Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of FAP
2.2.2. Azolla Encapsulation
2.2.3. Microsphere Characterisation
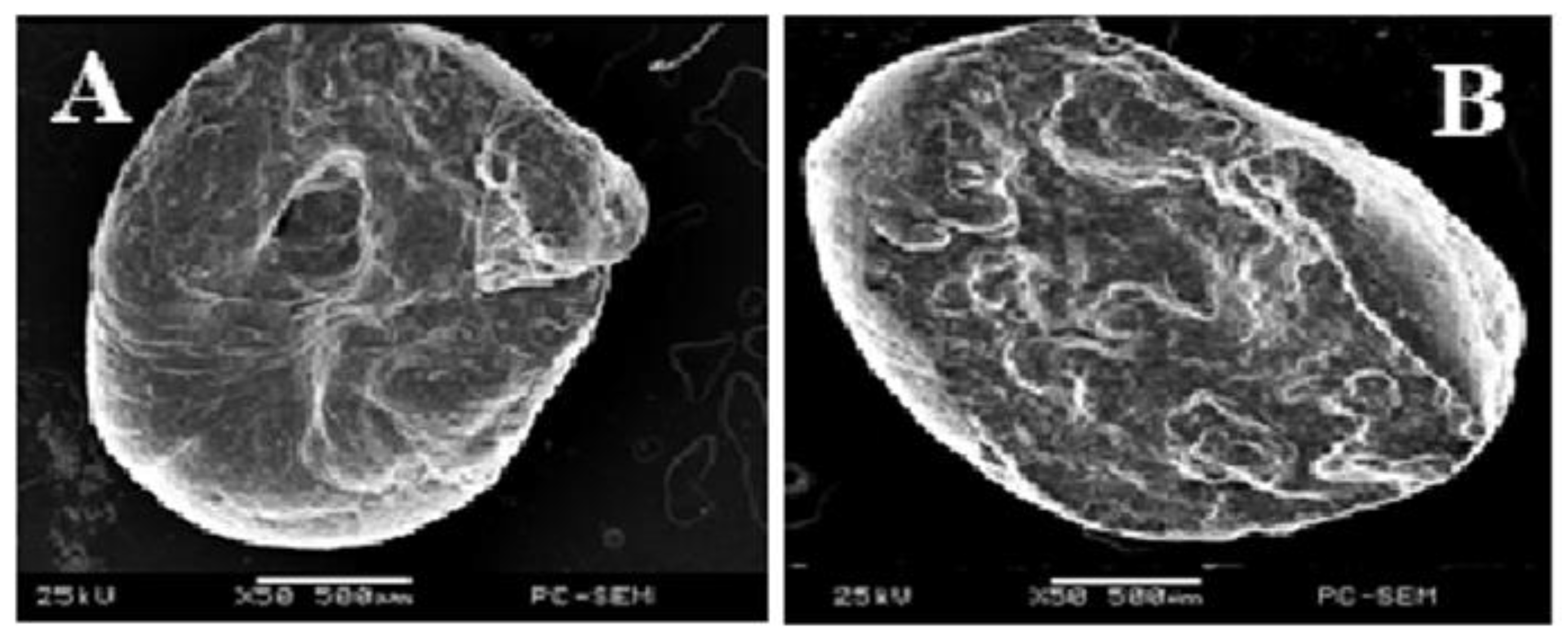
Microscopic Observations
Encapsulation Efficiency
Solubility
Stability of the FAP Microcapsule Antioxidant Activities
2.2.4. Fresh Macaroni Production
2.2.5. Fresh Macaroni Quality
Chemical Quality Indicators
Microbiological Analysis
Colour Measurement
Fresh Macaroni Cooking Attributes
Texture
Sensory Evaluation
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Microsphere Characterisation
3.1.1. Microscopic Observations
3.1.2. Encapsulation Efficiency (%) and Solubility
3.1.3. Stability versus Water Heat Treatment
3.2. Fresh Macaroni Quality
3.2.1. Chemical Quality Indicators
3.2.2. Colour Evaluation
3.2.3. Cooking Attributes
3.2.4. Fresh Macaroni Texture
3.2.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, D.; Pakhira, M.; Bera, S. A review on biology, cultivation and utilization of Azolla. Adv. Life Sci. 2016, 5, 11–15. [Google Scholar]
- Noor Nawaz, A.; Syed, J.; Dileep, N.; Rakesh, K.; Prashith Kekuda, T. Antioxidant activity of Azolla pinnata and Azolla rubra–A comparative study. Sch. Acad. J. Biosci. 2014, 2, 719–723. [Google Scholar]
- Nayak, N.; Padhy, R.N.; Singh, P.K. Evaluation of antibacterial and antioxidant efficacy of the fern Azolla caroliniana symbiotic with the cyanobacterium Anabaena azollae. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 555–569. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Sebestyén, Z.; Blazsó, M.; Berényi, B.; Kumar, J.; Krishna, B.B.; Bhaskar, T.; Czégény, Z. Comparison of hydrothermal carbonization and torrefaction of azolla biomass: Analysis of the solid products. J. Anal. Appl. Pyrolysis 2020, 149, 104844. [Google Scholar] [CrossRef]
- Tran, T.L.N.; Miranda, A.F.; Abeynayake, S.W.; Mouradov, A. Differential production of phenolics, lipids, carbohydrates and proteins in stressed and unstressed aquatic plants, Azolla filiculoides and Azolla pinnata. Biology 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Sci. 2013, 5, 743–749. [Google Scholar]
- Selvaraj, K.; Chowdhury, R.; Bhattacharjee, C. Optimization of the solvent extraction of bioactive polyphenolic compounds from aquatic fern Azolla microphylla using response surface methodology. Int. Food Res. J. 2014, 21, 1559. [Google Scholar]
- Miranda, A.F.; Biswas, B.; Ramkumar, N.; Singh, R.; Kumar, J.; James, A.; Roddick, F.; Lal, B.; Subudhi, S.; Bhaskar, T.; et al. Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 2016, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Agnesi, E. The History of Macaroni. In Macaroni and Noodle Technology; Kruger, J.E., Matsuo, R.B., Dick, J.D., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1996; pp. 1–12. [Google Scholar]
- Bergman, C.; Gualberto, D.; Weber, C. Development of high-temperature dried soft wheat macaroni supplemented with cowpea (Vigna unguiculata (L.) Walp). Cooking quality, colour and sensory evaluation. Cereal Chem. 1994, 71, 523–527. [Google Scholar]
- Sharma, R.; Dar, B.; Sharma, S.; Singh, B. In Vitro digestibility, cooking quality, bio-functional composition, and sensory properties of pasta incorporated with potato and pigeonpea flour. Int. J. Gastron. Food Sci. 2021, 23, 100300. [Google Scholar] [CrossRef]
