Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Flour Characterization
2.3. Lactic Acid Bacteria (LAB)
2.4. Sourdough Preparation
2.5. Analyses of Sourdoughs
2.6. Bread Preparation
2.7. Degree of Immersion of the Doughs
2.8. Rheological Properties of the Doughs
2.9. Bread Quality
2.9.1. Physical Properties
2.9.2. Crumb Elasticity
2.10. Sensory Analysis
2.11. Statistical Analyses
3. Results and Discussion
3.1. Flour Composition
3.2. Formulation of the Control Gluten-Free Dough Matrix
3.3. Sourdough Fermentation
3.4. Development of Gluten-Free Bread with Sourdough
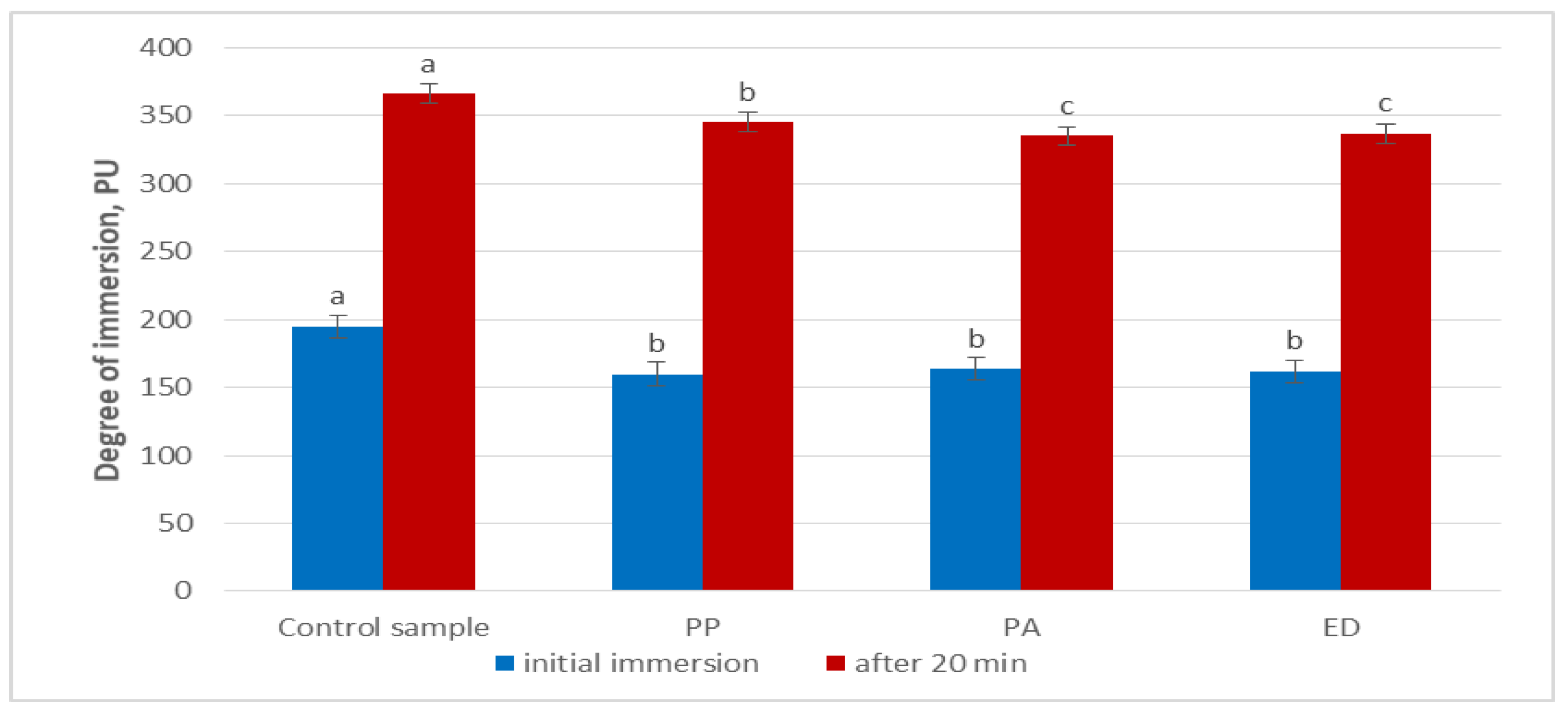
3.4.1. Degree of Immersion in the Nongluten Sourdoughs
3.4.2. Effect of Sourdoughs on the Rheological Characteristics of Nongluten Dough
3.4.3. Quality Assessment of Gluten-Free Breads Leavened with Sourdough
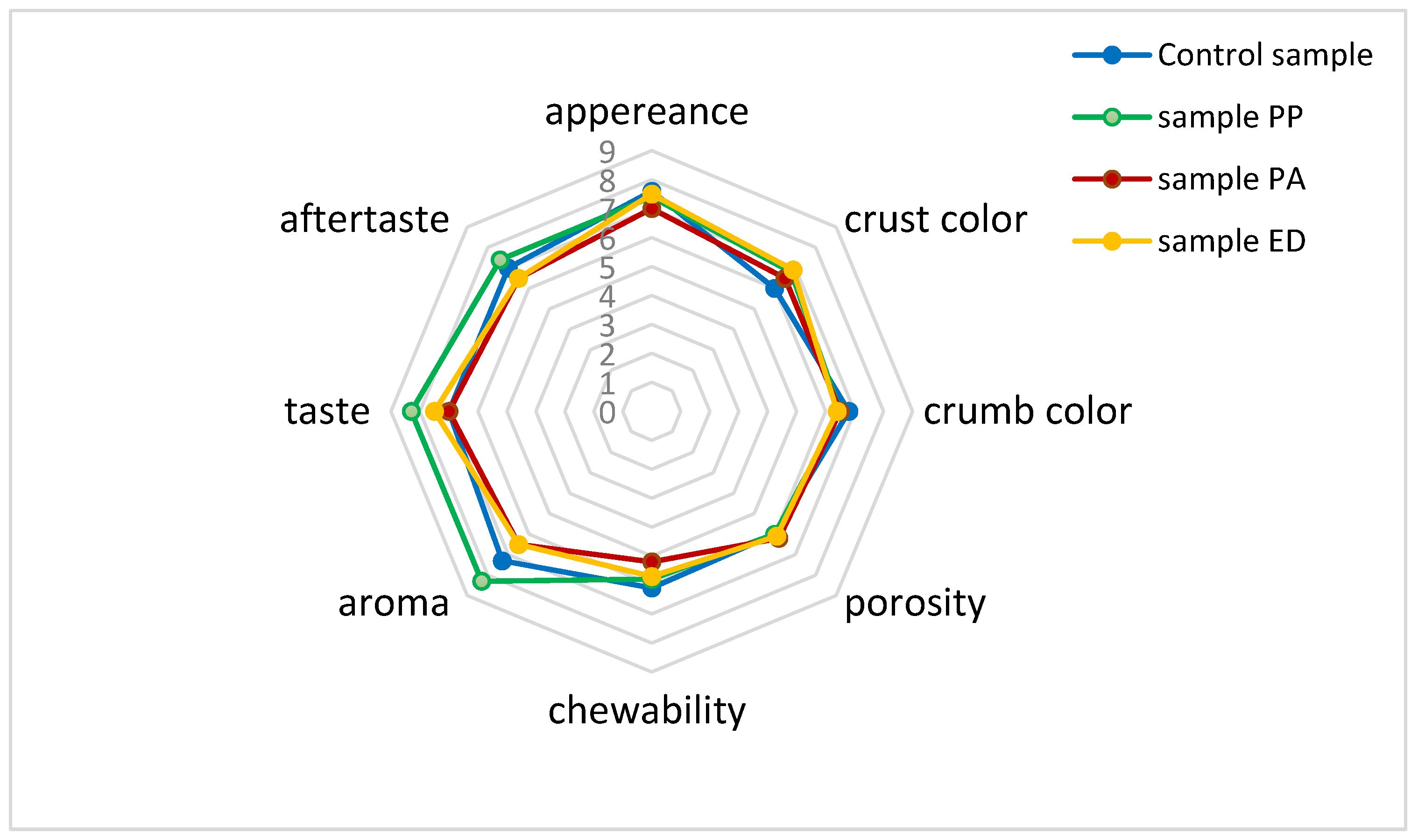
3.4.4. Sensory Profile of Nongluten Breads with Sourdough
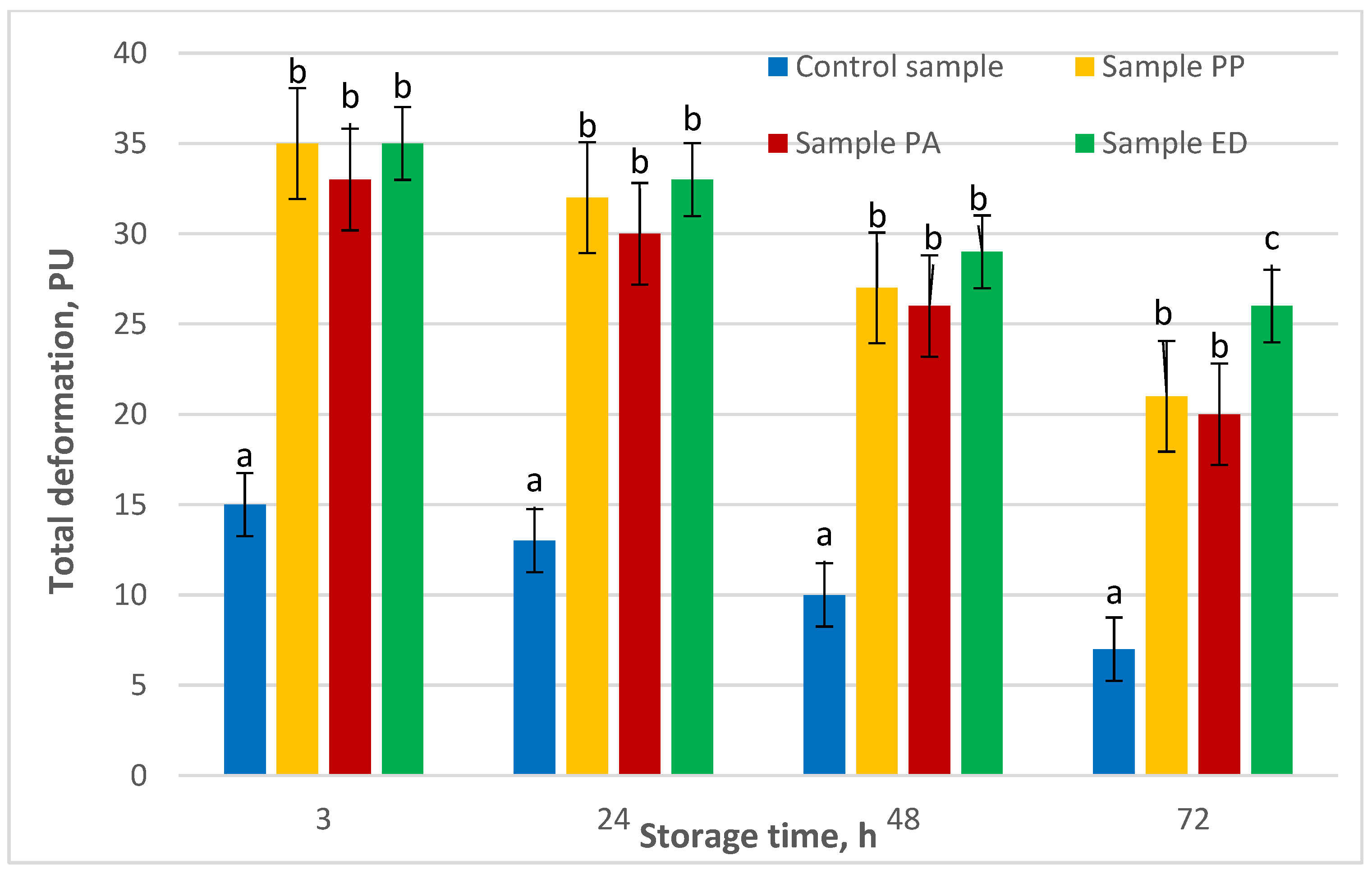
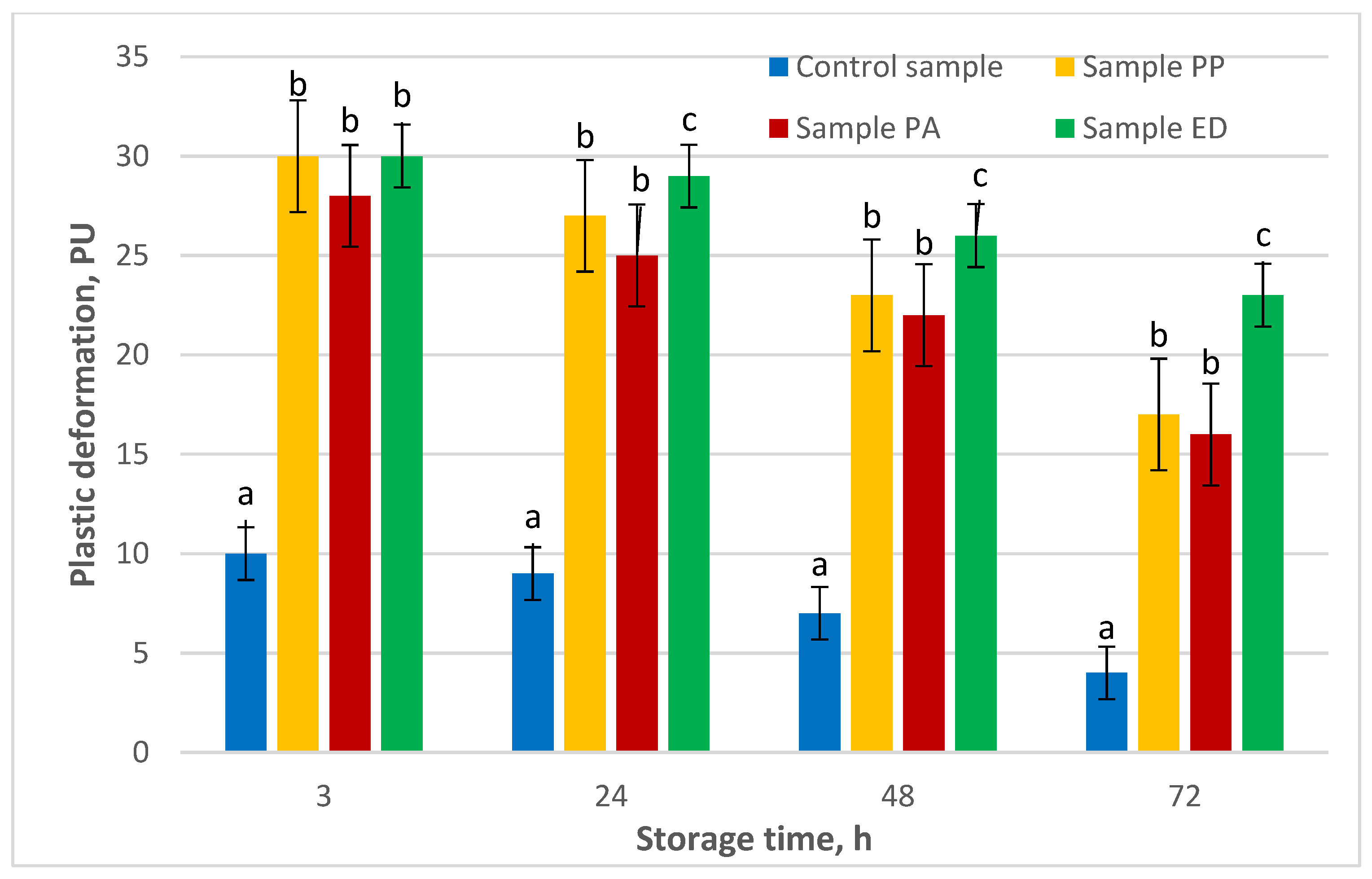
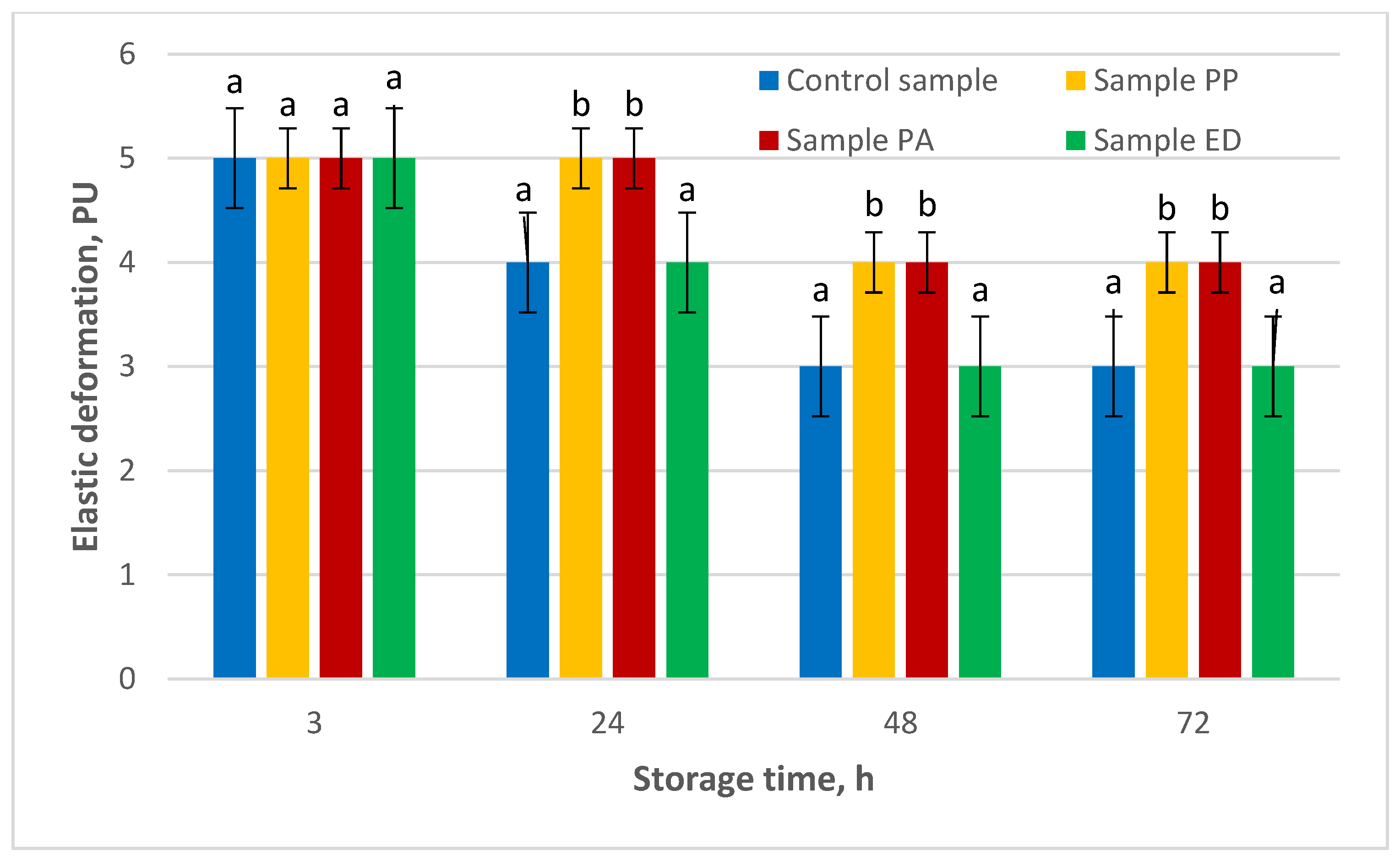
3.4.5. Shelf-Life Estimation of Nongluten Bread with Sourdough
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cappelli, A.; Oliva, N.; Cini, E. A systematic review of gluten-free dough and bread: Dough rheology, bread characteristics, and improvement strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Composition and Activity of Microbiota in Sourdough and Their Effect on Bread Quality and Safety. In Trends in Wheat and Bread Making; Galanakis, C.M., Ed.; Elsevier-Academic Press: Cambridge, MA, USA, 2020; p. 469. [Google Scholar]
- Rocha, J.M. Microbiological and Lipid Profiles of Broa: Contributions for the Characterization of a Traditional Portuguese Bread. Ph.D. Thesis, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal, 2011; p. 705. [Google Scholar]
- Galle, S.; Schwab, C.; dal Bello, F.; Coffey, A.; Ganzle, M.G.; Arendt, E.K.K. Influence of in situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Conte, A.; del Nobile, A.M. Overview on the general approaches to improve gluten-free pasta and bread. Foods 2016, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skendi, A.; Papageorgiou, M.; Varzakas, T. High protein substitutes for gluten in gluten-free bread. Foods 2021, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.M.; Pimentel, T.C.; de Rezende, T.A.M.; Silva, J.S.; Falcao, H.G.; Ida, E.I.; Egea, M.B. Gluten-free bread: Effect of soy and corn co-products on the quality parameters. Eur. Food Res. Technol. 2019, 245, 1365–1376. [Google Scholar] [CrossRef]
