Sensory Capacities and Eating Behavior: Intriguing Results from a Large Cohort of Italian Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.2.1. Personal and Lifestyle Characteristics
2.2.2. Sensory Measurements and Reduced Sensory Capacities Definition
2.2.3. Eating Behavior
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Prevalence of Reduced Sensory Capacities and Factors Influencing Sensory Ability
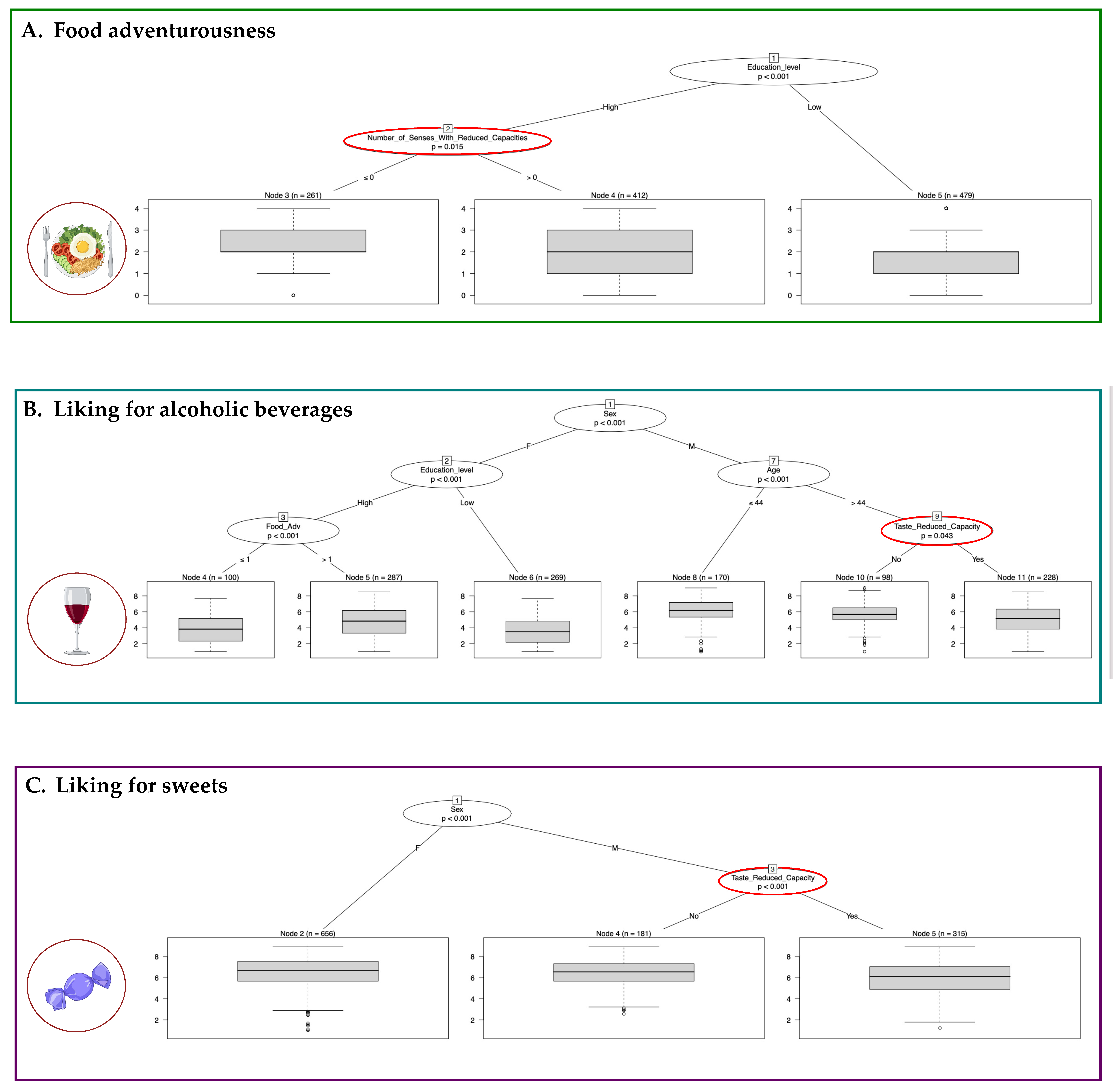
3.3. Effect of Reduced Sensory Capacities on Food Liking and Food Adventurousness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birch, L.L. Development of food preferences. Annu. Rev. Nutr. 1999, 19, 41–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mendonça, S.N.T.G.; Brandão, H.C.A.D.N.T.M.; Brandão, W.A.P.L.N.T.M.; Quintino, C.A.A.; De Francisco, A.; Teixeira, E. Food Preferences of Middle Aged and Elderly Subjects in a Brazilian City. J. Nutr. Health Aging 2013, 17, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Hann, C. Food Preferences and Reported Frequencies of Food Consumption as Predictors of Current Diet in Young Women. Am. J. Clin. Nutr. 1999, 70, 28–36. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, C.; Ribas, L.; Serra-Majem, L.; Aranceta, J. Food Preferences of Spanish Children and Young People: The EnKid Study. Eur. J. Clin. Nutr. 2003, 57, S45–S48. [Google Scholar] [CrossRef] [PubMed]
- Wądołowska, L.; Babicz-Zielińska, E.; Czarnocińska, J. Food Choice Models and Their Relation with Food Preferences and Eating Frequency in the Polish Population: POFPRES Study. Food Policy 2008, 33, 122–134. [Google Scholar] [CrossRef]
- Pallister, T.; Sharafi, M.; Lachance, G.; Pirastu, N.; Mohney, R.P.; MacGregor, A.; Feskens, E.J.M.; Duffy, V.; Spector, T.D.; Menni, C. Food Preference Patterns in a UK Twin Cohort. Twin Res. Hum. Genet. 2015, 18, 793–805. [Google Scholar] [CrossRef] [Green Version]
- Laureati, M.; Bergamaschi, V.; Pagliarini, E. School-Based Intervention with Children. Peer-Modeling, Reward and Repeated Exposure Reduce Food Neophobia and Increase Liking of Fruits and Vegetables. Appetite 2014, 83, 26–32. [Google Scholar] [CrossRef]
