Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Experimental Design
2.4. Extraction Assays
2.4.1. Ultrasound-Assisted Extraction (UAE)
2.4.2. Conventional Solvent Extraction (CSE)
2.5. Determination of Antioxidant Capacity
2.6. Determination of Total Phenolic Content and Total Flavonoid Content
2.7. Identification of Antioxidant Phytochemicals in Kiwifruit by UPLC–MS/MS
2.8. Quantification of Main Phytochemicals in Kiwifruit by HPLC
2.9. Statistical Analysis
3. Results and Discussion
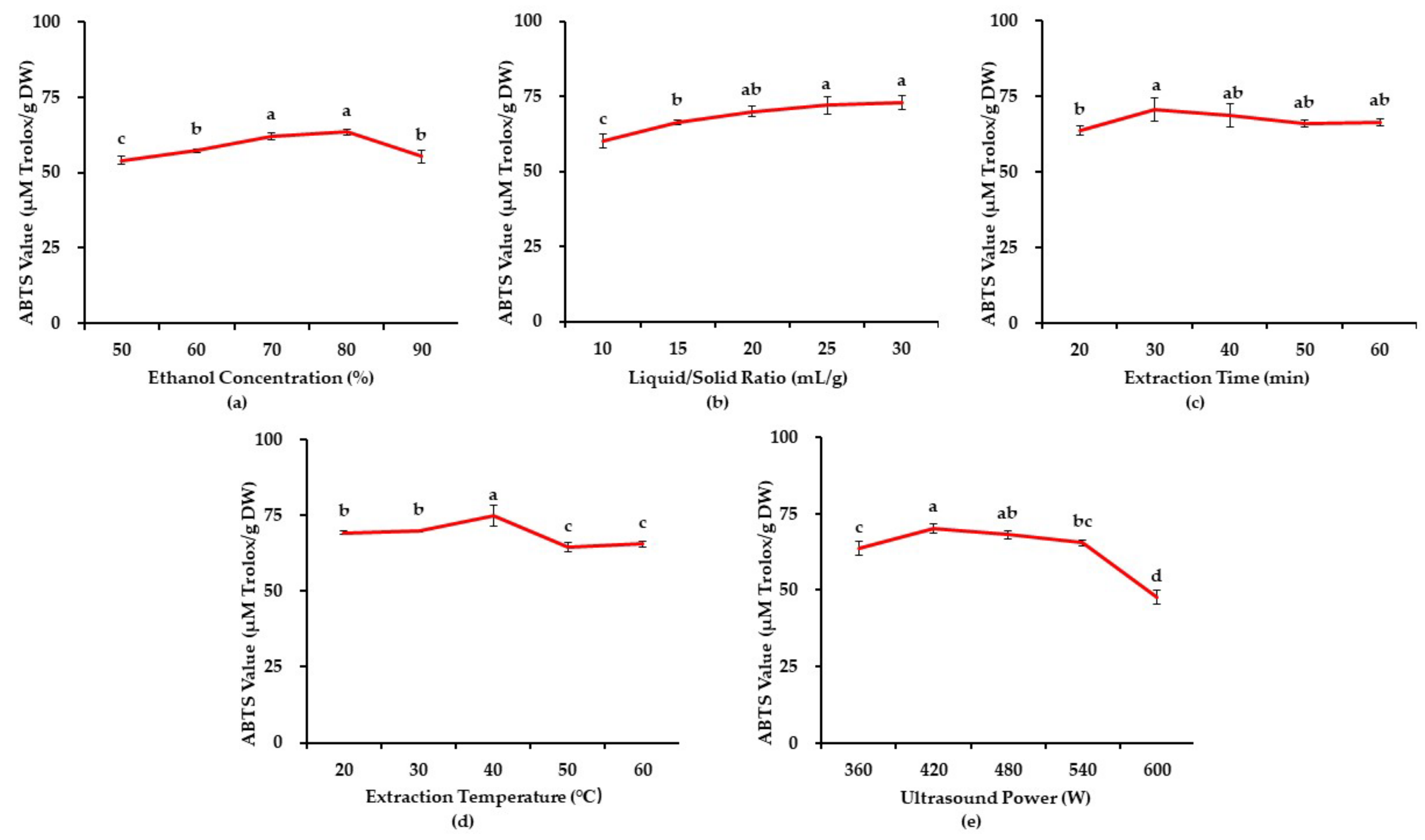
3.1. Results of Single-Factor Experiment
3.1.1. The Effects of Ethanol Concentration
3.1.2. The Effects of Liquid/Solid Ratio
3.1.3. The Effects of Extraction Time
3.1.4. The Effects of Extraction Temperature
3.1.5. The Effects of Ultrasound Power
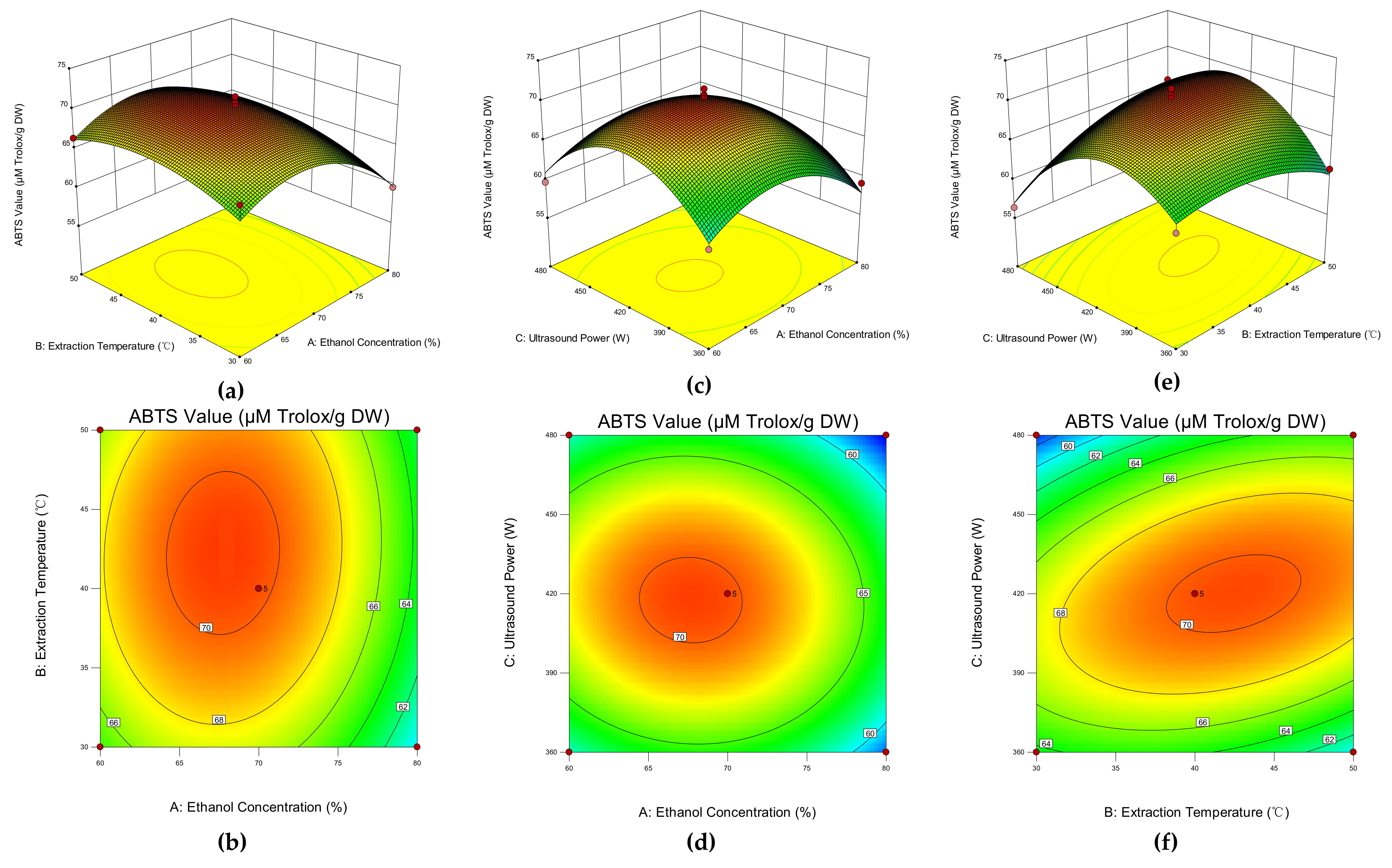
3.2. Response Surface Methodology
3.2.1. Response Surface Design and Experimental Results
3.2.2. Fitting the Model
3.2.3. Model Analysis
3.2.4. Verification of the Optimal Extraction Condition
3.3. Comparison of Extraction Methods
3.4. Total Phenolic and Flavonoid Contents of Kiwifruit
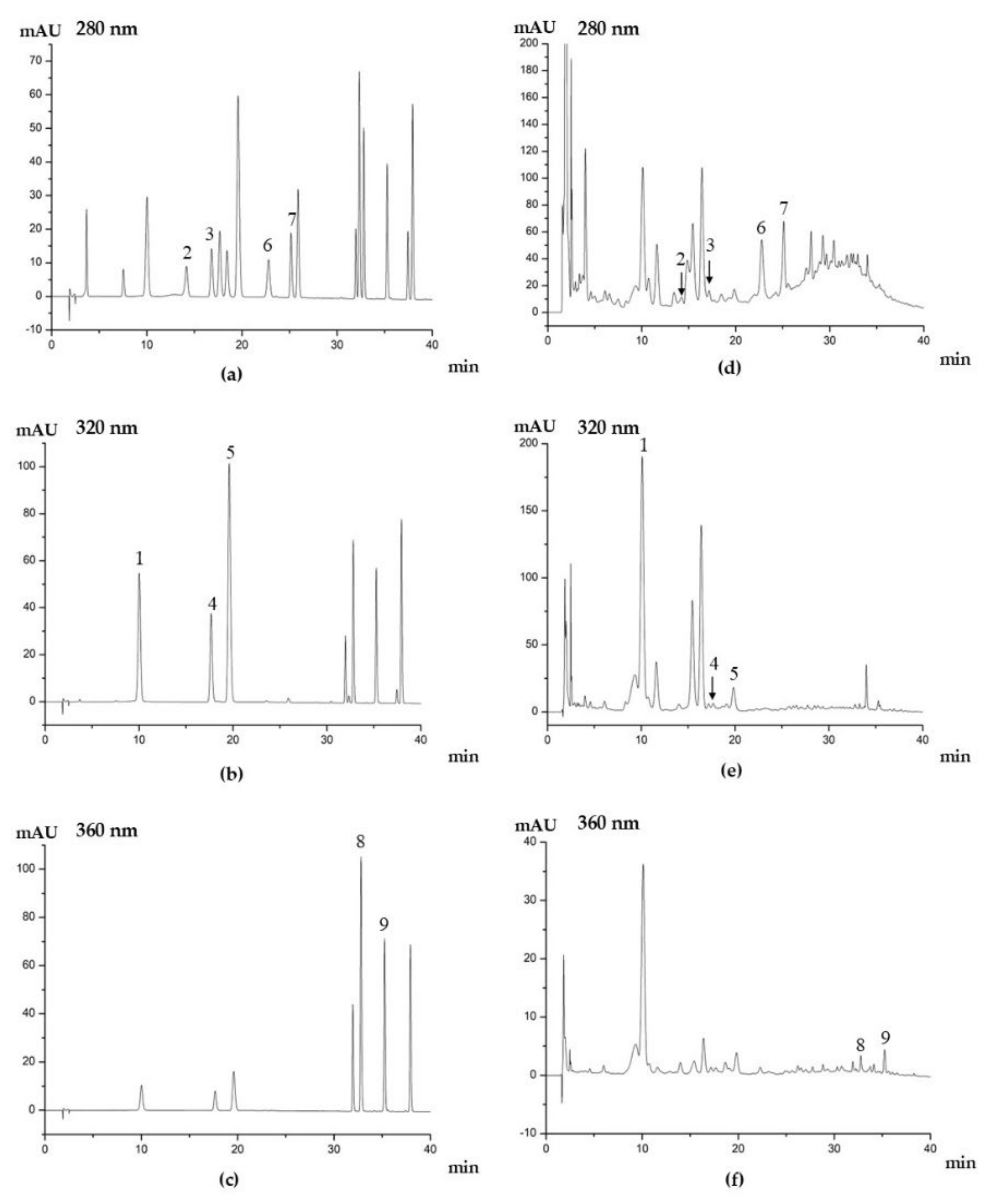
3.5. Identification of Antioxidant Phytochemicals in Kiwifruit
| No. | RT (min) | [M–H]− | MS2 Ion Fragments (m/z) | Formula | Identified Compounds | References |
|---|---|---|---|---|---|---|
| 1 | 0.90 | 175 | 115, 87 | C6H8O6 | Ascorbic acid | [47] |
| 2 | 0.98 | 115 | 71 | C4H4O4 | Fumaric acid | [49,51] |
| 3 | 1.01 | 133 | 115 | C4H6O5 | Malic acid | [49,51] |
| 4 | 1.17 | 129 | 85 | C5H6O4 | Itaconic acid | [50] |
| 5 | 1.3 | 191 | 111 | C6H8O7 | Citric acid | [49] |
| 6 | 1.38 | 117 | 73 | C4H6O4 | Succinic acid | [49,51] |
| 7 | 2.61 | 218 | 88 | C9H17NO5 | D-Pantothenic acid | [48] |
| 8 | 3.17 | 315 | 153 | C13H16O9 | Protocatechuic acid-O-hexoside | [46,55] |
| 9 | 3.80 | 353 | 191, 179, 135 | C16H18O9 | Neochlorogenic acid | [25,55] |
| 10 | 4.97 | 339 | 177 | C15H18O9 | Esculin | [58] |
| 11 | 7.16 | 289 | 245, 179, 205 | C15H14O6 | Catechin | [25,46] |
| 12 | 7.26 | 341 | 179, 135 | C15H18O9 | Caffeic acid-O-hexoside | [55] |
| 13 | 7.90 | 177 | 133 | C9H6O4 | Esculetin | [46] |
| 14 | 8.75 | 179 | 135 | C9H8O4 | Caffeic acid | [25,54] |
| 15 | 10.07 | 353 | 191, 179, 135 | C16H18O9 | Chlorogenic acid | [25,55] |