- Han, A.; Romero, H.M.; Nishijima, N.; Ichimura, T.; Handa, A.; Xu, C.; Zhang, Y. Effect of egg white solids on the rheological properties and bread making performance of gluten-free batter. Food Hydrocoll. 2019, 87, 287–296. [Google Scholar] [CrossRef]
- Pico, J.; Reguilón, M.P.; Bernal, J.; Manuel Gómez, M. Effect of rice, pea, egg white and whey proteins on crust quality of rice flour-corn starch based gluten-free breads. J. Cereal Sci. 2019, 86, 92–101. [Google Scholar] [CrossRef]
- Taghdir, M.; Mazloomi, S.M.; Honar, N.; Sepandi, M.; Ashourpour, M.; Salehi, M. Effect of soy flour on nutritional, physicochemical, and sensory characteristics of gluten-free bread. Food Sci. Nutr. 2017, 5, 439–445. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Turturică, M.; Rocha, J.M.; Bahrim, G.-E. Statistical approach to potentially enhance the postbiotication of gluten-free sourdough. Appl. Sci. 2021, 11, 5306. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.-E. Selection of wild lactic acid bacteria strains as promoters of postbiotics in gluten-free sourdoughs. Microorganisms 2020, 8, 643. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Mayrhofer, S.; Domig, K.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Vallons, K.J.R.; Ryan, L.A.M.; Arendt, E.K. Promoting structure formation by high pressure in gluten-free flours. LWT Food Sci. Technol. 2011, 44, 1672–1680. [Google Scholar] [CrossRef]
- Bender, D.; Gratz, M.; Vogt, S.; Fauster, T.; Wicki, B.; Pichler, S.; Kinner, M.; Jäger, H.; Schoenlechner, R. Ohmic heating—A novel approach for gluten-free bread baking. Food Bioprocess Technol. 2019, 12, 1603–1613. [Google Scholar] [CrossRef] [Green Version]
- Cappa, C.; Lucisano, M.; Raineri, A.; Fongaro, L.; Foschino, R.; Mariotti, M. Gluten-free bread: Influence of sourdough and compressed yeast on proofing and baking properties. Foods 2016, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Li, X.; Zhang, Y.; Yang, W.; Ma, G.; Ma, N.; Hu, Q.; Pei, F. A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Control 2020, 109, 106914. [Google Scholar] [CrossRef]
- Teleky, B.E.; Martau, G.A.; Vodnar, D.C. Physicochemical effects of Lactobacillus plantarum and Lactobacillus casei cocultures on soy-wheat flour dough fermentation. Foods 2020, 9, 1894. [Google Scholar] [CrossRef]
- Moore, M.M.; Heinbockel, M.; Dockery, P.; Ulmer, H.M.; Arendt, E.K. Network formation in gluten-free bread with application of transglutaminase. Cereal Chem. 2006, 83, 28–36. [Google Scholar] [CrossRef]
