Antibacterial Properties of TMA against Escherichia coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Determination of Antibacterial Activity of TMA against E. coli
2.3. Egg Collection and Storage
2.4. Determination of TMA Content in Yolk
2.5. Determination of Lysozyme Activity and Content
2.6. Determination of Cuticle Quality
2.7. Determination of Total Bacterial Count (TBC)
2.8. Statistical Analysis
3. Results and Discussion
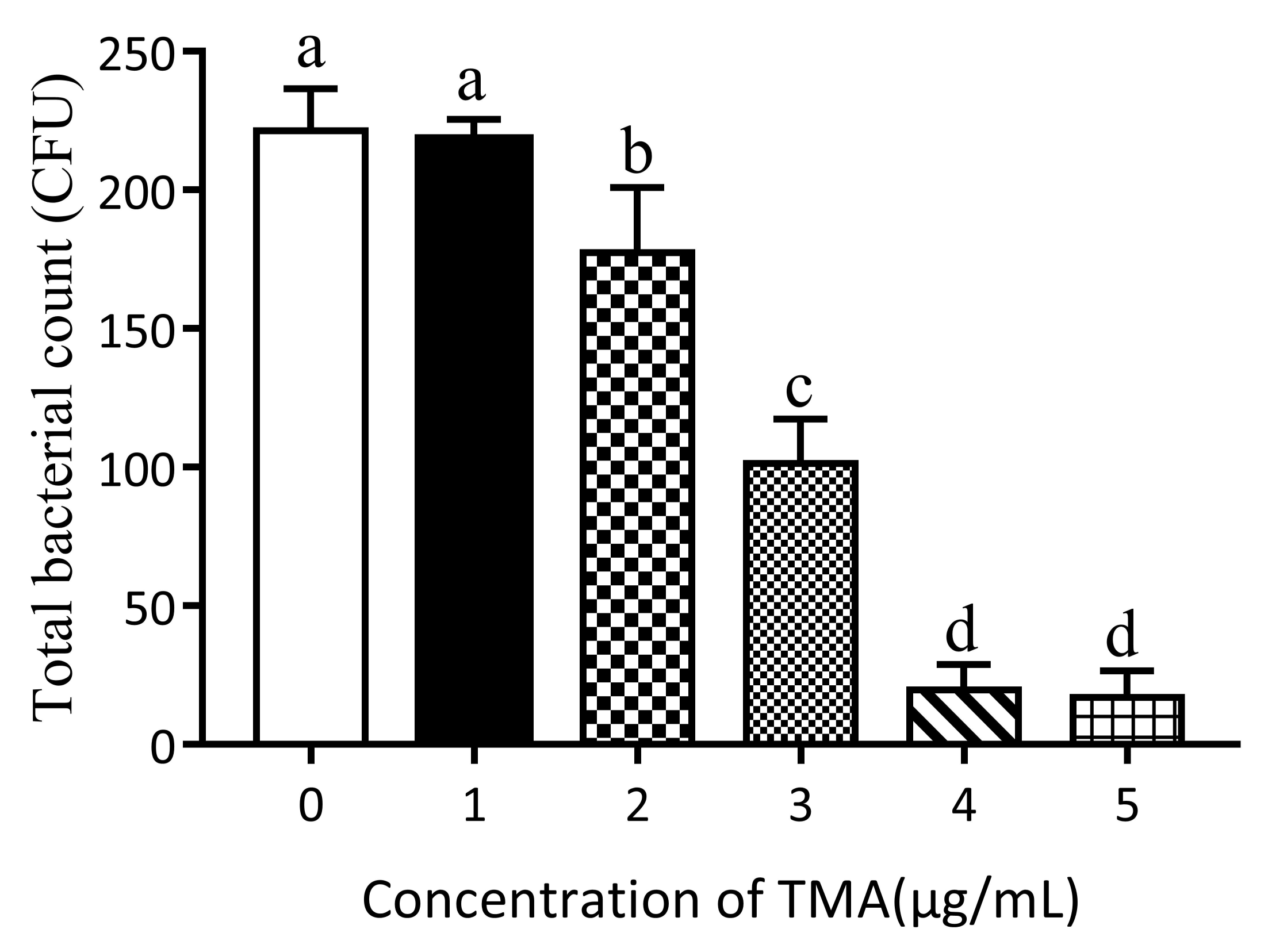
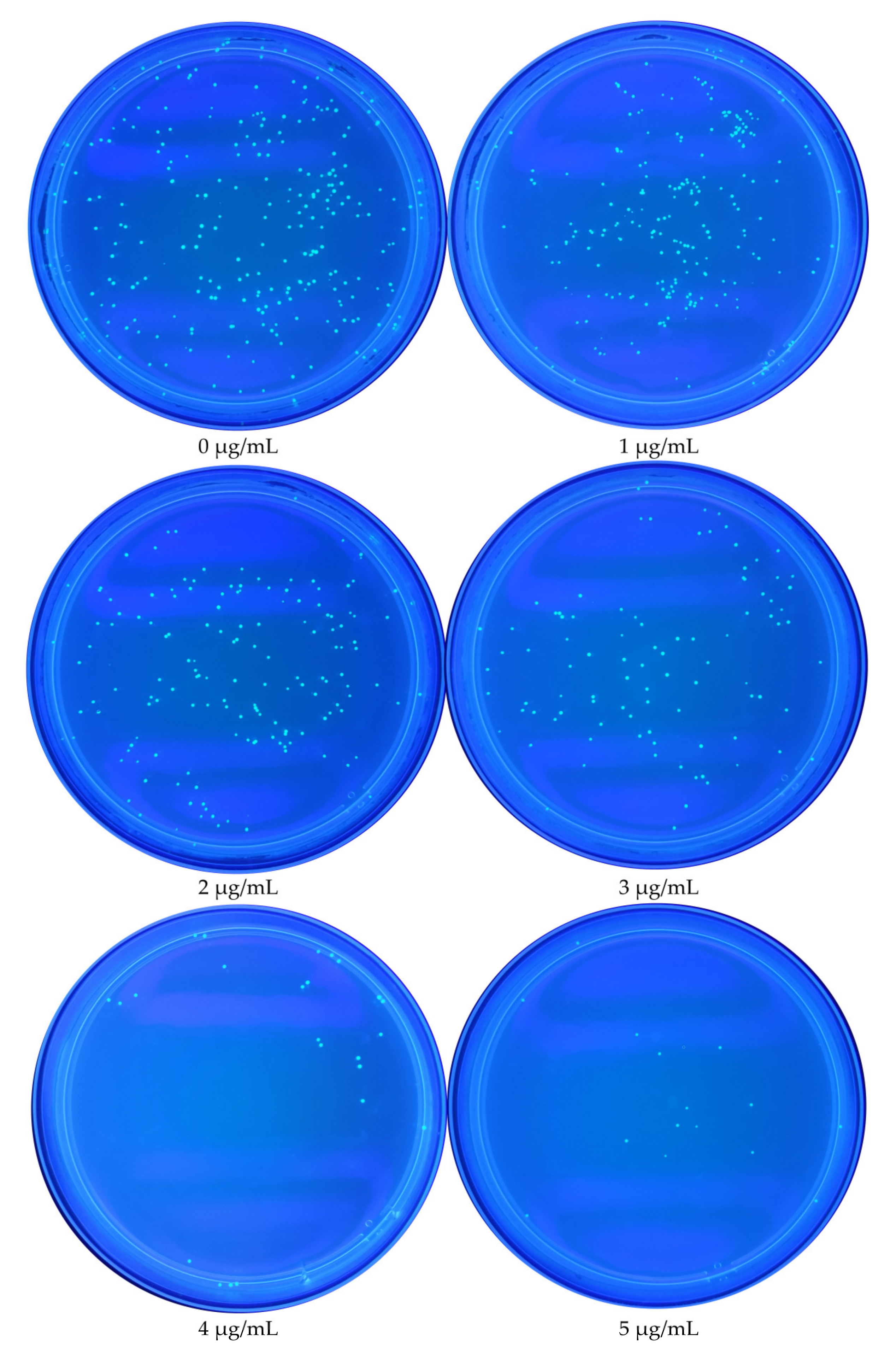
3.1. Effect of TMA Concentration on E. coli Growth
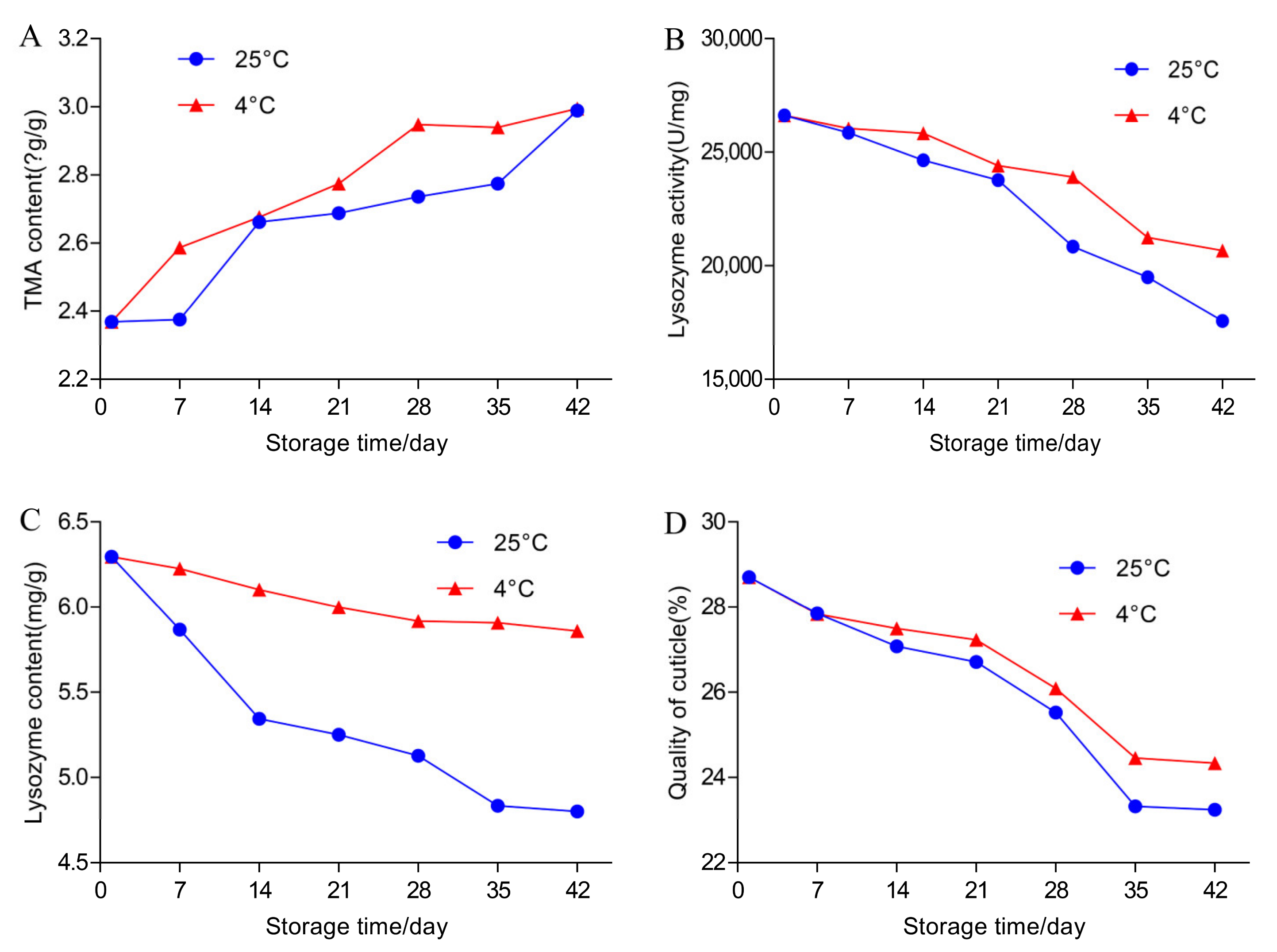
3.2. Effects of Temperature and Storage Duration on TMA Content and TBC in Egg Yolks
3.3. Effects of Temperature and Storage Duration on the Content and Activity of Lysozyme and TBC in Egg White
3.4. Effects of Temperature and Storage Duration on Egg Cuticle Quality and TBC on Eggshell Surface
3.5. Other Chemical Defense Mechanisms in the Egg White and Yolk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Board, R.G.; Halls, N.A. The cuticle: A barrier to liquid and particle penetration of the shell of the hen’s egg. Poult. Sci. 1973, 14, 69–97. [Google Scholar] [CrossRef]
- Gautron, J.; Stapane, L.; Roy, N.L.; Nys, Y.; Rodrigueznavarro, A.B.; Hincke, M.T. Correction to: Avian eggshell biomineralization: An update on its structure, mineralogy and protein tool kit. BMC Mol. Cell Biol. 2021, 22, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.M.; Mcdade, K.; Burchmore, R.; Law, A.; Wilson, P.W.; Schmutz, M.; Preisinger, R.; Dunn, I.C. Enhancing the egg’s natural defence against bacterial penetration by increasing cuticle deposition. Anim. Genet. 2013, 44, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Rose-Martel, M.; Du, J.; Hincke, M.T. Proteomic analysis provides new insight into the chicken eggshell cuticle. J. Proteomics 2012, 75, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Bolder, N.M.; Kan, C.A. Microbiology of the Avian Egg; Springer: New York, NY, USA, 1994; pp. 94–112. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Guo, Y.; Li, W.; Song, J.; Xu, G.; Yang, N.; Zheng, J. Impact of cuticle quality and eggshell thickness on egg antibacterial efficiency. Poult. Sci. 2019, 98, 940–948. [Google Scholar] [CrossRef]