| 16 | 11.07 | 577 | 407, 289, 245 | C30H26O12 | Procyanidin dimer B-type isomer | [46,59] |
| 17 | 11.65 | 577 | 407, 289, 245 | C30H26O12 | Procyanidin dimer B-type isomer | [46,59] |
| 18 | 13.69 | 289 | 245, 179, 125 | C15H14O6 | Epicatechin | [25,46,55] |
| 19 | 26.44 | 463 | 301, 151 | C21H20O12 | Quercetin 3-O-glucoside | [46,55] |
| 20 | 29.33 | 447 | 301, 151 | C21H20O11 | Quercetin 3-O-rhamnoside | [46,55] |
3.6. Quantification of Ascorbic Acid and Phenolic Compounds in Kiwifruit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.-Y.; Yuan, Q.; Yang, Y.-L.; Han, Q.-H.; He, J.-L.; Zhao, L.; Zhang, Q.; Liu, S.-X.; Lin, D.-R.; Wu, D.-T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef] [PubMed]
- Guroo, I.; Wani, S.A.; Wani, S.M.; Ahmad, M.; Mir, S.A. A review of production and processing of kiwifruit. J. Food Process. Technol. 2017, 8, 100699. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Wang, Y.; Jiang, Z.; Wang, S.; Huang, H. Vitamin C, flower color and ploidy variation of hybrids from a ploidy-unbalanced Actinidia interspecific cross and SSR characterization. Euphytica 2010, 175, 133–143. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen kiwifruit (Actinidia) cultivars—comparative analysis of fifteen kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-E.; Cho, C.-H.; Kang, H.; Heo, H.J.; Cho, Y.-S.; Kim, D.-O. Kiwifruit of Actinidia eriantha cv. Bidan has in vitro antioxidative, anti-inflammatory and immunomodulatory effects on macrophages and splenocytes isolated from male BALB/C mice. Food Sci. Biotechnol. 2018, 27, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.M.; You, L.-J.; Chen, C.; Zhao, Z.-G.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles and antioxidant and antiproliferative activities of kiwifruit (Actinidia deliciosa) cultivars. J. Food Biochem. 2019, 43, e13025. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Q.; Zhang, Q.; Tuo, X.; Fan, D.; Deng, J.; Yang, H. Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 2019, 125, 85–94. [Google Scholar] [CrossRef]
