Chemical Characterization of the Oil Separated by Mechanical Pressing from Strychnos madagascariensis Dried Fruit Pulp Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sampling and Oil Extraction
2.3. Fatty Acids Composition
2.4. Triacylglycerols, Diacylglycerols, and Free Fatty Acids
2.5. Phytosterols
2.6. Tocols and Carotenes
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acids Composition
3.2. Triacylglycerols, Diacylglycerols, and Free Fatty Acids
3.3. Minor Bioactive Constituents
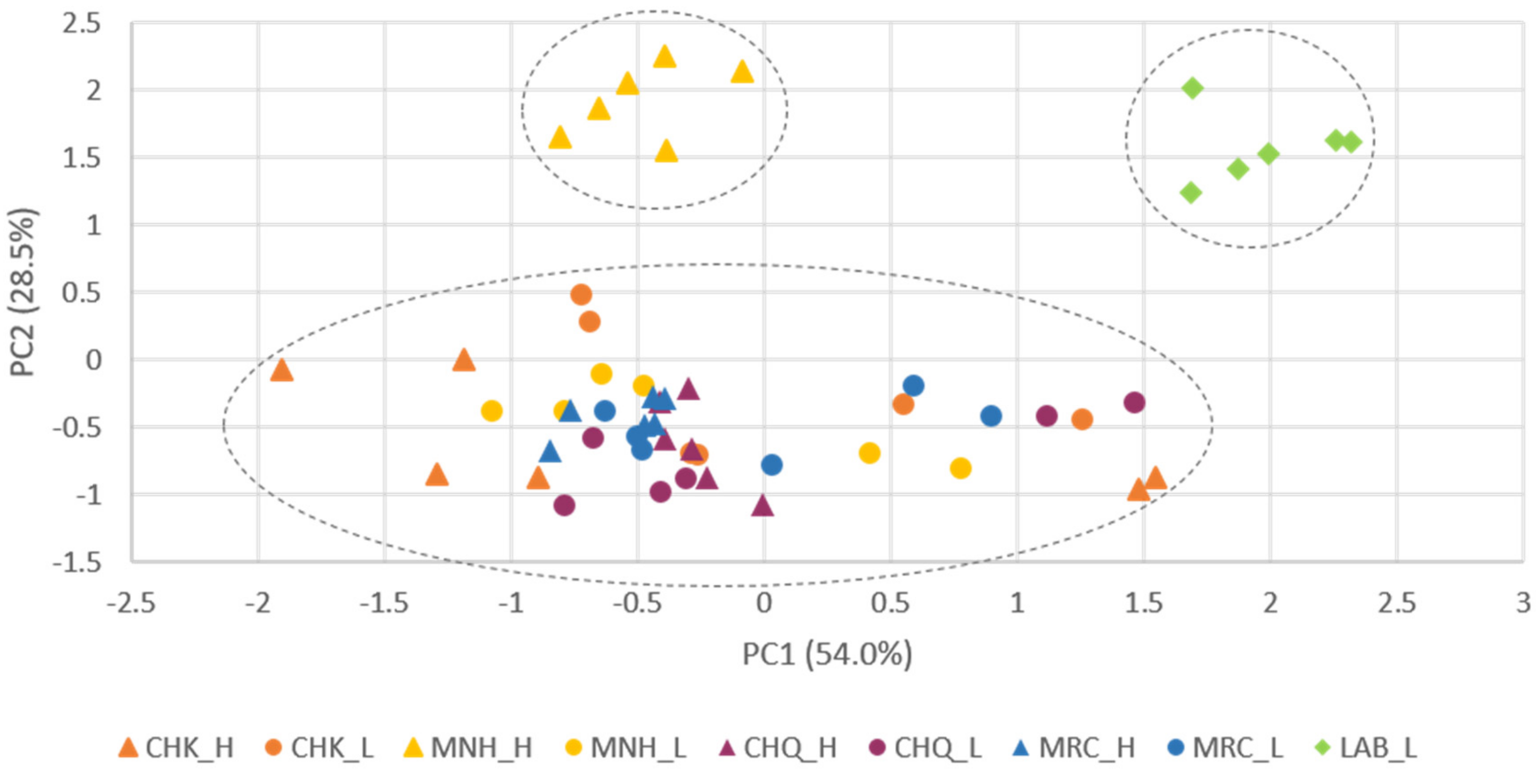
3.4. Samples Classification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngadze, R.T.; Linnemann, A.R.; Nyanga, L.K.; Fogliano, V.; Verkerk, R. Local processing and nutritional composition of indige-nous fruits: The case of monkey orange (Strychnos spp.) from Southern Africa. Food Rev. Int. 2017, 33, 123–142. [Google Scholar] [CrossRef] [Green Version]
- van Wyk, B.; Gericke, N. People’s Plants. A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- van Rayne, K.K.; Adebo, O.A.; Wokadala, O.C.; Ngobese, N.Z. The potential of Strychnos spp. L. Utilization in Food Insecurity Alleviation: A review. Food Rev. Int. 2021, 1–15. [Google Scholar] [CrossRef]
- Ngadze, R.T.; Verkerk, R.; Nyanga, L.K.; Fogliano, V.; Linnemann, A.R. Improvement of traditional processing of local monkey orange (Strychnos spp) fruits to enhance nutrition security in Zimbabwe. Food Sec. 2017, 9, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Ngemakwe, P.N.; Remize, F.; Thaoge, M.L.; Sivakumar, D. Phytochemical and nutritional properties of underutilised fruits in the southern African region. South Afr. J. Bot. 2017, 113, 137–149. [Google Scholar] [CrossRef]
- Oboh, M.O.; Osunsanmi, F.O.; Zharare, G.E.; Mosa, R.A.; Ojo, M.C.; Opoku, A.R. In vitro Antioxidant and Antidiabetic Potential of Crude Extracts from the Seed Coat and Fruit Pulp of Strychnos madagascariensis. Pharmacogn. J. 2020, 12, 1504–1511. [Google Scholar] [CrossRef]