- Valdes, S.A.; Vieira, L.G.; Ferreira, C.H.; Dos, S.M.; Ribeiro, P.R.; Abreu, F.E.; Santos, A.L. Effects of exposure to methyl parathion on egg hatchability and eggshell chemical composition in Podocnemis expansa (Testudines, Podocnemididae). Zool. Sci. 2015, 32, 135–140. [Google Scholar] [CrossRef]
- Julien, L.A.; Baron, F.; Bonnassie, S.; Nau, F.; Guerin, C.; Jan, S.; Andrews, S. The anti-bacterial iron-restriction defence mechanisms of egg white; the potential role of three lipocalin-like proteins in resistance against Salmonella. Biometals 2019, 32, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Peris-Vicente, J.; Garcia-Ferrer, D.; Mishra, P.; Albiol-Chiva, J.; Durgbanshi, A.; Carda-Broch, S.; Bose, D.; Esteve-Romero, J. Procedure for the screening of eggs and egg products to detect oxolonic acid, ciprofloxacin, enrofloxacin, and sarafloxacin using micellar liquid chromatography. Antibiotics 2019, 8, 226. [Google Scholar] [CrossRef]
- Baron, F.; Jan, S.; Gonnet, F.; Pasco, M.; Jardin, J.; Giudici, B.; Gautier, M.; Guérin-Dubiard, C.; Nau, F. Ovotransferrin plays a major role in the strong bactericidal effect of egg white against the Bacillus cereus group. J. Food Prot. 2014, 77, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y. Recent advances in egg protein functionality in the food system. World’s Poult. Sci. J. 2002, 58, 31–39. [Google Scholar] [CrossRef]
- Blake, C.C.; Mair, G.A.; North, A.C.; Phillips, D.C.; Sarma, V.R. On the conformation of the hen egg-white lysozyme molecule. Proc. R. Soc. Lond. Ser. B 1967, 167, 365–377. [Google Scholar] [CrossRef]
- Rose, M.E.; Orlans, E.; Buttress, N. Immunoglobulin classes in the hen’s egg: Their segregation in yolk and white. Eur. J. Immunol. 1974, 4, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Gaines, S.; Fenelon, L.; Mcpartlin, J.; Farrelly, C. A lipoprotein-derived antimicrobial factor from hen-egg yolk is active against Streptococcus species. J. Food Sci. 2002, 67, 3096–3103. [Google Scholar] [CrossRef]