- Moore, M.M.; Juga, B.; Schober, T.J.; Arendt, E.K. Effect of lactic acid bacteria on properties of gluten-free sourdoughs, batters, and quality and ultrastructure of gluten-free bread. Cereal Chem. 2007, 84, 357–364. [Google Scholar] [CrossRef]
- Ngemakwe, P.H.; le Roes-Hill, M.; Jideani, V.A. Advances in gluten-free bread technology. Food Sci. Technol. Int. 2015, 21, 256–276. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef]
- Moroni, A.V.; dal Bello, F.; Arendt, E.K. Sourdough in gluten-free bread-making: An ancient technology to solve a novel issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef]
- Di Cagno, R.; de Angelis, M.; Lavermicocca, P.; de Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Deora, N.S.; Deswal, A.; Mishra, H.N. Alternative approaches towards gluten-free dough development: Recent trends. Food Eng. Rev. 2014, 6, 89–104. [Google Scholar] [CrossRef]
- Ren, Y.; Linter, B.R.; Linforth, R.; Foster, T.J. A comprehensive investigation of gluten free bread dough rheology, proving and baking performance and bread qualities by response surface design and principal component analysis. Food Funct. 2020, 11, 5333–5345. [Google Scholar] [CrossRef]
- Bartkiene, E.; Özogul, F.; Rocha, J.M. Bread sourdough lactic acid bacteria—Technological, antimicrobial, toxin-degrading, immune system- and faecal microbiota-modelling biological agents for the preparation of food, nutraceuticals and feed. Foods 2020, 11, 452. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yılmaz, B.; Şahin, T.O.; Güneşliol, B.E.; Ayten, S.; Russo, P.; Spano, G.; Rocha, J.M.; Bartkiene, E.; Özogul, F. Dairy lactic acid bacteria and their potential function in dietetics: The food-gut-health axis. Foods 2021, 10, 3099. [Google Scholar] [CrossRef]
- Sharma, H.; Özogul, F.; Bartkiéne, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2021, 30, 1–23. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the valorisation and functionalization of by-products and wastes from cereal-based processing industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of milk permeate with selected lactic acid bacteria strains and apple by-products into beverages with antimicrobial properties and enriched with galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT Food Sci. Technol. 2020, 118, 108769. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Zannini, E. Amaranth. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 439–473. [Google Scholar]