- Ivanišová, E.; Košec, M.; Brindza, J.; Grygorieva, O.; Tokár, M. Green Barley as an Ingredient in Pasta: Antioxidant Activity and Sensory Characteristics Evaluation. Contemp. Agric. 2018, 67, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Kowalczewski, P.Ł.; Pauter, P.; Smarzyński, K.; Różańska, M.B.; Jeżowski, P.; Dwiecki, K.; Mildner-Szkudlarz, S. Thermal processing of pasta enriched with black locust flowers affect quality, phenolics, and antioxidant activity. J. Food Processing Preserv. 2019, 43, e14106. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT Food Sci. Technol. 2014, 58, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-enriched pasta as functional food rich in protein and antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar]
- Poorghasem, H.; Babakhani, A.; Rostamzad, H. Effect of Green Algae, Ulva intestinalis on Antioxidant Activity of Pasta. J. Fish. 2017, 70, 309–318. [Google Scholar]
- Tello-Ireland, C.; Lemus-Mondaca, R.; Vega-Gálvez, A.; López, J.; Di Scala, K. Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT Food Sci. Technol. 2011, 44, 2112–2118. [Google Scholar] [CrossRef]
- Chan, E.-S.; Lee, B.-B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. Approved Methods of American Association of Cereal Chemists; American Association of Cereal Chemists: St. Paul, MN, USA, 2010. [Google Scholar]
- Association of Official of Analytical Chemists. Official Methods of Analysis, 18th ed.; Association of Official of Analytical Chemists: Arlington, VA, USA, 2010. [Google Scholar]
- Yan, M.; Liu, B.; Jiao, X.; Qin, S. Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.; Ramos, A.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Martino, M. Release of yerba mate antioxidants from corn starch–alginate capsules as affected by structure. Carbohydr. Polym. 2014, 99, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Essa, R.Y.; Mohamed, E.E. Research Article Improvement of Functional and Technological Characteristics of Spaghetti by the Integration of Pomegranate Peels Powder. Am. J. Food Technol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Guiné, R.; Almeida, C.; Correia, P. Effect of packaging and conservation conditions on some physical-chemical properties of almonds. J. Hyg. Eng. Des. 2014, 8, 82–87. [Google Scholar]
- American Public Heath Association. Compendium of Methods for the Microbiological Examination of Food, 4th ed.; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Minolta Corporation. Precise Color Communication: Color Control from Feeling to Instrumentation; Minolta: Osaka, Japan, 1993. [Google Scholar]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Dutcosky, S.D. Análise Sensorial de Alimentos, 4th ed.; Champagnat: Curitiba, Brazil, 2013; p. 531. ISBN 9788554945473. [Google Scholar]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics, 2nd ed.; McGraw-Hill: New York, NY, USA, 1996; p. 120. [Google Scholar]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Suzery, M.; Setyawan, D.; Majid, D.; Sutanto, H. Encapsulation of phycocyanin-alginate for high stability and antioxidant activity. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012030. [Google Scholar] [CrossRef] [Green Version]