- Absalomé, M.A.; Massara, C.C.; Alexandre, A.A.; Gervais, K.; Chantal, G.G.; Ferdinand, D.; Rhedoor, A.J.; Coulibaly, I.; George, T.G.; Brigitte, T.; et al. Biochemical properties, nutritional values, health benefits and sustainability of palm oil. Biochimie 2020, 178, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Kamatou, G.P.P.; Komane-Mofokeng, B.; Viljoen, A.M.; Beckett, K.K. African seed oils of commercial importance—Cosmetic applications. South Afr. J. Bot. 2011, 77, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Boukandoul, S.; Casal, S.; Cruz, R.; Pinho, C.; Zaidi, F. Algerian Moringa oleifera whole seeds and kernels oils: Characterization, oxidative stability, and antioxidant capacity. Eur. J. Lipid Sci. Technol. 2017, 119, 1600410. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem 2012, 132, 439–446. [Google Scholar] [CrossRef]
- Regulation (EEC) No. 2568/91. The characteristics of olive oil and olive residue oil and on the relevant methods of analysis. OJ L 1991, 248, 59.
- ISO 18395:2005; Animal and Vegetable Fats and Oils—Determination of Monoacylglycerols, Diacylglycerols, Triacylglycerols and Glycerol by High-Performance Size-Exclusion Chromatography (HPSEC). International Organization for Standardization: Geneva, Switzerland, 2005.
- Cruz, R.; Casal, S. Direct analysis of vitamin A, vitamin E, carotenoids, chlorophylls and free sterols in animal and vegetable fats in a single normal-phase liquid chromatographic run. J. Chrom. A 2018, 1565, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Codex Stan 210-1999; Standard for Named Vegetable Oils. Food and Agriculture Organization of the United States (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 2015.