- Kalac, P.; Krausova, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food. Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Rêgo, I.O.; Menezes, L.D.; Figueiredo, T.C.; Oliveira, D.D.; Rocha, J.S.; Lara, L.J.; Lima, A.L.; Souza, M.R.; Cançado, S.V. Bioactive amines and microbiological quality in pasteurized and refrigerated liquid whole egg. Poult. Sci. 2014, 93, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.E.; Figueiredo, T.C.; Souza, M.R.; Oliveira, A.L.; Cancado, S.V.; Gloria, M.B. Bioactive amines and quality of egg from Dekalb hens under different storage conditions. Poult. Sci. 2009, 88, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zheng, J.; Qu, L.; Lian, L.; Xu, G.; Yang, N. Molecular cloning, sequence characterization, SNP detection, and tissue expression analysis of duck FMO3 gene. Mol. Cell. Biochem. 2013, 379, 141–145. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Song, J.; Shi, X.; Chen, X.; Yang, F.; Pi, J.; Zhang, H.; Xu, G.; Zheng, J. Analysis of fishy taint in duck eggs reveals the causative constituent of the fishy odor and factors affecting the perception ability of this odor. Poult. Sci. 2019, 98, 5198–5207. [Google Scholar] [CrossRef]
- Kubena, L.F.; Harvey, R.B.; Buckley, S.A.; Bailey, R.H.; Rottinghaus, G.E. Effects of long-term feeding of diets containing moniliformin, supplied by Fusarium fujikuroi culture material, and fumonisin, supplied by Fusarium moniliforme culture material, to laying hens. Poult. Sci. 1999, 78, 1499–1505. [Google Scholar] [CrossRef]
- Hobson-Frohock, A.; Land, D.G.; Griffiths, N.M.; Curtis, R.F. Egg taints: Association with trimethylamine. Nature 1973, 243, 304–305. [Google Scholar] [CrossRef]
- Ward, A.K.; Classen, H.L.; Buchanan, F.C. Fishy-egg tainting is recessively inherited when brown-shelled layers are fed canola meal. Poult. Sci. 2009, 88, 714–721. [Google Scholar] [CrossRef]
- Almas, K.; Al-Lafi, T.R. The natural toothbrush. World Health Forum 1995, 16, 206–210. Available online: http://www.who.int/iris/handle/10665/50441 (accessed on 10 January 2022). [PubMed]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. eLife 2016, 6, e21738. [Google Scholar] [CrossRef]