- Suzery, M.; Hadiyanto, H.; Majid, D.; Setyawan, D.; Sutanto, H. Improvement of Stability and Antioxidant Activities by Using Phycocyanin-Chitosan Encapsulation Technique. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 12052. [Google Scholar] [CrossRef]
- de Camargo Andrade-Molina, T.P.; Shirai, M.A.; Grossmann, M.V.E.; Yamashita, F. Active biodegradable packaging for fresh pasta. LWT Food Sci. Technol. 2013, 54, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Egyptian Organization for Standardization and Quality. ES: 286-1/ 2005; Marconi and Methods of Inspectation and Testing Part 1: Macaroni; Egyptian Organization for Standardization and Quality: Cairo, Egypt, 2005. [Google Scholar]
- Aranibar, C.; Pigni, N.B.; Martinez, M.; Aguirre, A.; Ribotta, P.; Wunderlin, D.; Borneo, R. Utilization of a partially-deoiled chia flour to improve the nutritional and antioxidant properties of wheat pasta. LWT 2018, 89, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Zardetto, S.; Dalla Rosa, M. Effect of extrusion process on properties of cooked, fresh egg pasta. J. Food Eng. 2009, 92, 70–77. [Google Scholar] [CrossRef]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni Suef Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Bastos, G.M.; Júnior, M.S.S.; Caliari, M.; de Araujo Pereira, A.L.; de Morais, C.C.; Campos, M.R.H. Physical and sensory quality of gluten-free spaghetti processed from amaranth flour and potato pulp. LWT Food Sci. Technol. 2016, 65, 128–136. [Google Scholar] [CrossRef]
- Piu, P.; Fois, S.; Sanna, M.; Roggio, T.; Catzeddu, P. Fresh pasta made with semolina based liquid sourdough: Technological properties and starch digestibility. Tec. Molit. 2018, 69, 496–502. [Google Scholar]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT Food Sci. Technol. 2015, 61, 41–46. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovanniello, V.; Del Nobile, M.A.; Padalino, L.; Lecce, L.; Borrelli, G.M.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Armellini, R.; Peinado, I.; Pittia, P.; Scampicchio, M.; Heredia, A.; Andres, A. Effect of saffron (Crocus sativus L.) enrichment on antioxidant and sensorial properties of wheat flour pasta. Food Chem. 2018, 254, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Hernández, A.; Beta, T.; Loarca-Piña, G.; Castaño-Tostado, E.; Nieto-Barrera, J.O.; Mendoza, S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016, 72, 84–90. [Google Scholar] [CrossRef]
- Doxastakis, G.; Papageorgiou, M.; Mandalou, D.; Irakli, M.; Papalamprou, E.; D’Agostina, A.; Resta, D.; Boschin, G.; Arnoldi, A. Technological properties and non-enzymatic browning of white lupin protein enriched spaghetti. Food Chem. 2007, 101, 57–64. [Google Scholar] [CrossRef]
- Desai, A.; Brennan, M.A.; Brennan, C.S. The effect of semolina replacement with protein powder from fish (Pseudophycis bachus) on the physicochemical characteristics of pasta. LWT 2018, 89, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Sissons, M.; Egan, N.; Gianibelli, M. New insights into the role of gluten on durum pasta quality using reconstitution method. Cereal Chem. 2005, 82, 601–608. [Google Scholar] [CrossRef]