- Riley, T.; Petersen, K.; Kris-Etherton, P. Health aspects of high-oleic oils. In High Oleic Oils; Flider, F.J., Ed.; AOCS Press: Urbana, IL, USA, 2022; Chapter 9; pp. 201–243. ISBN 9780128229125. [Google Scholar] [CrossRef]
- Wren, S.; Stucki, A. Organic essential oils, indigenous cold pressed oils, herbs and spices in Sub-Saharan Africa. Int. J. Arom. 2003, 23, 71–81. [Google Scholar] [CrossRef]
- Ojewole, J.A.O.; Mawoza, T.; Chiwororo, W.D.H.; Owira, P.M.O. Sclerocarya birrea (A. Rich.) Hochst. [‘Marula’] (Anacardiaceae): A review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phyt. Res. 2010, 24, 633–639. [Google Scholar] [CrossRef]
- Saeed, A.E.M.; Bashier, R.S.M. Physico-chemical analysis of Ximenia americana L., seed oil and structure elucidation of some chemical constituents of its seed oil and fruit pulp. J. Pharm. Phyt. 2010, 2, 49–55. [Google Scholar]
- Lee, R.; Balick, M.J. Palms, people and health. Explore 2008, 4, 59–62. [Google Scholar] [CrossRef]
- Morcillo, F.; Cros, D.; Billotte, N.; Ngando-Ebongue, G.F.; Domonhédo, H.; Pizot, M.; Cuéllar, T.; Espéout, S.; Dhouib, R.; Bourgis, F.; et al. Improving palm oil quality through identification and mapping of the lipase gene causing oil deterioration. Nat. Commun. 2013, 4, 2160. [Google Scholar] [CrossRef] [Green Version]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- Birringer, M.; Siems, K.; Maxones, A.; Frank, J.; Lorkowski, S. Natural 6-hydroxy-chromanols and -chromenols: Structural diversity, biosynthetic pathways and health implications. RSC Adv. 2018, 8, 4803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Xie, L.; Liu, R.; Chang, M.; Zhang, H.; Jin, Q.; Wang, X. Revisiting the 4, 4-dimethylsterols profile from different kinds of vegetable oils by using GC-MS. LWT 2020, 124, 109163. [Google Scholar] [CrossRef]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.N.S.; Nair, R.V.R.; Nair, A.P.R.; Nair, A.S.; Thyagarajan, S.; Johnson, A.J.; Baby, S. Antidiabetes constituents, cycloartenol and 24-methylenecycloartanol, from Ficus krishnae. PLoS ONE 2020, 15, e0235221. [Google Scholar] [CrossRef] [PubMed]
| Chókwé | Manhiça | Chicualacuala | Marracuene | Control | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50 °C * | |
| C6:0 | 0.22 ± 0.09 | 0.28 ± 0.02 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.21 ± 0.04 | 0.24 ± 0.03 | 0.25 ± 0.02 | 0.23 ± 0.01 |
| C8:0 | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 |
| C14:0 | 0.13 ± 0.01 | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.15 ± 0.01 |