- Percie, D.S.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Bernier, S.P.; Letoffe, S.; Delepierre, M.; Ghigo, J.M. Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol. Microbiol. 2011, 81, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Honkatukia, M.; Reese, K.; Preisinger, R.; Tuiskula-Haavisto, M.; Weigend, S.; Roito, J.; Maki-Tanila, A.; Vilkki, J. Fishy taint in chicken eggs is associated with a substitution within a conserved motif of the FMO3 gene. Genomics 2005, 86, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lewko, L.; Krawczyk, J.; Calik, J. Effect of genotype and some shell quality traits on lysozyme content and activity in the albumen of eggs from hens under the biodiversity conservation program. Poult. Sci. 2021, 100, 100863. [Google Scholar] [CrossRef]
- Dembczynski, R.; Bialas, W.; Regulski, K.; Jankowski, T. Lysozyme extraction from hen egg white in an aqueous two-phase system composed of ethylene oxide–propylene oxide thermoseparating copolymer and potassium phosphate. Process Biochem. 2010, 45, 369–374. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Letoffe, S.; Audrain, B.; Bernier, S.P.; Delepierre, M.; Ghigo, J.M. Aerial exposure to the bacterial volatile compound trimethylamine modifies antibiotic resistance of physically separated bacteria by raising culture medium pH. mBio 2014, 5, e913–e944. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, H.; He, X.; Thomas, B.W.; Lupwayi, N.Z.; Hao, X.; Thomas, M.C.; Shi, X. Fertilization shapes bacterial community structure by alteration of soil pH. Front. Microbiol. 2017, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, T.W.; Unruh, J.B.; Ruse, J.K.; Restuccia, N.G. Solubility of iron, manganese, and magnesium sulfates and glucoheptonates in two alkaline soils. Soil Sci. Soc. Am. J. 2016, 80, 765–770. [Google Scholar] [CrossRef]
- Ratzke, C.; Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 2018, 16, e2004248. [Google Scholar] [CrossRef]