- Hazley, D.; McCarthy, S.N.; Stack, M.; Walton, J.; McNulty, B.A.; Flynn, A.; Kearney, J.M. Food Neophobia and Its Relationship with Dietary Variety and Quality in Irish Adults: Findings from a National Cross-Sectional Study. Appetite 2022, 169, 105859. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Rasmussen, M.A.; Prescott, J. Relationships between Food Neophobia and Food Intake and Preferences: Findings from a Sample of New Zealand Adults. Appetite 2017, 116, 410–422. [Google Scholar] [CrossRef]
- Falciglia, G.A.; Couch, S.C.; Gribble, L.S.; Pabst, S.M.; Frank, R. Food Neophobia in Childhood Affects Dietary Variety. J. Am. Diet. Assoc. 2000, 100, 1474–1481. [Google Scholar] [CrossRef]
- Mathieu, M.-E.; Reid, R.E.R.; King, N.A. Sensory Profile of Adults with Reduced Food Intake and the Potential Roles of Nutrition and Physical Activity Interventions. Adv. Nutr. 2019, 10, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.-X.; Ong, P.K.C.; Huang, D. Odor-Specific Loss of Smell Sensitivity with Age as Revealed by the Specific Sensitivity Test. Chem. Senses 2016, 41, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, I.G.; Kim, S.Y.; Kim, M.-S.; Park, B.; Kim, J.-H.; Choi, H.G. Olfactory Dysfunction Is Associated with the Intake of Macronutrients in Korean Adults. PLoS ONE 2016, 11, e0164495. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Mattes, R.D. Nutrition and Taste and Smell Dysfunction. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Sue, C.M.; Kifley, A.; Mitchell, P. The Association Between Olfactory Impairment and Total Mortality in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67A, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, J.M. Effects of Ageing on Smell and Taste. Postgrad. Med. J. 2006, 82, 239–241. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Sim, S.; Kim, H.-J.; Choi, H.G. Low-Fat and Low-Protein Diets Are Associated with Hearing Discomfort among the Elderly of Korea. Br. J. Nutr. 2015, 114, 1711–1717. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, S.S. Critical Illness and Changes in Sensory Perception. Proc. Nutr. Soc. 2007, 66, 331–345. [Google Scholar] [CrossRef]