- Monro, J.A.; Paturi, G. Kiwifruit skin and flesh contributions to fecal bulking and bacterial abundance in rats. Plant Foods Hum. Nutr. 2020, 75, 525–531. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Liu, Y.; Yang, X. Consumption of two whole kiwifruit (Actinide chinensis) per day improves lipid homeostasis, fatty acid metabolism and gut microbiota in healthy rats. Int. J. Biol. Macromol. 2020, 156, 186–195. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Barboni, T.; Cannac, M.; Chiaramonti, N. Effect of cold storage and ozone treatment on physicochemical parameters, soluble sugars and organic acids in Actinidia deliciosa. Food Chem. 2010, 121, 946–951. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, Y.; Jo, Y.H.; Kim, S.B.; Yeon, S.W.; Kim, J.G.; Turk, A.; Song, J.Y.; Kim, Y.; Hwang, B.Y.; et al. Organic acid conjugated phenolic compounds of hardy kiwifruit (Actinidia arguta) and their NF-κB inhibitory activity. Food Chem. 2020, 308, 125666. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch.: A review of chemistry and pharmacology. Front. Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Ultrasonic treatment increases extraction rate of common bean (Phaseolus vulgaris L.) antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Luo, Q.; Wu, D.-T.; Zou, L.; Liu, Y.; Li, H.-B.; Gan, R.-Y. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2021, in press. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, K.W.; Wu, D.-T.; Liu, H.-Y.; Li, H.-B.; Zhang, J.-R.; Gan, R.-Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef]
- Wu, D.T.; Feng, K.L.; Huang, L.; Gan, R.Y.; Hu, Y.C.; Zou, L. Deep eutectic solvent-assisted extraction, partially structural characterization, and bioactivities of acidic polysaccharides from lotus leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.-Y.; Li, S.-H.; Ma, W.; Wu, D.-T.; Li, H.-B.; Xiao, A.-P.; Liu, L.-L.; Zhu, F.; Gan, R.-Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. TrAC Trends Anal. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.-Y.; Xia, Y.; Guo, H.; He, X.-Q.; Li, H.; Wu, D.-T.; Geng, F.; Lin, F.-J.; Li, H.-B.; et al. Screening and process optimization of ultrasound-assisted extraction of main antioxidants from sweet tea (Lithocarpus litseifolius [Hance] Chun). Food Biosci. 2021, 43, 101277. [Google Scholar] [CrossRef]