- Mcmillan, D.G.; Velasquez, I.; Nunn, B.L.; Goodlett, D.R.; Hunter, K.A.; Lamont, I.; Sander, S.G.; Cook, G.M. Acquisition of iron by alkaliphilic Bacillus species. Appl. Environ. Microbiol. 2010, 76, 6955–6961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, S.E.; Pham, C.A.; Zambri, M.P.; Mckillip, J.; Carlson, E.; Elliot, M. Streptomyces volatile compounds influence exploration and microbial community dynamics by altering iron availability. mBio 2019, 10, e171. [Google Scholar] [CrossRef]
- Hattab, F.N. Meswak: The natural toothbrush. J. Clin. Dent. 1997, 8, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hilal, A.; Nizar, A. Therapeutic properties of meswak chewing sticks: A review. Afr. J. Biotechnol. 2012, 11, 14850–14857. [Google Scholar] [CrossRef]
- Ahmad, H.; Rajagopal, K. Biological activities of Salvadora persica L. (Meswak). Med. Aromat. Plants 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Zhong, H.; Luo, Y.; Sun, J.; Wang, C.; Wang, Q.; Gao, G.; Zhang, K.; Li, Q.; Wang, H.; Li, J.; et al. Goose FMO3 gene cloning, tissue expression profiling, polymorphism detection and association analysis with trimethylamine level in the egg yolk. Gene 2017, 632, 25–35. [Google Scholar] [CrossRef]
- Li, X.; Yuan, G.; Chen, X.; Guo, Y.; Yang, N.; Pi, J.; Zhang, H.; Zheng, J. Fishy odor and TMA content levels in duck egg yolks. J. Food Sci. 2018, 83, 39–45. [Google Scholar] [CrossRef]
- Nijs, E.A.; Hicks, L.C.; Leizeaga, A.; Tietema, A.; Rousk, J. Soil microbial moisture dependences and responses to drying–rewetting: The legacy of 18 years drought. Glob. Chang. Biol. 2019, 25, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.G.; Zhang, H.J.; Yue, H.Y.; Xu, L.; Ji, F.; Xu, L.; Qi, G.H. Trimethylamine deposition in the egg yolk from laying hens with different FMO3 genotypes. Poult. Sci. 2013, 92, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.C.; Viegas, R.P.; Lara, L.J.; Baiao, N.C.; Souza, M.R.; Heneine, L.G.; Cancado, S.V. Bioactive amines and internal quality of commercial eggs. Poult. Sci. 2013, 92, 1376–1384. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Liao, Y.; Brown, M.B.; Martin, G.P. Turbidimetric and HPLC assays for the determination of formulated lysozyme activity. J. Pharm. Pharmacol. 2001, 53, 549–554. [Google Scholar] [CrossRef]