| C15:0 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.09 ± 0.00 |
| C16:0 | 19.9 ± 0.24 | 20.1 ± 0.34 | 19.9 ± 0.22 | 20.0 ± 0.17 | 19.9 ± 0.27 | 19.9 ± 0.10 | 19.9 ± 0.19 | 20.0 ± 0.04 | 19.5 ± 0.08 |
| C17:0 | 0.09 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 |
| C18:0 | 4.46 ± 0.09 | 4.45 ± 0.18 | 4.53 ± 0.09 | 4.57 ± 0.05 | 4.51 ± 0.07 | 4.61 ± 0.06 | 4.56 ± 0.11 | 4.54 ± 0.02 | 4.33 ± 0.04 |
| C20:0 | 0.61 ± 0.01 | 0.64 ± 0.03 | 0.62 ± 0.01 | 0.62 ± 0.01 | 0.63 ± 0.02 | 0.63 ± 0.01 | 0.61 ± 0.01 | 0.62 ± 0.02 | 0.65 ± 0.01 |
| C22:0 | 0.31 ± 0.01 | 0.30 ± 0.03 | 0.30 ± 0.01 | 0.30 ± 0.02 | 0.31 ± 0.01 | 0.32 ± 0.03 | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.35 ± 0.01 |
| C24:0 | 0.18 ± 0.01 | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.01 |
| Total SFA | 26.2 ± 0.3 a | 26.6 ± 0.5 A | 26.4 ± 0.3 a | 26.5 ± 0.2 A | 26.4 ± 0.3 a | 26.5 ± 0.1 A | 26.4 ± 0.2 a | 26.5 ± 0.1 A | 25.9 ± 0.1 bB |
| C16:1 | 1.59 ± 0.02 | 1.60 ± 0.04 | 1.58 ± 0.03 | 1.61 ± 0.02 | 1.59 ± 0.04 | 1.60 ± 0.02 | 1.60 ± 0.03 | 1.61 ± 0.01 | 1.55 ± 0.02 |
| C18:1 | 62.74 ± 0.17 | 62.36 ± 0.17 | 62.67 ± 0.18 | 62.58 ± 0.14 | 62.53 ± 0.13 | 62.50 ± 0.09 | 62.61 ± 0.26 | 62.49 ± 0.06 | 62.66 ± 0.11 |
| C20:1 | 0.30 ± 0.02 | 0.30 ± 0.03 | 0.31 ± 0.02 | 0.30 ± 0.02 | 0.31 ± 0.03 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.34 ± 0.02 |
| Total MUFA | 64.9 ± 0.1 | 64.5 ± 0.2 B | 64.7 ± 0.2 | 64.7 ± 0.1 A | 64.7 ± 0.1 | 64.6 ± 0.1 A | 64.7 ± 0.2 | 64.6 ± 0.1 A | 64.8 ± 0.1 A |
| C18:2 | 6.80 ± 0.08 | 6.85 ± 0.24 | 6.79 ± 0.07 | 6.76 ± 0.03 | 6.84 ± 0.13 | 6.78 ± 0.03 | 6.81 ± 0.10 | 6.75 ± 0.02 | 7.14 ± 0.06 |
| C18:3 | 1.71 ± 0.04 | 1.71 ± 0.07 | 1.70 ± 0.04 | 1.70 ± 0.01 | 1.73 ± 0.06 | 1.72 ± 0.02 | 1.74 ± 0.05 | 1.70 ± 0.03 | 1.83 ± 0.02 |
| Total PUFA | 8.7 ± 0.1 b | 8.7 ± 0.3 B | 8.7 ± 0.1 b | 8.7 ± 0.1 B | 8.8 ± 0.2 b | 8.7 ± 0.1 B | 8.8 ± 0.2 b | 8.7 ± 0.1 B | 9.2 ± 0.1 aA |
| Total trans | 0.24 ± 0.01 a | 0.28 ± 0.02 A | 0.22 ± 0.02 b | 0.23 ± 0.02 B | 0.20 ± 0.03 b | 0.21 ± 0.02 C | 0.21 ± 0.03 b | 0.21 ± 0.01 BC | 0.18 ± 0.01 cC |
| Chókwé | Manhiça | Chicualacuala | Marracuene | Control | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50–60 °C | 60–70 °C | 50 °C * | |
| Trialylglycerols (%) | 66 ± 1 ab | 69 ± 5 B | 66 ± 1 ab | 75 ± 1A | 67 ± 3 b | 67 ± 3 B | 65 ± 0 b | 66 ± 1 B | 74 ± 0 aA |
| Diacylglycerols (%) | 9 ± 1 b | 9 ± 1 | 9 ± 0 ab | 8 ± 0 | 9 ± 0 ab | 9 ± 0 | 10 ± 1 b | 9 ± 1 | 9 ± 1 b |
| Free fatty acids (%) | 25 ± 1 a | 22 ± 5 A | 25 ± 1 a | 17 ± 1 B | 24 ± 2 a | 24 ± 3 A | 26 ± 1 a | 25 ± 0 A | 17 ± 1 bB |