- Lu, H.; Guo, L.; Zhang, L.; Xie, C.; Li, W.; Gu, B.; Li, K. Study on quality characteristics of cassava flour and cassava flour short biscuits. Food Sci. Nutr. 2020, 8, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Skendi, A.; Mouselemidou, P.; Papageorgiou, M.; Papastergiadis, E. Effect of acorn meal-water combinations on technological properties and fine structure of gluten-free bread. Food Chem. 2018, 253, 119–126. [Google Scholar] [CrossRef]
- Angelov, A.; Yaneva-Marinova, T.; Gotcheva, V. Oats as a matrix of choice for developing fermented functional beverages. J. Food Sci. Technol. 2018, 55, 2351–2360. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Proximate composition, mineral content and fatty acids analyses of aromatic and non-aromatic Indian rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2013, 1312, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M.; Malcata, F.X. Microbial ecology dynamics in Portuguese broa sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Behavior of the complex micro-ecology in maize and rye flour and mother-dough for broa throughout storage. J. Food Qual. 2016, 39, 218–233. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 2012, 31, 72–88. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional Portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef]
- Dereje, N.; Bekele, G.; Nigatu, Y.; Worku, Y.; Holland, R.P. Glycemic index and load of selected Ethiopian foods: An experimental study. J. Diabetes Res. 2019, 5, 8564879. [Google Scholar] [CrossRef] [Green Version]
- Gebru, Y.A.; Hyun-II, J.; Young-Soo, K.; Myung-Kon, K.; Kwang-Pyo, K. Variations in amino acid and protein profiles in white versus brown teff (Eragrostis tef) seeds, and effect of extraction methods on protein yields. Foods 2019, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Fliedel, G.; Marti, A.; Thiebaut, S. Caracterisation et valorization du sorgho. Bibliogr. CIRAD 1996, 6, 349. [Google Scholar]
- Arendt, E.K.; Zannini, E. 10-Teff Cereal Grains for the Food and Beverage Industries; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 351–368. [Google Scholar]
- Dingeo, C.; Difonzo, G.; Paradiso, V.M.; Rizzello, C.G.; Pontonio, E. Teff type-I sourdough to produce gluten-free muffin. Microorganisms 2020, 8, 1149. [Google Scholar] [CrossRef]
- Bultosa, G.; Hall, A.N.; Taylor, J.R.N. Physico-chemical characterization of grain teff (Eragrostis tef (Zucc.) Trotter) starch. Starch 2002, 54, 461–468. [Google Scholar] [CrossRef]
- Campo, E.; del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016, 67, 75–82. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef]