- Weth, F.; Schroeder, T.; Buxtorf, U.P. Determination of lysozyme content in eggs and egg products using SDS-gel electrophoresis. Z. Lebensm. Unters. Forsch. 1988, 187, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Board, R.G. Review article: The course of microbial infection of the hen’s egg. J. Appl. Bacteriol. 1966, 29, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Mendoncam, A.F.; Ismail, H.; Ahn, D.U. Ethylenediaminetetraacetate and lysozyme improves antimicrobial activities of ovotransferrin against Escherichia coli O157:H7. Poult. Sci. 2009, 88, 406. [Google Scholar] [CrossRef] [PubMed]
- Lesnierowski, G.; Borowiak, R. Effect of environmental conditions on change in properties of lysozyme in hen egg white. Zywn. Nauka Technol. Jakosc 2012, 19, 77–87. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Davies, G.J.; Laine, R.; Withers, S.G. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 2001, 412, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, J.; Huang, M.; Xu, Q.; Ma, M. The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol. 2016, 92, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Feeney, R.E.; Anderson, J.S.; Azari, P.R.; Bennett, N.; Rhodes, M.B. The comparative biochemistry of avian egg white proteins. J. Biol. Chem. 1960, 235, 2307. [Google Scholar] [CrossRef]
- Mayes, F.J.; Takeballi, M.A. Microbial contamination of the hen’s egg: A review. J. Food Prot. 1983, 46, 1092–1098. [Google Scholar] [CrossRef]
- D’Alba, L.; Maia, R.; Hauber, M.E.; Shawkey, M.D. The evolution of eggshell cuticle in relation to nesting ecology. Proc. R. Soc. B 2016, 283, 687. [Google Scholar] [CrossRef] [PubMed]
- Reu, K.D.; Grijspeerdt, K.; Heyndrickx, M.; Messens, W.; Uyttendaele, M.; Debevere, J.; Herman, L. Influence of eggshell condensation on eggshell penetration and whole egg contamination with Salmonella enterica serovar enteritidis. J. Food Prot. 2006, 69, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A.; Silversides, F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000, 79, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Curtis, P.A.; Anderson, K.E.; Jones, F.T. Microbial contamination in inoculated shell eggs: II. effects of layer strain and egg storage. Poult. Sci. 2004, 83, 95–100. [Google Scholar] [CrossRef]