- Gao, Y.; Ping, H.; Li, B.; Li, Y.; Zhao, F.; Ma, Z. Characterization of free, conjugated, and bound phenolics in early and late ripening kiwifruit cultivars. J. Sci. Food Agric. 2021, 101, 4743–4750. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.-Y.; Gan, R.-Y.; Zheng, J.; Li, Y.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Optimization of ultrasound-assisted extraction of antioxidant polyphenols from the seed coats of red sword bean (Canavalia gladiate (Jacq.) DC.). Antioxidants 2019, 8, 200. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, D.-D.; Shang, A.; Gan, R.-Y.; Li, H.-B. Influences of microwave-assisted extraction parameters on antioxidant activity of the extract from Akebia trifoliata peels. Foods 2021, 10, 1432. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Zhang, D.; Farha, A.K.; Yang, X.; Li, H.-B.; Kong, K.-W.; Zhang, J.-R.; Chan, C.-L.; Lu, W.-Y.; Corke, H.; et al. Phytochemicals, essential oils, and bioactivities of an underutilized wild fruit Cili (Rosa roxburghii). Ind. Crops Prod. 2020, 143, 111928. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, Y.S.; Park, Y.K.; Ham, K.S.; Kang, S.G.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Phytochemical analysis of two main varieties of persimmon and kiwifruit and their antioxidative and quenching capacities. Eur. Food Res. Technol. 2020, 246, 1259–1268. [Google Scholar] [CrossRef]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Xu, D.-P.; Xu, X.-R.; Chen, Y.-M.; Ling, W.-H.; Chen, F.; Li, H.-B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Methods 2015, 8, 1207–1214. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.-N.; Li, H.-B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21, 18. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.-L.; Jiang, J.-G.; Lin, Q.-S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef]
- Li, Y.; Cao, S.-Y.; Lin, S.-J.; Zhang, J.-R.; Gan, R.-Y.; Li, H.-B. Polyphenolic profile and antioxidant capacity of extracts from Gordonia axillaris fruits. Antioxidants 2019, 8, 150. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green extraction of antioxidant polyphenols from green tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, J.-M.; Mao, Q.-Q.; Shang, A.; Li, B.-Y.; Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Wei, X.-L.; Gan, R.-Y.; et al. Effects of tannase and ultrasound treatment on the bioactive compounds and antioxidant activity of green tea extract. Antioxidants 2019, 8, 362. [Google Scholar] [CrossRef]