| Carotenes # (mg/100 g) | 9 ± 2 b | 8 ± 1 C | 10 ± 1 c | 14 ± 1 A | 8 ± 1 c | 8 ± 1 BC | 10 ± 1 c | 10 ± 1 B | 12 ± 1 aA |
| Tocols ## (mg/100 g) | 30 ± 3 bc | 24 ± 2 D | 27 ± 2 b | 34 ± 0 A | 26 ± 1 c | 26 ± 2 D | 26 ± 3 b | 26 ± 1 C | 35 ± 2 aB |
| Sterols ### (mg/100 g) | 435 ± 3 a | - | 423 ± 4 b | - | 427 ± 3 a | - | 427 ± 7 ab | - | 452 ± 4 c |
| Triterpene alcohols #### (mg/100 g) | 826 ± 1 | - | 825 ± 9 | - | 818 ± 6 | - | 815 ± 12 | - | 826 ± 6 |
| Communities | Extraction Temperatures | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Z | p | η2 | Z | p | η2 | Z | p | η2 | |
| Total SFA | 0.023 | 0.995 | 0.002 | 3.102 | 0.085 | 0.064 | 1.843 | 0.153 | 0.109 |
| Total MUFA | 0.490 | 0.691 | 0.032 | 5.986 | 0.018 | 0.117 | 4.804 | 0.005 | 0.243 |
| Total PUFA | 0.954 | 0.423 | 0.060 | 0.035 | 0.852 | 0.001 | 0.530 | 0.664 | 0.034 |
| Total trans | 17.876 | 0.000 | 0.544 | 1.896 | 0.175 | 0.04 | 3.871 | 0.015 | 0.205 |
| Triacylglycerols | 8.395 | 0.000 | 0.359 | 22.679 | 0.000 | 0.335 | 9.766 | 0.000 | 0.394 |
| Diacylglycerols | 3.419 | 0.025 | 0.186 | 8.007 | 0.007 | 0.151 | 2.007 | 0.126 | 0.118 |
| Free fatty acids | 8.116 | 0.000 | 0.351 | 20.134 | 0.000 | 0.309 | 10.606 | 0.000 | 0.414 |
| Carotenes | 16.572 | 0.000 | 0.525 | 1.236 | 0.272 | 0.027 | 24.846 | 0.000 | 0.624 |
| Tocols | 31.541 | 0.000 | 0.678 | 8.221 | 0.006 | 0.154 | 15.266 | 0.000 | 0.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemane, S.S.I.; Casal, S.; Cruz, R.; Pinho, T.; Khan, M.; Pinho, O.; Viegas, O. Chemical Characterization of the Oil Separated by Mechanical Pressing from Strychnos madagascariensis Dried Fruit Pulp Flour. Foods 2022, 11, 474. https://doi.org/10.3390/foods11030474
Chemane SSI, Casal S, Cruz R, Pinho T, Khan M, Pinho O, Viegas O. Chemical Characterization of the Oil Separated by Mechanical Pressing from Strychnos madagascariensis Dried Fruit Pulp Flour. Foods. 2022; 11(3):474. https://doi.org/10.3390/foods11030474
Chicago/Turabian StyleChemane, Sandra S. I., Susana Casal, Rebeca Cruz, Teresa Pinho, Maida Khan, Olívia Pinho, and Olga Viegas. 2022. "Chemical Characterization of the Oil Separated by Mechanical Pressing from Strychnos madagascariensis Dried Fruit Pulp Flour" Foods 11, no. 3: 474. https://doi.org/10.3390/foods11030474
APA StyleChemane, S. S. I., Casal, S., Cruz, R., Pinho, T., Khan, M., Pinho, O., & Viegas, O. (2022). Chemical Characterization of the Oil Separated by Mechanical Pressing from Strychnos madagascariensis Dried Fruit Pulp Flour. Foods, 11(3), 474. https://doi.org/10.3390/foods11030474