- Schiffman, S.S. Effects of Aging on the Human Taste System. Ann. N. Y. Acad. Sci. 2009, 1170, 725–729. [Google Scholar] [CrossRef]
- Schiffman, S.; Graham, B. Taste and Smell Perception Affect Appetite and Immunity in the Elderly. Eur. J. Clin. Nutr. 2000, 54, S54–S63. [Google Scholar] [CrossRef]
- Kern, D.W.; Wroblewski, K.E.; Schumm, L.P.; Pinto, J.M.; McClintock, M.K. Field Survey Measures of Olfaction: The Olfactory Function Field Exam (OFFE). Field Methods 2014, 26, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, J.E.; Campbell, V.A. Vision Impairment and Hearing Loss Among Community-Dwelling Older Americans: Implications for Health and Functioning. Am. J. Public Health 2004, 94, 823–829. [Google Scholar] [CrossRef]
- Murphy, C. Prevalence of Olfactory Impairment in Older Adults. JAMA 2002, 288, 2307. [Google Scholar] [CrossRef] [PubMed]
- Welge-Lüssen, A. Ageing, Neurodegeneration, and Olfactory and Gustatory Loss. B-ENT 2009, 13 (Suppl. S5), 129–132. [Google Scholar]
- Chia, E.-M. Association Between Vision and Hearing Impairments and Their Combined Effects on Quality of Life. Arch Ophthalmol. 2006, 124, 1465. [Google Scholar] [CrossRef] [Green Version]
- Kiely, K.M.; Anstey, K.J.; Luszcz, M.A. Dual Sensory Loss and Depressive Symptoms: The Importance of Hearing, Daily Functioning, and Activity Engagement. Front. Hum. Neurosci. 2013, 7, 837. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zong, G.; Doty, R.L.; Sun, Q. Prevalence and Risk Factors of Taste and Smell Impairment in a Nationwide Representative Sample of the US Population: A Cross-Sectional Study. BMJ Open 2016, 6, e013246. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.; Lopez, K.J.; Wroblewski, K.E.; Huisingh-Scheetz, M.; Kern, D.W.; Chen, R.C.; Schumm, L.P.; Dale, W.; McClintock, M.K.; Pinto, J.M. Global Sensory Impairment in Older Adults in the United States. J. Am. Geriatr. Soc. 2016, 64, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory Dysfunction Predicts 5-Year Mortality in Older Adults. PLoS ONE 2014, 9, e107541. [Google Scholar] [CrossRef] [Green Version]
- Khil, L.; Wellmann, J.; Berger, K. Determinants of Single and Multiple Sensory Impairments in an Urban Population. Otolaryngol. Head Neck Surg. 2015, 153, 364–371. [Google Scholar] [CrossRef]
- Concas, M.P.; Catamo, E.; Biino, G.; Toniolo, D.; Gasparini, P.; Robino, A. Factors Associated with Food Liking and Their Relationship with Metabolic Traits in Italian Cohorts. Food Qual. Prefer. 2019, 75, 64–70. [Google Scholar] [CrossRef]
- Vuckovic, D.; Mezzavilla, M.; Cocca, M.; Morgan, A.; Brumat, M.; Catamo, E.; Concas, M.P.; Biino, G.; Franzè, A.; Ambrosetti, U.; et al. Whole-Genome Sequencing Reveals New Insights into Age-Related Hearing Loss: Cumulative Effects, Pleiotropy and the Role of Selection. Eur. J. Hum. Genet. 2018, 26, 1167–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concas, M.P.; Cocca, M.; Francescatto, M.; Battistuzzi, T.; Spedicati, B.; Feresin, A.; Morgan, A.; Gasparini, P.; Girotto, G. The Role of Knockout Olfactory Receptor Genes in Odor Discrimination. Genes 2021, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Catamo, E.; Tornese, G.; Concas, M.P.; Gasparini, P.; Robino, A. Differences in Taste and Smell Perception between Type 2 Diabetes Mellitus Patients and Healthy Controls. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 193–200. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Vennemann, M.M.; Hummel, T.; Berger, K. The Association between Smoking and Smell and Taste Impairment in the General Population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef]
- Zhao, L.; Kirkmeyer, S.V.; Tepper, B.J. A Paper Screening Test to Assess Genetic Taste Sensitivity to 6-n-Propylthiouracil. Physiol. Behav. 2003, 78, 625–633. [Google Scholar] [CrossRef]