- Dexter, J.; Matsuo, R.; MacGregor, A. Relationship of instrumental assessment of spaghetti cooking quality to the type and the amount of material rinsed from cooked spaghetti. J. Cereal Sci. 1985, 3, 39–53. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
| Sample | Unheated * | Heated * |
|---|---|---|
| Azolla | 99.18 ± 1.04 Aa | 62.91 ± 1.50 Bb |
| Azolla microsphere | 91.26 ± 1.35 Ba | 80.49 ± 1.73 Ab |
| Parameters | Fresh Macaroni Type | |||
|---|---|---|---|---|
| CM | Macaroni with EB | Macaroni with FAP Microcapsules | Macaroni with FAP | |
| Moisture (%) | 29.88 ± 2.47 a | 30.91 ± 2.85 a | 31.30 ± 2.06 a | 30.72 ± 3.11 a |
| Water activity | 0.97 ± 0.001 a | 0.90 ± 0.003 c | 0.94 ± 0.002 b | 0.95 ± 0.004 b |
| Parameters | Fresh Macaroni Type | |||
|---|---|---|---|---|
| CM | Macaroni with EB | Macaroni with FAP Microcapsules | Macaroni with FAP | |
| L* | 61.75 ± 0.82 b | 64.98 ± 0.71 a | 33.18 ± 0.65 c | 31.07 ± 0.59 c |
| a* | 1.17 ± 0.06 a | 0.95 ± 0.05 a | −5.61 ± 0.09 b | −8.49 ± 1.03 c |
| b* | 16.02 ± 0.88 a | 14.73 ± 0.86 b | 6.62 ± 0.34 c | 5.97 ± 0.54 c |
| Parameters | Fresh Macaroni Type | |||
|---|---|---|---|---|
| CM | Macaroni with EB | Macaroni with FAP Microcapsules | Macaroni with FAP | |
| Cooking loss (%) | 7.56 ± 0.29 a | 8.10 ± 0.42 a | 8.53 ± 0.39 a | 8.29 ± 0.50 a |
| Swelling index (%) | 1.23 ± 0.02 a | 1.68 ± 0.03 a | 1.64 ± 0.02 a | 1.58 ± 0.04 a |
| Water absorption (%) | 95.62 ± 1.08 a | 82.40 ± 1.11 b | 79.85 ± 1.16 b | 85.93 ± 1.07 ab |
| Weight increase (%) | 19.33 ± 1.32 a | 18.23 ± 1.48 b | 17.86 ± 1.19 b | 18.60 ± 1.20 ab |
| Firmness (N) | 1.66 ± 0.03 c | 1.85 ± 0.04 b | 2.38 ± 0.01 a | 1.92 ± 0.03 b |
| Adhesiveness (N) | 2.88 ± 0.25 a | 3.10 ± 0.29 a | 2.89 ± 0.18 a | 2.71 ± 0.34 a |
| Parameters | Fresh Macaroni Type | |||
|---|---|---|---|---|
| CM | Macaroni with EB | Macaroni with FAP Microcapsules | Macaroni with FAP | |
| Colour | 7.71 ± 0.93 b | 7.18 ± 1.04 b | 8.23 ± 0.39 a | 8.07 ± 0.66 ab |
| Taste | 7.42 ± 1.18 a | 7.11 ± 1.20 a | 7.30 ± 0.95 a | 7.27 ± 1.13 a |
| Texture | 7.81 ± 1.14 b | 7.39 ± 0.99 bc | 8.60 ± 0.12 a | 7.88 ± 1.09 ab |
| Odour | 8.56 ± 0.24 a | 7.98 ± 0.67 b | 8.43 ± 0.30 a | 7.04 ± 0.97 c |
| Overall acceptability | 7.88 ± 0.87 a | 7.42 ± 0.98 b | 8.14 ± 0.44 a | 7.57 ± 0.96 b |
| Acceptance index (%) | 87.56 b | 82.44 c | 90.44 a | 84.11 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsebaie, E.M.; Asker, G.A.; Mousa, M.M.; Kassem, M.M.; Essa, R.Y. Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder. Foods 2022, 11, 707. https://doi.org/10.3390/foods11050707
Elsebaie EM, Asker GA, Mousa MM, Kassem MM, Essa RY. Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder. Foods. 2022; 11(5):707. https://doi.org/10.3390/foods11050707
Chicago/Turabian StyleElsebaie, Essam Mohamed, Galila Ali Asker, Mona Metwally Mousa, Mona Morgan Kassem, and Rowida Younis Essa. 2022. "Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder" Foods 11, no. 5: 707. https://doi.org/10.3390/foods11050707
APA StyleElsebaie, E. M., Asker, G. A., Mousa, M. M., Kassem, M. M., & Essa, R. Y. (2022). Technological and Sensory Aspects of Macaroni with Free or Encapsulated Azolla Fern Powder. Foods, 11(5), 707. https://doi.org/10.3390/foods11050707






