High Prevalence of Klebsiella pneumoniae in Greek Meat Products: Detection of Virulence and Antimicrobial Resistance Genes by Molecular Techniques
Abstract
1. Introduction
2. Materials and Methods
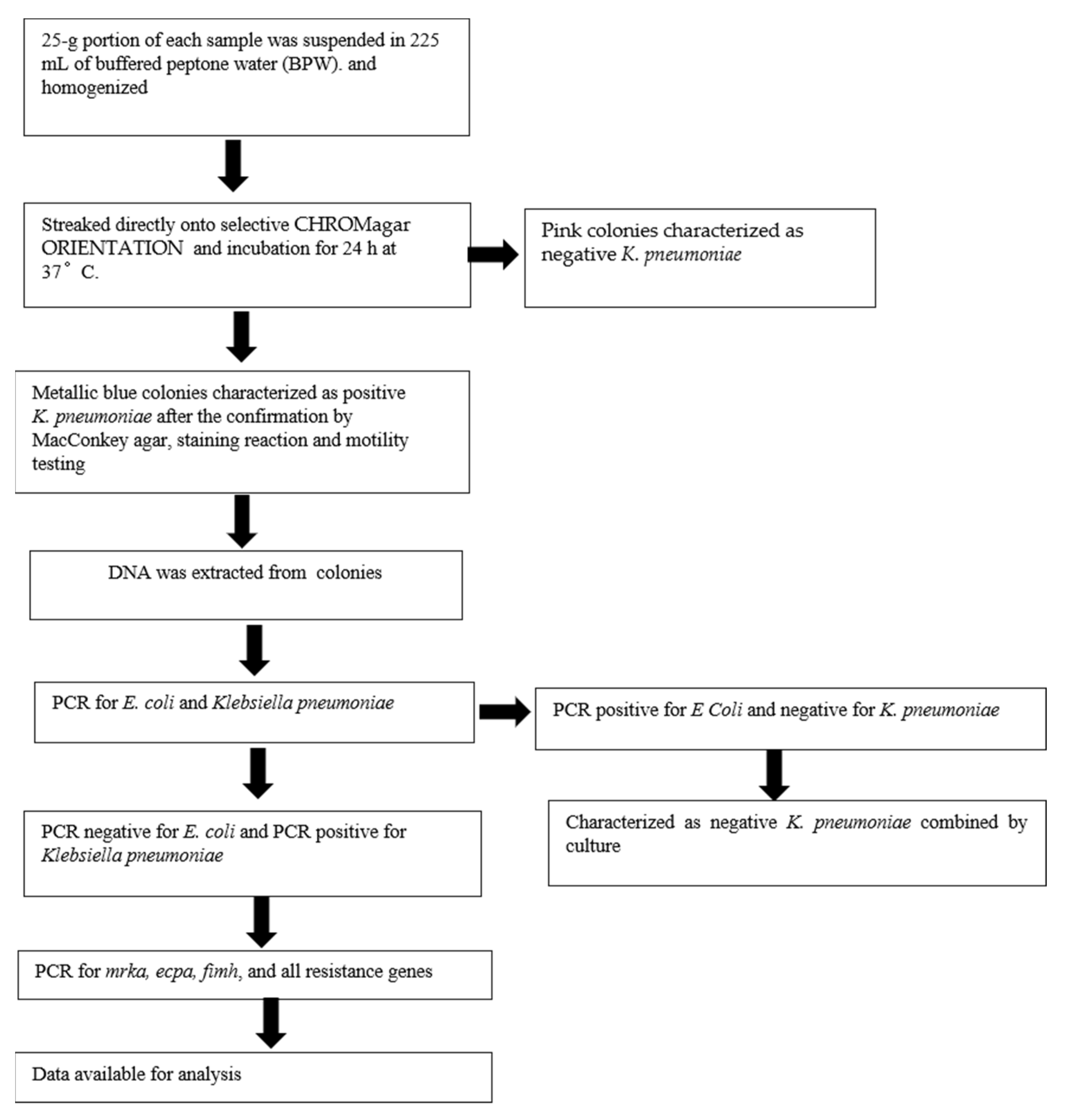
2.1. General Workflow
2.2. Sample Collection
2.3. Detection of K. pneumoniae Colonies
2.4. DNA Isolation
2.5. Confirmation of E. coli and K. pneumoniae by PCR
2.6. Detection of the Virulence Genes ecpA, fimH, mrkA and the Resistance Genes blaKPC, blaNDM, blaVIM, bla IMP and blaOXA-48 by Multiplex PCR
2.7. Phenotypic Analysis
2.8. Statistical Analysis
3. Results
3.1. Phenotypic Analysis
3.2. Virulence and Resistance Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Palmer, A.; Kishony, R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet. 2013, 14, 243–248. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10 (Suppl. 12), S122–S129. [Google Scholar] [CrossRef]
- Ochman, H.; Lawrence, J.G.; Groisman, E.A. Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405, 299–304. [Google Scholar] [CrossRef]
- Domingues, S.; da Silva, G.J.; Nielsen, K.M. Integrons: Vehicles and pathways for horizontal dissemination in bacteria. Mob. Genet. Elem. 2012, 2, 211–223. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the Antibiotic Resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Jones, M.E.; Thornsberry, C.; Friedland, I.R.; Sahm, D.F. Trends in antimicrobial susceptibilities among Enterobacteriaceae isolated from hospitalized patients in the United States from 1998 to 2001. Antimicrob. Agents Chemother. 2003, 47, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.K.; Fung, C.P.; Chang, F.Y.; Lee, N.; Yeh, K.M.; Koh, T.H.; Ip, M. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J. Clin. Microbiol. 2011, 49, 3761–3765. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.P.; Lin, Y.T.; Lin, J.C.; Chen, T.L.; Yeh, K.M.; Chang, F.Y.; Chuang, H.C.; Wu, H.S.; Tseng, C.P.; Siu, L.K. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg. Infect. Dis. 2012, 18, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Calbo, E.; Freixas, N.; Xercavins, M.; Riera, M.; Nicolás, C.; Monistrol, O.; Solé, M.D.M.; Sala, M.R.; Vila, J.; Garau, J. Foodborne Nosocomial Outbreak of SHV1 and CTX-M-15-producing Klebsiella pneumoniae: Epidemiology and Control. Clin. Infect. Dis. 2011, 52, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Falomir, M.P.; Rico, H.; Gozalbo, D. Enterobacter and Klebsiella Species Isolated from Fresh Vegetables Marketed in Valencia (Spain) and Their Clinically Relevant Resistances to Chemotherapeutic Agents. Foodborne Pathog. Dis. 2013, 10, 1002–1007. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, S.; Tran, Q.; Sung, K.; Khan, A.; Adamu, I.; Steele, R. Isolation and characterization of multidrug-resistant Klebsiella spp. isolated from shrimp imported from Thailand. Int. J. Food Microbiol. 2012, 155, 179–184. [Google Scholar] [CrossRef]
- Wu, H.; Liu, B.-G.; Liu, J.-H.; Pan, Y.-S.; Yuan, L.; Hu, G.-Z. Phenotypic and molecular characterization of CTX-M-14 extended-spectrum β-lactamase and plasmid-mediated ACT-like AmpC β-lactamase produced by Klebsiella pneumoniae isolates from chickens in Henan Province, China. Genet. Mol. Res. 2012, 11, 3357–3364. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal car-riage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. 2017, 65, 208–215. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zheng, W.; Zhang, X.; Yu, J.; Gao, Q.; Hou, Y.; Huang, X. PCR detection of Klebsiella pneumoniae in infant formula based on 16S–23S internal transcribed spacer. Int. J. Food Microbiol. 2008, 125, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.; Perry, C.; Elgohari, S.; Hampton, C.V. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J. Med. Microbiol. 2010, 59, 541–547. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Nakavuma, J.L.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Eddie, W.M. Prevalence of pathogenic Klebsiella pneumoniae based on PCR capsular typing harbouring carbapenemases encoding genes in Uganda tertiary hospitals. Antimicrob. Resist. Infect. Control 2021, 10, 57. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement. CLSI Document M100-S24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Barus, T.; Hanjaya, I.; Sadeli, J.; Lay, B.W.; Suwanto, A.; Yulandi, A. Genetic Diversity of Klebsiella spp. Isolated from Tempe based on Enterobacterial Repetitive Intergenic Consensus-Polymerase Chain Reaction (ERIC-PCR). Hayati J. Biosci. 2013, 20, 171–176. [Google Scholar] [CrossRef][Green Version]