- Pirastu, N.; Kooyman, M.; Traglia, M.; Robino, A.; Willems, S.M.; Pistis, G.; Amin, N.; Sala, C.; Karssen, L.C.; Van Duijn, C.; et al. A Genome-Wide Association Study in Isolated Populations Reveals New Genes Associated to Common Food Likings. Rev. Endocr. Metab. Disord. 2016, 17, 209–219. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.S. Correlation and Causation. J. Agric. Res. 1921, 20, 557–585. [Google Scholar]
- Tabachnick. Using Multivariate Statistics; Pearson: Boston, MA, USA, 2001; Volume XXVI. [Google Scholar]
- Sobel, M.E. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociol. Methodol. 1982, 13, 290–312. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Strobl, C.; Malley, J.; Tutz, G. An Introduction to Recursive Partitioning: Rationale, Application, and Characteristics of Classification and Regression Trees, Bagging, and Random Forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaka, M.; Ikebe, K.; Uota, M.; Ogawa, T.; Okada, T.; Inomata, C.; Takeshita, H.; Mihara, Y.; Gondo, Y.; Masui, Y.; et al. Age and Sex Differences in the Taste Sensitivity of Young Adult, Young-Old and Old-Old Japanese: Age and Sex Differences in Taste Sense. Geriatr. Gerontol. Int. 2016, 16, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nishiwaki, Y.; Takebayashi, T. Are you conscious of any age-related taste impairment? prevalence of and factors associated with taste impairment in japan: Letters to the editor. J. Am. Geriatr. Soc. 2011, 59, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter Taste Markers Explain Variability in Vegetable Sweetness, Bitterness, and Intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J. 6-n-Propylthiouracil: A Genetic Marker for Taste, with Implications for Food Preference and Dietary Habits. Am. J. Hum. Genet. 1998, 63, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; D’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the Bitter-Taste Receptor Gene TAS2R38, and Adiposity in a Genetically Isolated Population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef]