- Abdul Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91. [Google Scholar] [CrossRef]
- Drzewiecki, J.; Latocha, P.; Leontowicz, H.; Leontowicz, M.; Park, Y.S.; Najman, K.; Weisz, M.; Ezra, A.; Gorinstein, S. Analytical methods applied to characterization of Actinidia arguta, Actinidia deliciosa, and Actinidia eriantha kiwi fruit cultivars. Food Anal. Methods 2016, 9, 1353–1366. [Google Scholar] [CrossRef][Green Version]
- Schaefer, A.; Hossain, M. Extraction of organic acids from kiwifruit juice using a supported liquid membrane process. Bioprocess Eng. 1996, 16, 25–33. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, X.; Li, G.; He, X.; Yu, X.; Yu, X.; Xiao, Q.; Xiang, Z.; Wang, C. Chemical constituents of radix Actinidia chinensis planch by UPLC–QTOF–MS. Biomed. Chromatogr. 2021, 35, e5103. [Google Scholar] [CrossRef]
- Santos, J.; Mendiola, J.A.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef]
- Zhang, Q.; Ford, L.A.; Goodman, K.D.; Freed, T.A.; Hauser, D.M.; Conner, J.K.; Vroom, K.E.T.; Toal, D.R. LC–MS/MS method for quantitation of seven biomarkers in human plasma for the assessment of insulin resistance and impaired glucose tolerance. J. Chromatogr. B 2016, 1038, 101–108. [Google Scholar] [CrossRef]
- Fiori, J.; Amadesi, E.; Fanelli, F.; Tropeano, C.V.; Rugolo, M.; Gotti, R. Cellular and mitochondrial determination of low molecular mass organic acids by LC–MS/MS. J. Pharm. Biomed. Anal. 2018, 150, 33–38. [Google Scholar] [CrossRef]
- Tan, B.; Malu, S.; Roth, K.D. Development of ion pairing LC-MS/MS method for itaconate and cis-aconitate in cell extract and cell media. J. Chromatogr. B 2020, 1146, 122120. [Google Scholar] [CrossRef]
- Pawlak, M.; Klupczynska, A.; Kokot, Z.J.; Matysiak, J. Extending metabolomic studies of Apis mellifera Venom: LC-MS-based targeted analysis of organic acids. Toxins 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yan, P.; Du, K.; Li, M.; Xie, Y.; Gao, P. Nutritional component analyses of kiwifruit in different development stages by metabolomic and transcriptomic approaches. J. Sci. Food Agric. 2020, 100, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.S.K.; Singh, R.P. Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour. Technol. 2002, 85, 69–71. [Google Scholar] [CrossRef]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the presence and contents of phytochemicals in honey samples: Phenolic compounds as Indicators to Identify their botanical origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Parkar, S.G.; Simmons, L.; Herath, T.D.; Phipps, J.E.; Trower, T.M.; Hedderley, D.I.; McGhie, T.K.; Blatchford, P.; Ansell, J.; Sutton, K.H.; et al. Evaluation of the prebiotic potential of five kiwifruit cultivars after simulated gastrointestinal digestion and fermentation with human faecal bacteria. Int. J. Food Sci. Technol. 2018, 53, 1203–1210. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Scognamiglio, M.; Monaco, P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J. Agric. Food Chem. 2009, 57, 4148–4155. [Google Scholar] [CrossRef]
- Yun, E.-S.; Park, S.-K.; Kim, B.-S.; Chae, Y.-Z.; Cho, S.-M.; Yi, H.; Cho, H.-J.; Shin, H.-C. Determination of the esculetin contents of medicinal plants by liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 1247–1251. [Google Scholar] [CrossRef]
- Zhao, T.; He, J.; Wang, X.; Ma, B.; Wang, X.; Zhang, L.; Li, P.; Liu, N.; Lu, J.; Zhang, X. Rapid detection and characterization of major phenolic compounds in Radix Actinidia chinensis Planch by ultra-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 98, 311–320. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production and Applications; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Duxford, UK, 2019; pp. 191–246. [Google Scholar]
- Park, Y.S.; Ham, K.-S.; Park, Y.-K.; Leontowicz, H.; Leontowicz, M.; Namieśnik, J.; Katrich, E.; Gorinstein, S. The effects of treatment on quality parameters of smoothie-type ‘Hayward’ kiwi fruit beverages. Food Control 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Kvesitadze, G.I.; Kalandiya, A.G.; Papunidze, S.G.; Vanidze, M.R. Identification and quantification of ascorbic acid in kiwi fruit by high-performance liquid chromatography. Appl. Biochem. Microbiol. 2001, 37, 215–218. [Google Scholar] [CrossRef]
- Hettihewa, S.K.; Hemar, Y.; Rupasinghe, H.P.V. Flavonoid-rich extract of Actinidia macrosperma (a wild kiwifruit) inhibits angiotensin-converting enzyme in vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef] [PubMed]
| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ethanol concentration (%) | X1 | 60 | 70 | 80 |
| Extraction temperature (°C) | X2 | 30 | 40 | 50 |
| Ultrasound Power (W) | X3 | 360 | 420 | 480 |
| Run | X1 (%) | X2 (°C) | X3 (W) | Y (μM Trolox/g DW) | |
|---|---|---|---|---|---|
| Experimental Data | Predicted Results | ||||
| 1 | 0(70) | 1(50) | 1(480) | 65.54 ± 0.50 | 64.46 |
| 2 | 0(70) | 0(40) | 0(420) | 69.26 ± 0.25 | 70.22 |
| 3 | 1(80) | 1(50) | 0(420) | 60.88 ± 0.81 | 62.69 |
| 4 | 0(70) | 0(40) | 0(420) | 69.56 ± 0.67 | 70.22 |
| 5 | 1(80) | 0(40) | −1(360) | 59.13 ± 1.31 | 57.91 |
| 6 | −1(60) | 0(40) | −1(360) | 60.57 ± 0.82 | 61.30 |
| 7 | −1(60) | 0(40) | 1(480) | 59.72 ± 1.44 | 60.95 |
| 8 | −1(60) | 1(50) | 0(420) | 66.41 ± 0.39 | 66.27 |
| 9 | 1(80) | −1(30) | 0(420) | 59.64 ± 1.16 | 59.79 |
| 10 | 1(80) | 0(40) | 1(480) | 56.44 ± 1.05 | 55.70 |
| 11 | 0(70) | 0(40) | 0(420) | 70.24 ± 1.60 | 70.22 |
| 12 | 0(70) | 0(40) | 0(420) | 70.66 ± 1.27 | 70.22 |
| 13 | 0(70) | −1(30) | −1(360) | 62.50 ± 1.77 | 63.58 |
| 14 | 0(70) | 1(50) | −1(360) | 60.99 ± 2.00 | 60.40 |
| 15 | 0(70) | 0(40) | 0(420) | 71.38 ± 1.26 | 70.22 |
| 16 | −1(60) | −1(30) | 0(420) | 66.67 ± 0.39 | 64.85 |
| 17 | 0(70) | −1(30) | 1(480) | 56.37 ± 2.48 | 56.96 |
| Effects | Source | Sum of Square | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|---|
| Total effect | Model | 404.03 | 9 | 44.89 | 18.89 | 0.0004 a |
| Linear effect | X1 | 37.36 | 1 | 37.36 | 15.72 | 0.0054 a |
| X2 | 9.33 | 1 | 9.33 | 3.92 | 0.0880 | |
| X3 | 3.27 | 1 | 3.27 | 1.38 | 0.2790 | |
| Interactive effect | X1X2 | 0.56 | 1 | 0.56 | 0.23 | 0.6435 |
| X1X3 | 0.86 | 1 | 0.86 | 0.36 | 0.5671 | |
| X2X3 | 28.53 | 1 | 28.53 | 12.01 | 0.0105 a | |
| Quadratic effect | X12 | 89.22 | 1 | 89.22 | 37.54 | 0.0005 a |
| X22 | 20.70 | 1 | 20.70 | 8.71 | 0.0214 a | |
| X32 | 186.30 | 1 | 186.30 | 78.39 | <0.0001 a | |
| Residual | 16.64 | 7 | 2.38 | |||
| Lack of Fit | 13.74 | 3 | 4.58 | 6.34 | 0.0533 | |
| Pure Error | 2.89 | 4 | 0.72 | |||
| Corrected Total | 420.66 | 16 | ||||
| R2 | 0.9605 | |||||
| Adjusted R2 | 0.9096 |