- Calhau, V.; Boaventura, L.; Ribeiro, G.; Mendonça, N.; da Silva, G.J. Molecular characterization of Klebsiella pneumoniae isolated from renal transplanted patients: Virulence markers, extended-spectrum β-lactamases, and genetic relatedness. Diagn. Microbiol. Infect. Dis. 2014, 79, 393–395. [Google Scholar] [CrossRef]
- Cao, X.; Xu, X.; Zhang, Z.; Shen, H.; Chen, J.; Zhang, K. Molecular characterization of clinical multidrug-resistant Klebsiella pneumoniae isolates. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, H.; Qin, L.; Pang, Z.; Qin, T.; Ren, H.; Pan, Z.; Zhou, J. Frequency, Antimicrobial Resistance and Genetic Diversity of Klebsiella pneumoniae in Food Samples. PLoS ONE 2016, 11, e0153561. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Jeng, Y.-Y.; Chen, T.-L.; Fung, C.-P. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: Clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect. Dis. 2010, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Hartantyo, S.H.P.; Chau, M.L.; Koh, T.H.; Yap, M.; Yi, T.; Cao, D.Y.H.; Gutiérrez, R.A.; Ng, L.C. Foodborne Klebsiella pneumoniae: Virulence Potential, Antibiotic Resistance, and Risks to Food Safety. J. Food Prot. 2020, 83, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Puspanadan, S.; Afsah-Hejri, L.; Loo, Y.Y.; Nillian, E.; Kuan, C.H.; Goh, S.G.; Chang, W.S.; Lye, Y.L.; John, Y.H.T.; Rukayadi, Y.; et al. Detection of Klebsiella pneumoniae in raw vegetables using Most Probable Number-Polymerase Chain Reaction (MPN431 PCR). Int. Food Res. J. 2012, 19, 1757–1762. [Google Scholar]
| Target Genes | Primer Sequence | Ampillicon Size (bp) |
|---|---|---|
| blaIMP-1 | F: 5′-TGA GCA AGT TAT CTG TAT TC-3′ | 139 |
| R:5′-TTA GTT GCT TGG TTT TGA TG-3 | ||
| blaOXA-48 | F: 5′-TTG GTG GCA TCG ATT ATC GG-3′ | 281 |
| R: 5′-GAG CAC TTC TTT TGT GAT GGC-3′ | ||
| fimH | F: 5′-CGC CTG GTC CTT TGC CTG CA-3′ | 817 |
| R: 5′-CTG CAC GTT GCC GGC GGT AA-3′ | ||
| ecpA | F: 5′-AAT GGT TCA CCG GGA CAT CAT GTC C-3′ | 759 |
| R: 5′-AAG GAT GAA ATA TCG CCG ACA TCC-3′ | ||
| mrkA | F: 5′-GTT AAC GGC GGC CAG GGC AGC GA-3′ R: 5′-AGG TGA AAC GCG CGC CAT CA-3′ | 382 |
| blaVIM | F: 5′- GAT GGT GTT TGG TCG CAT A-3′ | 390 |
| R: 5′-CGA ATG CGC AGC ACC AG-3′ | ||
| blaNDM | F: 5′-GGT TTG GCG ATC TGG TTT TC-3′ | 521 |
| R: 5′- CGG AAT GGC TCA TCA CGA TC-3′ | ||
| blaKPC | F: 5′-ATG TCA CTG TAT CGC CGT CT-3′ | 538 |
| R: 5′-TTT TCA GAG CCT TAC TGC CC-3′ | ||
| E. coli | F: 5′-TGATTGAAGCAGAAGCCTGC R: 5′-CGCCAATCCACATCTGTGAA | 1.350 |