- Feeney, E.; O’Brien, S.; Scannell, A.; Markey, A.; Gibney, E.R. Genetic Variation in Taste Perception: Does It Have a Role in Healthy Eating? Proc. Nutr. Soc. 2011, 70, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A.; Henderson, S.A.; Cockroft, J.E. Genetic Sensitivity to 6-n-Propylthiouracil Has No Influence on Dietary Patterns, Body Mass Indexes, or Plasma Lipid Profiles of Women. J. Am. Diet. Assoc. 2007, 107, 1340–1348. [Google Scholar] [CrossRef]
- Zang, Y.; Han, P.; Burghardt, S.; Knaapila, A.; Schriever, V.; Hummel, T. Influence of olfactory dysfunction on the perception of food. Eur. Arch. Otorhinolaryngol. 2019, 276, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Lanier, S.A.; Hutchins, H.L.; Pescatello, L.S.; Johnson, M.K.; Bartoshuk, L.M. Food Preference Questionnaire as a Screening Tool for Assessing Dietary Risk of Cardiovascular Disease within Health Risk Appraisals. J. Am. Diet. Assoc. 2007, 107, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, A.J.; Sandell, M.A.; Vaarno, J.; Hoppu, U.; Puolimatka, T.; Kaljonen, A.; Lagström, H. Food Neophobia Associates with Lower Dietary Quality and Higher BMI in Finnish Adults. Public Health Nutr. 2015, 18, 2161–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuorila, H.; Lähteenmäki, L.; Pohjalainen, L.; Lotti, L. Food Neophobia among the Finns and Related Responses to Familiar and Unfamiliar Foods. Food Qual. Prefer. 2001, 12, 29–37. [Google Scholar] [CrossRef]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory Dysfunction and Related Nutritional Risk in Free-Living, Elderly Women. J. Am. Diet. Assoc. 1995, 95, 879–884. [Google Scholar] [CrossRef]
- Gopinath, B.; Russell, J.; Sue, C.M.; Flood, V.M.; Burlutsky, G.; Mitchell, P. Olfactory Impairment in Older Adults Is Associated with Poorer Diet Quality over 5 Years. Eur. J. Nutr. 2016, 55, 1081–1087. [Google Scholar] [CrossRef]
- Wildes, J.E.; Zucker, N.L.; Marcus, M.D. Picky eating in adults: Results of a web-based survey. Int. J. Eat. Disord. 2012, 45, 575–582. [Google Scholar] [CrossRef]