- Olivier, W.; Jaroslav, P.; Maxwell, T.H. Antimicrobial activity of the Anseriform outer eggshell and cuticle. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 640–649. [Google Scholar] [CrossRef]
- Messens, W.; Grijspeerdt, K.; Reu, K.D.; Ketelaere, B.D.; Mertens, K.; Bamelis, F.; Kemps, B.; Baerdemaeker, J.D.; Decuypere, E.; Herman, L. Eggshell penetration of various types of hens’ eggs by Salmonella enterica serovar Enteritidis. J. Food Prot. 2007, 70, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J. The eggshell cuticle of the laying hen. World’s Poult. Sci. J. 2014, 70, 693–708. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Domínguez-Gasca, N.; Muñoz, A.; Ortega-Huertas, M. Change in the chicken eggshell cuticle with hen age and egg freshness. Poult. Sci. 2013, 92, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Reu, K.D.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Legros, J.; Jan, S.; Bonnassie, S. The role of ovotransferrin in egg-white antimicrobial activity: A review. Foods 2021, 10, 823. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, Q.; Geng, F. Characterization of the interaction between hen egg white lysozyme and ovalbumin: Interaction between lysozyme and ovalbumin. Food Biosci. 2020, 36, 10674. [Google Scholar] [CrossRef]
- Zhen, Y.H.; Jin, L.J.; Guo, J.; Li, X.Y.; Lu, Y.N.; Chen, J. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Escherichia coli. Vet. Microbiol. 2008, 130, 126–133. [Google Scholar] [CrossRef]
- Choi, I.; Jung, C.; Seog, H.; Choi, H. Purification of phosvitin from egg yolk and determination of its physicochemical properties. Food Sci. Biotechnol. 2004, 13, 434–437. [Google Scholar] [CrossRef]


| Index | T (°C) | Storage Time (Day) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| TMA content (μg/g) | 25 4 | 2.369 ± 0.186 c 2.369 ± 0.186 c | 2.375 ± 0.68 c,y 2.587 ± 0.161 c,x | 2.662 ± 0.15 b 2.677 ± 0.146 bc | 2.688 ± 0.394 b 2.774 ± 0.127 b | 2.736 ± 0.568 b,y 2.948 ± 0.121 a,x | 2.775 ± 0.166 b,y 2.989 ± 0.043 a,x | 2.989 ± 1.116 a 2.995 ± 0.044 a |