| Klebsiella pneumoniae | F 5′-ATTTGAAGAGGTTGCAAACGAT-3′ R 5′-TTCACTCTGAAGTTTTCTTGTGTTC-3′ | 130 |
| Strains | Overall (* n = 110) | Chicken (* n = 35) | Bovine (* n = 35) | Pork (* n = 40) |
|---|---|---|---|---|
| Klebsiella pneumoniae | 90 (81.8) | 23 (65.7) | 32 (91.4) | 35 (87.5) |
| Escherichia coli | 20 (18.2) | 7 (20.0) | 3 (8.5) | 10 (25.0) |
| Carbapenemase | Positive Isolates Based on PCR (* n = 65) | Positive Isolates Based on NG-Test Carba 5 (* n = 54) |
|---|---|---|
| KPC | 5 (7.6) | 4 (7.4) |
| OXA-48 | 20 (30.7) | 17 (31.4) |
| IMP | 5 (7.6) | 5 (9.2) |
| VIM | 5 (7.6) | 5 (9.2) |
| NDM | 40 (61.5) | 32 (59.2) |
| Meat Specimens | ecpA (n = 30) | fimH-1 (n = 15) | mrkA (n = 65) |
|---|---|---|---|
| Chicken | 14 (46.7) | 5 (33.3) | 25 (38.5) |
| Bovine | 16 (53.3) | 5 (33.3) | 25 (38.5) |
| Pork | 0 (0.0) | 5 (33.3) | 15 (23.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theocharidi, N.A.; Balta, I.; Houhoula, D.; Tsantes, A.G.; Lalliotis, G.P.; Polydera, A.C.; Stamatis, H.; Halvatsiotis, P. High Prevalence of Klebsiella pneumoniae in Greek Meat Products: Detection of Virulence and Antimicrobial Resistance Genes by Molecular Techniques. Foods 2022, 11, 708. https://doi.org/10.3390/foods11050708
Theocharidi NA, Balta I, Houhoula D, Tsantes AG, Lalliotis GP, Polydera AC, Stamatis H, Halvatsiotis P. High Prevalence of Klebsiella pneumoniae in Greek Meat Products: Detection of Virulence and Antimicrobial Resistance Genes by Molecular Techniques. Foods. 2022; 11(5):708. https://doi.org/10.3390/foods11050708
Chicago/Turabian StyleTheocharidi, Nikoletta Argyro, Iliana Balta, Dimitra Houhoula, Andreas G. Tsantes, George P. Lalliotis, Angeliki C. Polydera, Haralambos Stamatis, and Panagiotis Halvatsiotis. 2022. "High Prevalence of Klebsiella pneumoniae in Greek Meat Products: Detection of Virulence and Antimicrobial Resistance Genes by Molecular Techniques" Foods 11, no. 5: 708. https://doi.org/10.3390/foods11050708
APA StyleTheocharidi, N. A., Balta, I., Houhoula, D., Tsantes, A. G., Lalliotis, G. P., Polydera, A. C., Stamatis, H., & Halvatsiotis, P. (2022). High Prevalence of Klebsiella pneumoniae in Greek Meat Products: Detection of Virulence and Antimicrobial Resistance Genes by Molecular Techniques. Foods, 11(5), 708. https://doi.org/10.3390/foods11050708






_Stamatis.png)