- Hultsch, D.F.; Dixon, R.A. Learning and Memory in Aging. In Handbook of the Psychology of Aging; Elsevier: Amsterdam, The Netherlands, 1990; pp. 258–274. [Google Scholar] [CrossRef]
- Kausler, D.H. Experimental Psychology, Cognition, and Human Aging; Springer: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Larsson, M.; Bäckman, L. Semantic Activation and Episodic Odor Recognition in Young and Older Adults. Psychol. Aging 1993, 8, 582–588. [Google Scholar] [CrossRef]
- Daniel, T.A.; Katz, J.S. Primacy and Recency Effects for Taste. J. Exp. Psychol. Learn. Mem. Cogn. 2018, 44, 399–405. [Google Scholar] [CrossRef]
- Mojet, J. Taste Perception with Age: Generic or Specific Losses in Supra-Threshold Intensities of Five Taste Qualities? Chem. Senses 2003, 28, 397–413. [Google Scholar] [CrossRef]
- Sulmont-Rosse, C.; Maitre, I.; Amand, M.; Symoneaux, R.; Van Wymelbeke, V.; Caumon, E.; Tavares, J.; Issanchou, S. Evidence for Different Patterns of Chemosensory Alterations in the Elderly Population: Impact of Age Versus Dependency. Chem. Senses 2015, 40, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doty, R.L.; Chen, J.H.; Overend, J. Taste Quality Confusions: Influences of Age, Smoking, PTC Taster Status, and Other Subject Characteristics. Perception 2017, 46, 257–267. [Google Scholar] [CrossRef] [PubMed]
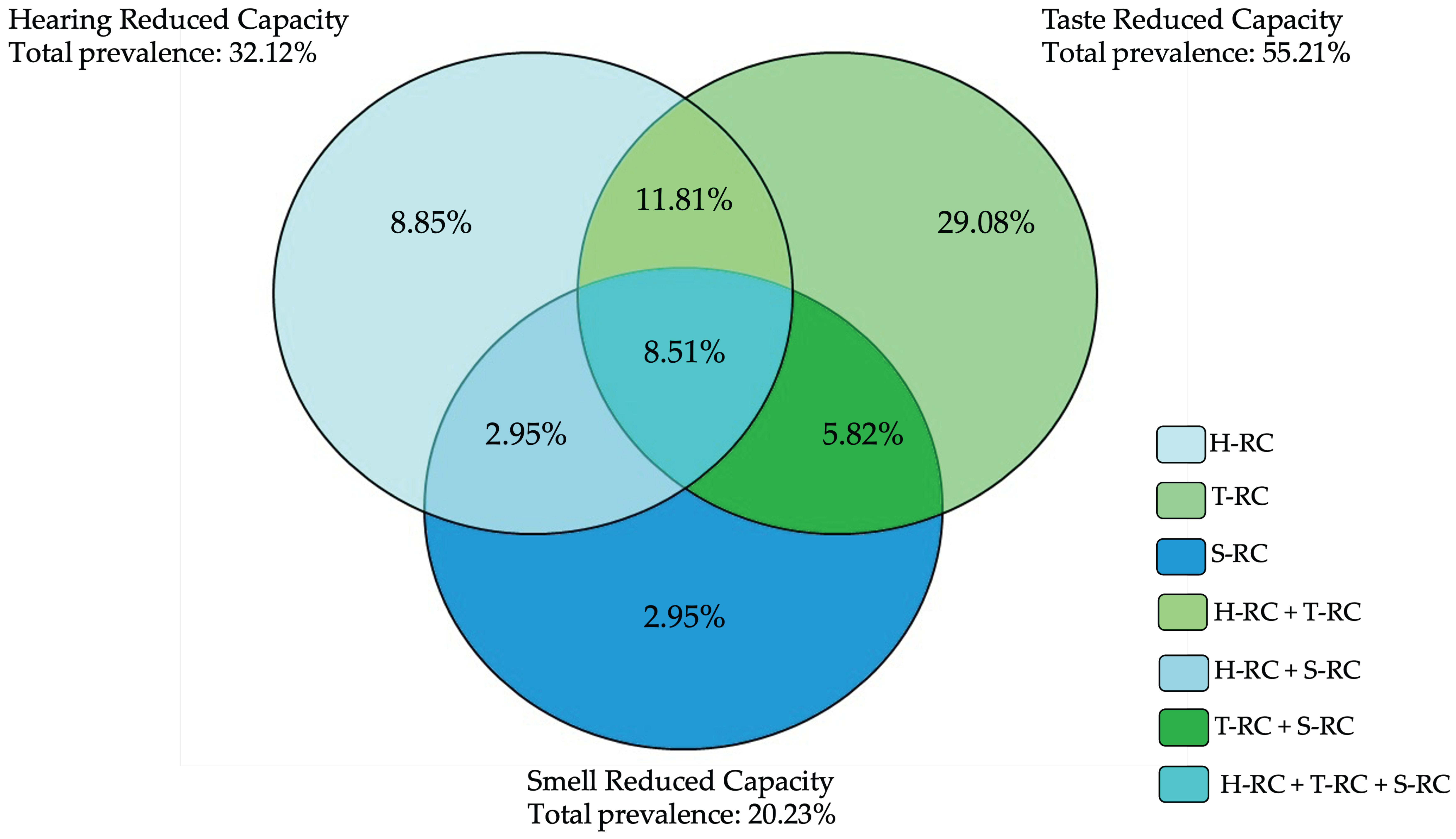
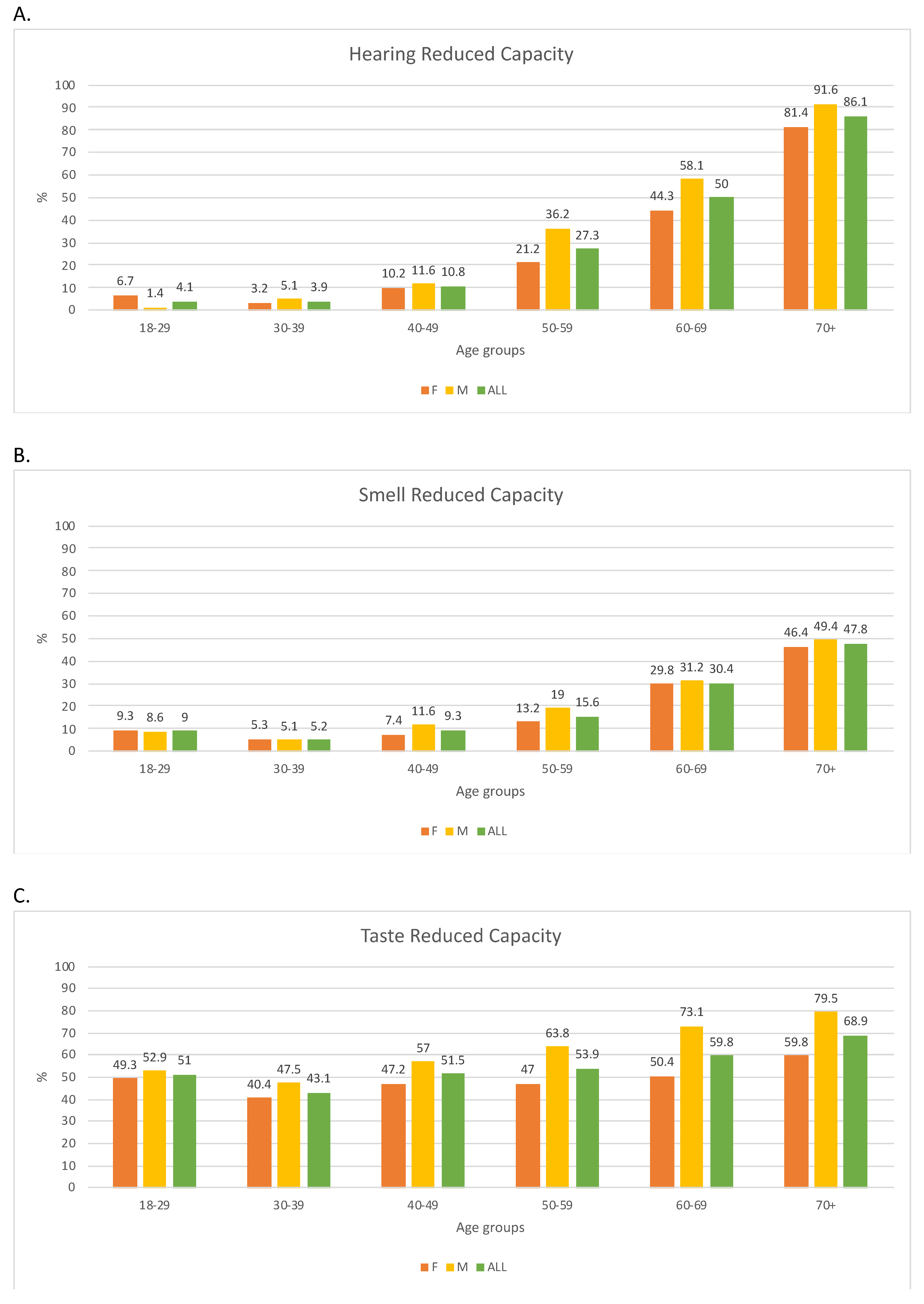
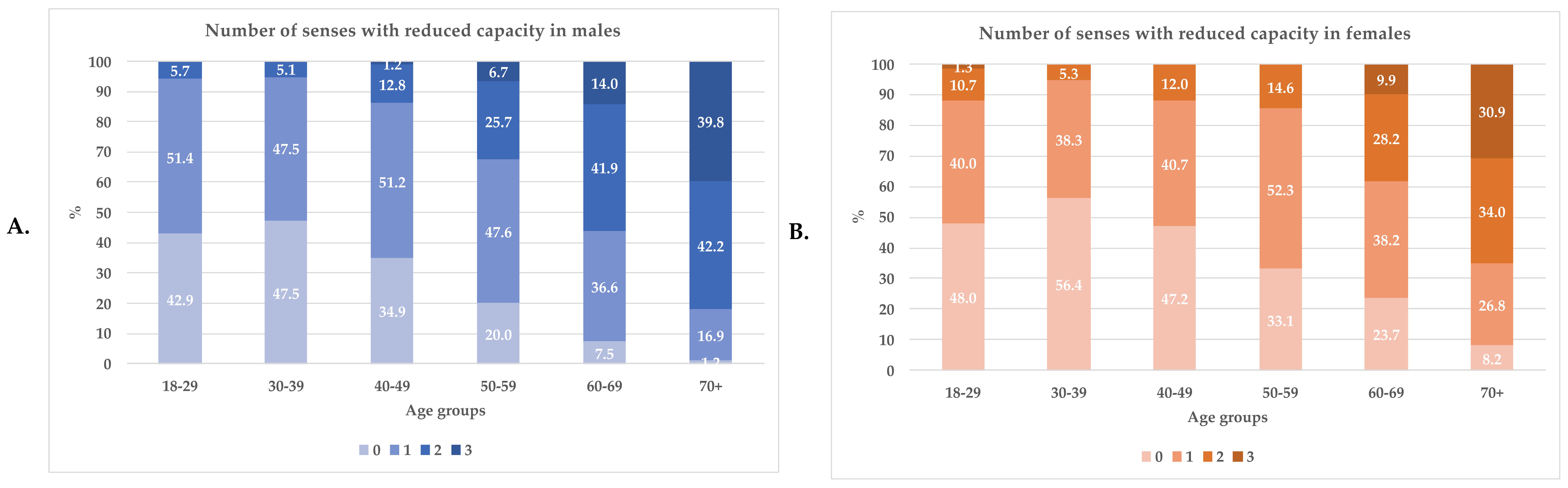
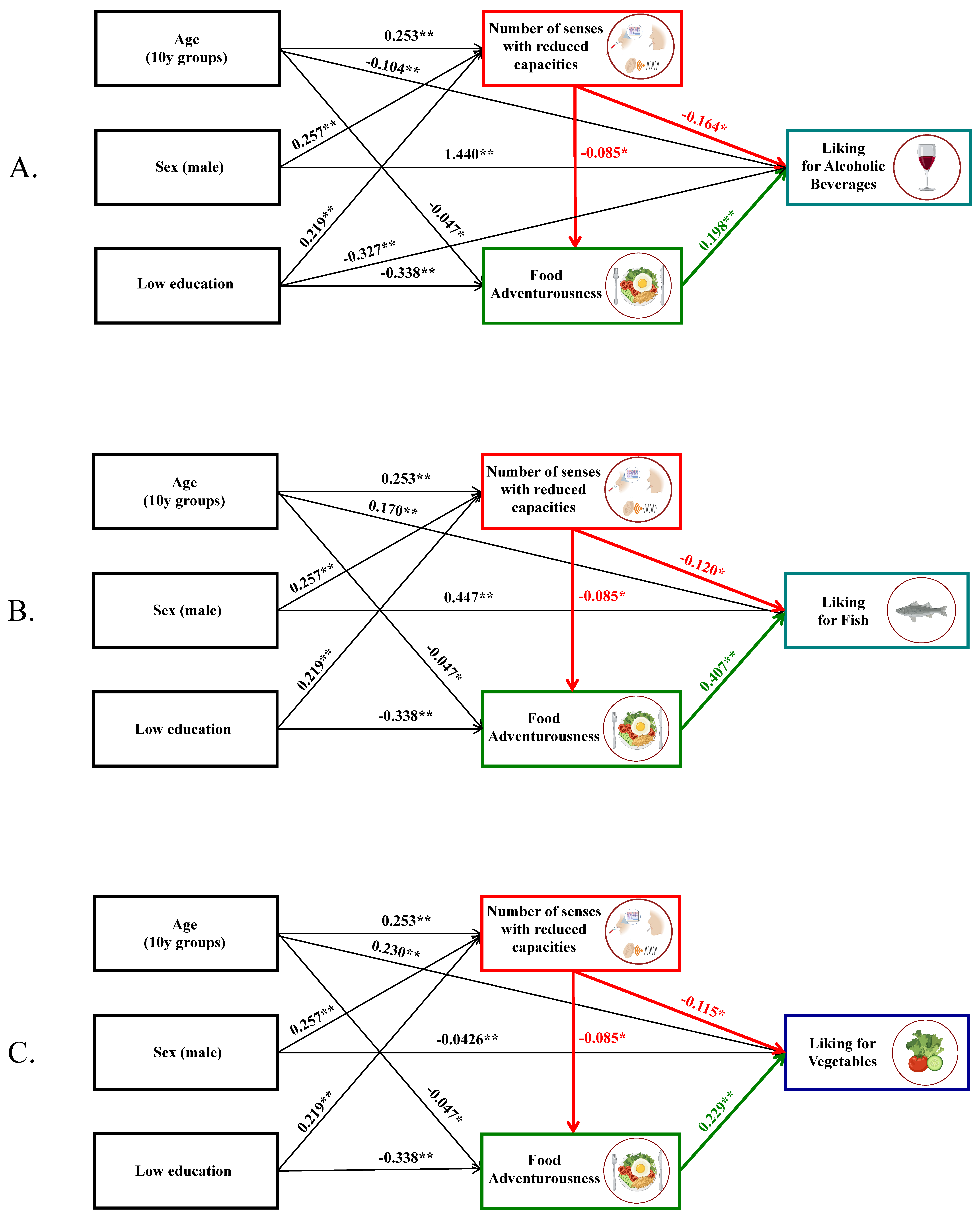
| All | No Sense with Reduced Capacity | At Least One Sense with Reduced Capacity | |
|---|---|---|---|
| N (% males) | 1152 (43.1) | 346 (33.8) | 806 (47.0) |
| Age groups, N (%) | |||
| 18–29 | 145 (12.6) | 66 (19.1) | 79 (9.80) |
| 30–39 | 153 (13.3) | 81 (23.4) | 72 (8.93) |
| 40–49 | 194 (16.8) | 81 (23.4) | 113 (14.0) |
| 50–59 | 256 (22.2) | 71 (20.5) | 185 (23.0) |
| 60–69 | 224 (19.4) | 38 (11.0) | 186 (23.1) |
| 70+ | 180 (15.6) | 9 (2.60) | 171 (21.2) |