| lysozyme activity (×105 U/mg) | 25 4 | 2.662 ± 0.119 a 2.662 ± 0.119 a | 2.586 ± 0.077 b,y 2.605 ± 0.106 a,x | 2.464 ± 0.083 c,y 2.584 ± 0.063 b,x | 2.377 ± 0.138 d,y 2.440 ± 0.043 b,x | 2.084 ± 0.145 d,y 2.391 ± 0.135 c,x | 1.949 ± 0.839 e,y 2.124 ± 0.112 c,x | 1.756 ± 0.029 e,y 2.066 ± 0.086 c,x |
| lysozyme content (mg/g) | 25 4 | 6.296 ± 0.215 a 6.296 ± 0.215 a | 5.867 ± 0.065 a,y 6.226 ± 0.183 ab,x | 5.344 ± 0.134 b,y 6.103 ± 0.141 bc,x | 5.251 ± 0.139 c,y 6.000 ± 0.193 cd,x | 5.128 ± 0.097 c,y 5.918 ± 0.155 d,x | 4.833 ± 0.045 d,y 5.908 ± 0.198 d,x | 4.800 ± 0.088 e,y 5.589 ± 0.098 d,x |
| quality of cuticle (%) | 25 4 | 28.701 ± 1.894 a 28.701 ± 1.894 a | 27.840 ± 2.337 a 27.853 ± 0.926 ab | 27.077 ± 2.206 ab,y 27.494 ± 2.610 ab,x | 26.708 ± 1.289 ab,y 27.231 ± 1.82 ab,x | 25.524 ± 1.612 b,y 26.092 ± 2.142 bc,x | 23.325 ± 0.894 c,y 24.460 ± 1.676 c,x | 23.244 ± 1.406 c,y 24.337 ± 1.299 c,x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Li, X.; Li, X.; He, Z.; Chen, X.; Song, J.; Zeng, L.; Liang, Q.; Li, J.; Xu, G.; et al. Antibacterial Properties of TMA against Escherichia coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs. Foods 2022, 11, 527. https://doi.org/10.3390/foods11040527
Shi X, Li X, Li X, He Z, Chen X, Song J, Zeng L, Liang Q, Li J, Xu G, et al. Antibacterial Properties of TMA against Escherichia coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs. Foods. 2022; 11(4):527. https://doi.org/10.3390/foods11040527
Chicago/Turabian StyleShi, Xuefeng, Xingzheng Li, Xianyu Li, Zhaoxiang He, Xia Chen, Jianlou Song, Lingsen Zeng, Qianni Liang, Junying Li, Guiyun Xu, and et al. 2022. "Antibacterial Properties of TMA against Escherichia coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs" Foods 11, no. 4: 527. https://doi.org/10.3390/foods11040527
APA StyleShi, X., Li, X., Li, X., He, Z., Chen, X., Song, J., Zeng, L., Liang, Q., Li, J., Xu, G., & Zheng, J. (2022). Antibacterial Properties of TMA against Escherichia coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs. Foods, 11(4), 527. https://doi.org/10.3390/foods11040527