- Barretto, R.; Buenavista, R.M.; Rivera, J.L.; Wang, S.; Vara Prasad, P.V.; Siliveru, K. Teff (Eragrostis tef) processing, utilization and future opportunities: A review. Int. J. Food Sci. Technol. 2021, 56, 3125–3137. [Google Scholar] [CrossRef]
- Moroni, A.V.; Arendt, E.K.; dal Bello, F. Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiol. 2011, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Harth, H.; van Kerrebroeck, S.; Vuyst, L. Impact of process conditions on the microbial community dynamics and metabolite production kinetics of teff sourdough fermentations under bakery and laboratory conditions. Food Sci. Nutr. 2018, 6, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Ogunsakin, O.A.; Banwo, K.; Ogunremi, O.R.; Sanni, A.I. Microbiological and physicochemical properties of sourdough bread from sorghum flour. Int. Food Res. J. 2015, 22, 2610–2618. [Google Scholar]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- AACC International. AACC Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Petkova, M.; Stefanova, P.; Gotcheva, V.; Kuzmanova, I.; Angelov, A. Microbiological and physicochemical characterization of traditional Bulgarian sourdoughs and screening of lactic acid bacteria for amylolytic activity. J. Chem. Technol. Metal. 2020, 55, 921–934. [Google Scholar]
- Petkova, M.; Stefanova, P.; Gotcheva, V.; Angelov, A. Isolation and characterisation of lactic acid bacteria and yeasts from typical Bulgarian sourdoughs. Microorganisms 2021, 9, 1346. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Petkova, N.; Teneva, D.; Denkova, Z.; Desev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- ICC. Standard Methods of the International Association for Cereal Science and Technology; ICC: Vienna, Austria, 2021. [Google Scholar]
- Novotni, D.; Čukelj, N.; Smerdel, B.; Bituh, M.; Dujmić, F.; Ćurić, D. Glycemic index and firming kinetics of partially baked frozen gluten-free bread with sourdough. J. Cereal Sci. 2012, 55, 120–125. [Google Scholar] [CrossRef]
- Kim, W.M.; Gyu-Hee Lee, G.H. Comparison of imported wheat flour bread making properties and korean wheat flour bread making properties made by various bread making methods. J. Korean Soc. Food Sci. Nutr. 2015, 44, 434–441. [Google Scholar] [CrossRef]
- Lönner, C.; Preve-Akesson, K. Effects of lactic acid bacteria on the properties of sour dough bread. Food Microbiol. 1989, 6, 19–35. [Google Scholar] [CrossRef]
- Zlateva, D.; Chochkov, R. Effect of Spirulina platensis on the crumb firming of wheat bread during storage. Ukr. Food J. 2019, 8, 851–860. [Google Scholar] [CrossRef]
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance. ISO: Geneva, Switzerland, 2017.