| Extraction Method | Ethanol Concentration | L/S Ratio | Extraction Time | Extraction Temperature | Ultrasonic Power | ABTS Value (μM Trolox/g DW) |
|---|---|---|---|---|---|---|
| UAE | 68% | 20:1 | 30 min | 42 °C | 420 W | 70.38 ± 1.38 |
| CSE | 68% | 20:1 | 30 min | 42 °C | None | 59.39 ± 1.40 |
| Compounds | Regression Equation | Linear Range (µg/mL) | Correlation Coefficient (R2) | Retention Time (min) | Content |
|---|---|---|---|---|---|
| Vitamin (mg/g DW) | |||||
| Ascorbic acid | Y = 26.536X + 81.753 | 10.00–100.00 | 0.9971 | 2.0 | 7.21 ± 0.08 |
| Phenolic compounds (µg/g DW) | |||||
| Neochlorogenic acid | Y = 38.141X − 126.62 | 5.88–29.41 | 0.9980 | 9.9 | 119.90 ± 1.73 |
| Procyanidin B1 | Y = 6.1598X − 7.2701 | 5.88–29.41 | 0.9984 | 14.1 | 15.93 ± 0.30 |
| Catechin | Y = 8.5506X − 9.5818 | 5.88–29.41 | 0.9997 | 17.1 | 16.68 ± 0.34 |
| Chlorogenic acid | Y = 39.002X − 66.271 | 3.53–17.65 | 0.9984 | 17.6 | 3.53 ± 0.17 |
| Caffeic acid | Y = 66.056X − 82.779 | 5.88–29.41 | 0.9996 | 19.8 | 6.93 ± 0.40 |
| Procyanidin B2 | Y = 8.1696X − 11.262 | 5.88–29.41 | 0.9991 | 22.7 | 166.67 ± 2.84 |
| Epicatechin | Y = 8.7362X − 5.9765 | 5.88–29.41 | 0.9998 | 25.1 | 110.28 ± 0.50 |
| Quercetin 3-O-glucoside | Y = 31.11X − 33.905 | 5.88–29.41 | 0.9997 | 32.7 | 2.04 ± 0.01 |
| Quercetin 3-O-rhamnoside | Y = 21.924X − 26.664 | 5.88–29.41 | 0.9992 | 35.2 | 3.37 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Y.-H.; Zhuang, Q.-G.; Li, Q.-H.; Du, K.; Wu, D.-T.; Li, H.-B.; Xia, Y.; Zhu, F.; Gan, R.-Y. Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit. Foods 2022, 11, 827. https://doi.org/10.3390/foods11060827
Mai Y-H, Zhuang Q-G, Li Q-H, Du K, Wu D-T, Li H-B, Xia Y, Zhu F, Gan R-Y. Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit. Foods. 2022; 11(6):827. https://doi.org/10.3390/foods11060827
Chicago/Turabian StyleMai, Ying-Hui, Qi-Guo Zhuang, Qiao-Hong Li, Kui Du, Ding-Tao Wu, Hua-Bin Li, Yu Xia, Fan Zhu, and Ren-You Gan. 2022. "Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit" Foods 11, no. 6: 827. https://doi.org/10.3390/foods11060827
APA StyleMai, Y.-H., Zhuang, Q.-G., Li, Q.-H., Du, K., Wu, D.-T., Li, H.-B., Xia, Y., Zhu, F., & Gan, R.-Y. (2022). Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit. Foods, 11(6), 827. https://doi.org/10.3390/foods11060827