| Education, N (%) | |||
| Elementary | 170 (14.8) | 17 (4.91) | 153 (19.0) |
| Lower secondary | 309 (26.8) | 68 (19.7) | 241 (30.0) |
| Upper secondary | 516 (44.8) | 182 (52.6) | 334 (41.4) |
| University | 157 (13.6) | 79 (22.8) | 78 (9.68) |
| Current smokers, N (%) | 224 (19.4) | 80 (23.1) | 144 (17.9) |
| High alcohol consumers, N (%) | 298 (25.9) | 79 (22.8) | 219 (27.2) |
| Food adv, median (IQR) | 2.0 (1; 3) | 2.0 (1.3; 3.0) | 2.0 (1.0; 3.0) |
| Liking groups, median (IQR) | |||
| Alcoholic beverages | 4.8 (3.3; 6.2) | 5.0 (3.7; 6.5) | 4.8 (3.2; 6.2) |
| Cheeses | 6.3 (5.3; 7.0) | 6.3 (5.3; 7.2) | 6.2 (5.3; 7.0) |
| Fish | 6.7 (5.3; 7.5) | 6.8 (5.5; 7.5) | 6.5 (5.3; 7.3) |
| Fruit | 7.0 (6.3; 7.7) | 7.0 (6.4; 7.7) | 7.0 (6.3; 7.7) |
| Meat | 6.8 (6.1; 7.5) | 7.0 (6.0; 7.6) | 6.8 (6.1; 7.5) |
| Sweets | 6.4 (5.4; 7.3) | 6.6 (5.6; 7.3) | 6.4 (5.3; 7.3) |
| Vegetables | 6.7 (5.9; 7.5) | 6.8 (5.9; 7.6) | 6.7 (5.9; 7.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concas, M.P.; Morgan, A.; Tesolin, P.; Santin, A.; Girotto, G.; Gasparini, P. Sensory Capacities and Eating Behavior: Intriguing Results from a Large Cohort of Italian Individuals. Foods 2022, 11, 735. https://doi.org/10.3390/foods11050735
Concas MP, Morgan A, Tesolin P, Santin A, Girotto G, Gasparini P. Sensory Capacities and Eating Behavior: Intriguing Results from a Large Cohort of Italian Individuals. Foods. 2022; 11(5):735. https://doi.org/10.3390/foods11050735
Chicago/Turabian StyleConcas, Maria Pina, Anna Morgan, Paola Tesolin, Aurora Santin, Giorgia Girotto, and Paolo Gasparini. 2022. "Sensory Capacities and Eating Behavior: Intriguing Results from a Large Cohort of Italian Individuals" Foods 11, no. 5: 735. https://doi.org/10.3390/foods11050735
APA StyleConcas, M. P., Morgan, A., Tesolin, P., Santin, A., Girotto, G., & Gasparini, P. (2022). Sensory Capacities and Eating Behavior: Intriguing Results from a Large Cohort of Italian Individuals. Foods, 11(5), 735. https://doi.org/10.3390/foods11050735