- Bower, J.A. Statistics for food science V: ANOVA and multiple comparisons. Nutr. Food Sci. 1998, 98, 41–48. [Google Scholar] [CrossRef]
- Puncha-arnon, S.; Uttapap, D. Rice starch vs. rice flour: Differences in their properties when modified by heat–moisture treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef]
- Gebru, Y.A.; Sbhatu, D.B.; Kim, K.P. Nutritional composition and health benefits of teff (Eragrostis tef (Zucc.) Trotter). J. Food Qual. 2020, 6, 9595086. [Google Scholar] [CrossRef]
- Di Monaco, R.; Torrieri, E.; Pepe, O.; Masi, P.; Cavella, S. Effect of sourdough with exopolysaccharide (EPS)-producing lactic acid bacteria (LAB) on sensory quality of bread during shelf life. Food Bioproc. Technol. 2015, 8, 691–701. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; di Nunzio, M.; Viadel, B.; Gallego, E.; Laure, D. Integrated evaluation of the potential health benefits of einkorn-based breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef] [Green Version]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Influence of levan-producing acetic acid bacteria on buckwheat-sourdough breads. Food Microbiol. 2017, 65, 95–104. [Google Scholar] [CrossRef]
- Hadaegh, H.; Seyyedain Ardabili, S.; Tajabadi Ebrahimi, M.; Chamani, M.; Azizi Nezhad, R. The impact of different lactic acid bacteria sourdoughs on the quality characteristics of toast bread. J. Food Qual. 2017, 11, 7825203. [Google Scholar] [CrossRef] [Green Version]
- Topuzova, Y.A.; Karadzhov, G.I.; Chonova, V.M. Basic raw materials used for production of gluten-free bakery and confectionery products. Sci. Works UFT 2012, 59, 439–443. [Google Scholar]
- Alaunyte, I.; Stojceska, V.; Plunkett, A.; Ainsworth, P.; Derbyshire, E. Improving the quality of nutrient-rich Teff (Eragrostis tef) breads by combination of enzymes in straight dough and sourdough breadmaking. J. Cereal Sci. 2012, 55, 22–30. [Google Scholar] [CrossRef]
- Hager, A.S.; Arendt, E.K. Influence of hydroxypropylmethylcellulose (HPMC), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocol. 2013, 32, 195–203. [Google Scholar] [CrossRef]
- Neela, S.; Fanta, S.W. Injera (An ethnic, traditional staple food of Ethiopia): A review on traditional practice to scientific developments. J. Ethn. Food 2020, 7, 32. [Google Scholar] [CrossRef]
- Arendt, E.K.; Morrissey, A.; Moore, M.M.; dal Bello, F. Gluten-Free Breads. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., dal Bello, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 289–319. [Google Scholar]
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and technological characterization of two C4 gluten-free cereals: Sorghum bicolor and Eragrostis tef. Cereal Chem. 2020, 97, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Arendt, E.K.; dal Bello, F. Functional Cereal Products for Those with Gluten Intolerance. In Technology of Functional Cereal Products; Hamaker, B.R., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 446–475. [Google Scholar]
- Duodu, K.G.; Taylor, J.R.N. The Quality of Breads Made with Non-Wheat Flours. In Breadmaking; Cauvain, S.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 754–782. [Google Scholar]
- Marti, A.; Marengo, M.; Bonomi, F.; Casiraghi, M.C.; Franzetti, L.; Pagani, M.A.; Iametti, S. Molecular features of fermented teff flour relate to its suitability for the production of enriched gluten-free bread. LWT 2017, 78, 296–302. [Google Scholar] [CrossRef]
- Yilmaz, H.O.; Arslan, M. Teff: Nutritional compounds and effects on human health. Acta Sci. Med. Sci. 2018, 2, 15–18. [Google Scholar]
- Olojede, A.O.; Sannic, A.I.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT Food Sci. Technol. 2020, 120, 108875. [Google Scholar] [CrossRef]
- Rico, D.; Ronda, F.; Villanueva, M.; Perez Montero, C.; Martin-Diana, A.B. Development of healthy gluten-free crackers from white and brown teff (Eragrostis tef Zucc.) flours. Heliyon 2019, 5, e02598. [Google Scholar] [CrossRef] [Green Version]
- Chanapamokkhot, H.; Thongngam, M. The chemical and physico-chemical properties of sorghum starch and flour. Kasetsart J. 2007, 41, 343–349. [Google Scholar]
- Pandya, T.S.; Srinivasan, R. Effect of hammer mill retention screen size on fiber separation from corn flour using the Elusieve process. Ind. Crops Prod. 2012, 35, 37–43. [Google Scholar] [CrossRef]
