.; Jiang, Q. Correlations between microbiota succession and flavor formation during fermentation of Chinese low-salt fermented common carp (Cyprinus carpio L.) inoculated with mixed starter cultures. Food Microbiol. 2020, 90, 103487. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Beauchamp, G.K. Salt enhances flavour by suppressing bitterness. Nat. Int. Wkly. J. Sci. 1997, 387, 563. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrAC Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, J.; Chen, W. Analysis of the relationship between microorganisms and flavour development in dry-cured grass carp by high-throughput sequencing, volatile flavour analysis and metabolomics. Food Chem. 2022, 368, 130889. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Kato, Y.; Yamazaki, J.; Kai, Y.; Mizukoshi, T.; Miyano, H.; Eto, Y. Determination and quantification of the kokumi peptide, γ-glutamyl-valyl-glycine in commercial soy Sauces. Food Chem. 2013, 141, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Ran, J.; Yu, C.; Yin, L.; Li, Z.; Liu, Y. The age-dependent variations for fatty acid composition and sensory quality of chicken meat and associations between gene expression patterns and meat quality. Livest. Sci. 2021, 254, 104736. [Google Scholar] [CrossRef]
- Anggo, A.D.; Ma’ruf, W.F.; Swastawati, F.; Rianingsih, L. Changes of amino and fatty acids in anchovy (Stolephorus Sp.) fermented fish paste with different fermentation periods. Procedia Environ. Sci. 2015, 23, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Khiabani, A.A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef]
- Schulz, M.; Brugnerotto, P.; Seraglio, S.K.T.; Gonzaga, L.V.; Borges, G.d.S.C.; Costa, A.C.O.; Fett, R. Aliphatic organic acids and sugars in seven edible ripening stages of jucara fruit (Euterpe edulis Martius). J. Food Compos. Anal. 2021, 95, 103683. [Google Scholar] [CrossRef]
- Zhao, M.-T.; Liu, Z.-Y.; Li, A.; Zhao, G.-H.; Xie, H.-K.; Zhou, D.-Y.; Wang, T. Gallic acid and its alkyl esters emerge as effective antioxidants against lipid oxidation during hot air drying process of Ostrea talienwhanensis. LWT 2021, 139, 110551. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, J.; Yin, H.; Duan, C.; Wang, B.; Li, W. Effects of dietary nicotinic acid supplementation on meat quality, carcass characteristics, lipid metabolism, and tibia parameters of Wulong geese. Poult. Sci. 2021, 100, 101430. [Google Scholar] [CrossRef]
- Kawai, M.; Sekine-Hayakawa, Y.; Okiyama, A.; Ninomiya, Y. Gustatory sensation of L- and D-amino acids in humans. Amino Acids 2012, 43, 2349–2358. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Lv, J.; Sun, Z.; Xu, W.; Ji, C.; Liang, H.; Li, S.; Yu, C.; Lin, X. Microbial succession and the changes of flavor and aroma in Chouguiyu, a traditional Chinese fermented fish. Food Biosci. 2020, 37, 100725. [Google Scholar] [CrossRef]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste enhancements between various amino acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Keska, P.; Stadnik, J. Taste-active peptides and amino acids of pork meat as components of dry-cured meat products: An in-silico study. J. Sens. Stud. 2017, 32, e12301. [Google Scholar] [CrossRef]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.-H.; Joo, S.-T. Volatile and nonvolatile taste compounds and their correlation with umami and flavor characteristics of chicken nuggets added with milkfat and potato mash. Food Chem. 2021, 343, 128499. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Nie, S.; Li, C.; Xiang, H.; Zhao, Y.; Chen, S.; Li, L.; Wu, Y. Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation. Foods 2022, 11, 944. https://doi.org/10.3390/foods11070944
Wang Y, Nie S, Li C, Xiang H, Zhao Y, Chen S, Li L, Wu Y. Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation. Foods. 2022; 11(7):944. https://doi.org/10.3390/foods11070944
Chicago/Turabian StyleWang, Yueqi, Shi Nie, Chunsheng Li, Huan Xiang, Yongqiang Zhao, Shengjun Chen, Laihao Li, and Yanyan Wu. 2022. "Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation" Foods 11, no. 7: 944. https://doi.org/10.3390/foods11070944
APA StyleWang, Y., Nie, S., Li, C., Xiang, H., Zhao, Y., Chen, S., Li, L., & Wu, Y. (2022). Application of Untargeted Metabolomics to Reveal the Taste-Related Metabolite Profiles during Mandarin Fish (Siniperca chuatsi) Fermentation. Foods, 11(7), 944. https://doi.org/10.3390/foods11070944