- Zannini, E.; Pontonio, E.; Waters, D.M.; Arendt, E.K. Applications of microbial fermentations for production of gluten-free products and perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ćuric, D.; Novotni, D.; Tusak, D.; Bauman, I.; Gabric, D. Gluten-free bread production by the corn meal and soybean flour extruded blend usage. Agric. Consp. Sci. 2007, 72, 227–232. [Google Scholar]
- Wolter, A.; Hager, A.S.; Zannini, E.; Czerny, M.; Arendt, E.K. Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads. Eur. Food Res. Technol. 2014, 239, 1–12. [Google Scholar] [CrossRef]
- Nami, Y.; Gharekhani, M.; Aalami, M.; Hejazi, M.A. Lactobacillus-fermented sourdoughs improve the quality of gluten-free bread made from pearl millet flour. J. Food Sci. Technol. 2019, 56, 4057–4067. [Google Scholar] [CrossRef]
- Edema, M.O.; Emmambux, M.N.; Taylor, J.R.N. Improvement of fonio dough properties through starch modification by sourdough fermentation. Starch 2013, 65, 730–737. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Boyle, D.L. Gluten-free sorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. J. Agric. Food Chem. 2007, 55, 5137–5146. [Google Scholar] [CrossRef]
- Sahin, A.W.; Wiertz, J.; Arendt, E.K. Evaluation of a new method to determine the water addition level in gluten-free bread systems. J. Cereal Sci. 2020, 93, 102971. [Google Scholar] [CrossRef]
- Tafti, A.G.; Peighardoust, S.H.; Behnam, F.; Bahrami, A.; Aghagholizadeh, R.; Ghamari, M.; Rafat, S.A. Effects of spray-dried sourdough on flour characteristics and rheological properties of dough. Czech J. Food Sci. 2013, 31, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.M.; dal Bello, F.; Arendt, E.K. Sourdough fermented by Lactobacillusplantarum FST 1.7 improves the quality and shelf life of gluten-free bread. Eur. Food Res. Technol. 2008, 226, 1309–1316. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef]
- Axel, C.; Rocker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, G.C.; de Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of selected sourdough strains of Lactobacillus for removing gluten and enhancing the nutritional properties of gluten-free bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef]
- Falade, A.T.; Emmambux, M.N.; Buys, E.M.; Taylor, J.R.N. Improvement of maize bread quality through modification of dough, rheological properties by lactic acid bacteria fermentation. J. Cereal Sci. 2014, 60, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, M.F.T.; Jalali, H.; Nafchi, A.M.; Nouri, L. Evaluating the effects of lactic acid bacteria and olive leaf extract on the quality of gluten-free bread. Gene Reports 2020, 21, 100771. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Tieking, M.; Gänzle, M.G. Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- Belz, M.C.E.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of taste and shelf life of yeasted low-salt bread containing functional sourdoughs using Lactobacillus amylovorus DSM 19280 and Weisella cibaria MG1. Int. J. Food Microbiol. 2019, 302, 69–79. [Google Scholar] [CrossRef]
| Ingredients | Variants | |||
|---|---|---|---|---|
| A | B | C | ||
| Quantity, % | ||||
| Teff flour | Gluten-free flour base | 40 | 40 | 40 |
| Rice flour | 40 | 40 | 40 | |
| Sorghum flour | 10 | 10 | 10 | |
| Corn flour | 10 | 10 | 10 | |
| Other ingredients, g/100 g Gluten-free flour base | ||||
| Water | 65 | 65 | 65 | |
| Baker’s yeast | 3.0 | 3.0 | 3.0 | |
| Salt | 1.5 | 1.5 | 1.5 | |
| Xanthan gum | 1.0 | 0.6 | 0.6 | |
| Guar gum | 1.0 | 1.0 | 1.0 | |
| Carboxymethyl cellulose (CMC) | 1.0 | 3.0 | ||
| Ingredients | Control Sample | Sample PP | Sample PA | Sample ED | |
|---|---|---|---|---|---|
| Quantity, % | |||||
| Teff flour | Gluten-free flour base | 40 | 32.8 | ||
| Rice flour | 40 | 40.0 | |||
| Sorghum flour | 10 | 10.0 | |||
| Corn flour | 10 | 10.0 | |||
| Other ingredients, g/100 g Gluten-free flour base | |||||
| Water | 65 | 52.4 | |||
| Baker’s yeast | 3.0 | - | |||
| Salt | 1.5 | 1.5 | |||
| Xanthan gum | 0.6 | 0.6 | |||
| Guar gum | 1.0 | 1.0 | |||
| Carboxymethyl cellulose (CMC) | 1.0 | 1.0 | |||
| Sourdough (Teff flour + Water + LAB) | 21.5 | ||||
| Samples | Rheological Characteristics | ||||
|---|---|---|---|---|---|
| Water Absorption, % | Consistency, FU | DDT, min | Stability, min | Degree of Softening, FU | |
| Control sample | 65 ± 3.56 a | 350 ± 1.41 a | 6.0 ± 0.82 a | 10.0 ± 0.82 a | 10 ± 0.82 a |
| Sample PP | 65 ± 5.65 a | 290 ± 1.83 b | 2.0 ± 0.82 b | 7.5 ± 0.08 b | 30 ± 0.82 b |
| Sample PA | 65 ± 0.82 a | 290 ± 2.58 b | 1.5 ± 0.22 b | 7.5 ± 0.08 b | 30 ± 0.82 b |
| Sample ED | 65 ± 5.72 a | 290 ± 0.82 b | 1.5 ± 0.08 b | 6.5 ± 0.29 c | 30 ± 0.82 b |
| Flour | Moisture Content, % | Protein Content, % (d.m.) | Fiber Content, % (d.m.) | Crude Fat, % (d.m.) | Starch Content, % (d.m.) |
|---|---|---|---|---|---|
| Teff | 10.18 ± 0.16 a | 10.20 ± 0.13 a | 12.39 ± 0.13 a | 3.09 ± 0.16 a | 74.5 ± 0.80 a |
| Rice | 10.00 ± 0.14 a | 6.99 ± 0.10 b | 1.47 ± 0.16 b | 0.59 ± 0.09 b | 84.7 ± 1.07 b |
| Sorghum | 11.04 ± 0.24 b | 12.49 ± 0.46 c | 9.56 ± 0.08 c | 4.54 ± 0.12 c | 72.2 ± 0.92 c |
| Corn | 9.05 ± 0.16 c | 4.20 ± 0.09 d | 3.74 ± 0.12 d | 3.73 ± 0.08 d | 73.4 ± 0.77 d |
| Dough Variant | Preliminary Degree of Immersion, PU | Degree of Immersion After 20 min, PU |
|---|---|---|
| A | 70 ± 1.63 a | 117 ± 3.74 a |
| B | 195 ± 4.55 b | 366 ± 0.82 b |
| C | 118 ± 1.63 c | 190 ± 3.46 c |
| Dough Variant | Height/Diameter | Specific Volume, cm3/g | Bake Loss, % | ||
|---|---|---|---|---|---|
| Floor Bread | Floor Bread | Pan Bread | Floor Bread | Pan Bread | |
| A | 0.45 ± 0.03 a | 0.90 ± 0.08 a | 1.12 ± 0.09 a | 22.61 ± 0.07 a | 20.68 ± 0.09 a |
| B | 0.45 ± 0.02 a | 1.40 ± 0.06 b | 1.85 ± 0.09 b | 20.87 ± 0.11 b | 17.72 ± 0.05 b |
| C | 0.36 ± 0.01 b | 0.95 ± 0.05 a | 0.88 ± 0.09 c | 21.30 ± 0.64 b | 16.14 ± 0.67 c |
| Strain/Time | Pediococcus acidilactici 02P108 (PA) | Pediococcus pentosaceus 12R2187 (PP) | Enteroccocus durans 09B374 (ED) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | TTA | log CFU/g | pH | TTA | log CFU/g | pH | TTA | log CFU/g | |
| 0 h | 5.92 ± 0.06 | 4.2 ± 0,25 | 3.00 ± 0.11 | 5.92 ± 0.18 | 4.2 ± 0.29 | 2.95 ± 0.19 | 5.92 ± 0.18 | 4.2 ± 0.40 | 2.90 ± 0.57 |
| 4 h | 5.48 ± 0.10 | 4.6 ± 0.26 | 3.08 ± 0.21 | 5.40 ± 0.23 | 4.7 ± 0.31 | 3.04 ± 0.18 | 5.26 ± 0.14 | 4.6 ± 0.31 | 2.95 ± 0.38 |
| 8 h | 5.26 ± 0.10 | 5.8 ± 0.13 | 4.63 ± 0.64 | 5.32 ± 0.18 | 5.6 ± 0.26 | 4.62 ± 0.31 | 5.12 ± 0.36 | 5.9 ± 0.26 | 4.71 ± 0.59 |
| 12 h | 4.88 ± 0.14 | 9.6 ± 0.31 | 6.26 ± 0.35 | 5.02 ± 0.28 | 8.8 ± 0.25 | 6.75 ± 0.21 | 4.65 ± 0.26 | 10.0 ± 0.26 | 6.86 ± 0.67 |
| 16 h | 4.32 ± 0.15 | 13.2 ± 0.25 | 6.79 ± 0.49 | 4.64 ± 0.19 | 13.4 ± 0.38 | 6.95 ± 0.47 | 4.28 ± 0.35 | 15.3 ± 0.29 | 7.00 ± 0.71 |
| 20 h | 4.12 ± 0.10 | 14.9 ± 0.45 | 8.15 ± 0.24 | 4.04 ± 0.38 | 14.2 ± 0.28 | 8.28 ± 0.26 | 4.15 ± 0.12 | 17.1 ± 0.31 | 8.53 ± 0.49 |
| 24 h | 4.02 ± 0.10 | 15.2 ± 0.26 | 8.72 ± 0.59 | 3.88 ± 0.27 | 14.6 ± 0.33 | 8.81 ± 0.51 | 4.10 ± 0.21 | 18.4 ± 0.34 | 8.83 ± 0.27 |
| a | a | a | a | a | a | a | b | a | |
| Dough Samples | Specific Volume, cm3/g | Height/ Diameter | Baking Loss, % | ||
|---|---|---|---|---|---|
| Floor Bread | Pan Bread | Floor Bread | Floor Bread | Pan Bread | |
| Control sample | 1.49 ± 0.01 a | 1.55 ± 0.01 a | 0.36 ± 0.01 a | 20.86 ± 0.06 a | 17.72 ± 0.05 a |
| Sample PP | 1.55 ± 0.06 a | 1.24 ± 0.03 b | 0.30 ± 0.04 a | 18.90 ± 0.06 b | 13.02 ± 0.03 b |
| Sample PA | 1.59 ± 0.01 b | 1.46 ± 0.01 c | 0.36 ± 0.03 a | 16.70 ± 0.01 c | 12.25 ± 0.17 c |
| Sample ED | 1.70 ± 0.03 c | 1.56 ± 0.02 a | 0.48 ± 0.04 b | 15.65 ± 0.04 d | 12.59 ± 0.07 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chochkov, R.; Savova-Stoyanova, D.; Papageorgiou, M.; Rocha, J.M.; Gotcheva, V.; Angelov, A. Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality. Foods 2022, 11, 1012. https://doi.org/10.3390/foods11071012
Chochkov R, Savova-Stoyanova D, Papageorgiou M, Rocha JM, Gotcheva V, Angelov A. Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality. Foods. 2022; 11(7):1012. https://doi.org/10.3390/foods11071012
Chicago/Turabian StyleChochkov, Rosen, Daniela Savova-Stoyanova, Maria Papageorgiou, João Miguel Rocha, Velitchka Gotcheva, and Angel Angelov. 2022. "Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality" Foods 11, no. 7: 1012. https://doi.org/10.3390/foods11071012
APA StyleChochkov, R., Savova-Stoyanova, D., Papageorgiou, M., Rocha, J. M., Gotcheva, V., & Angelov, A. (2022). Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality. Foods, 11(7), 1012. https://doi.org/10.3390/foods11071012









