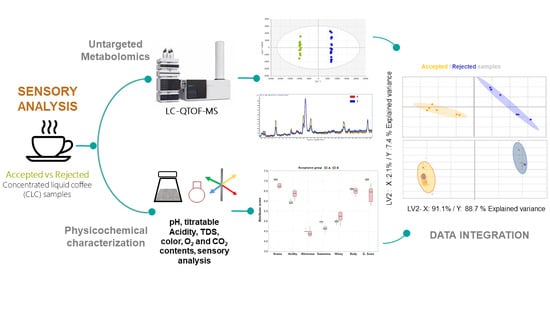
Exploring Chemical Markers Related to the Acceptance and Sensory Profiles of Concentrated Liquid Coffees: An Untargeted Metabolomics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Untargeted Metabolomic Analysis
2.2.1. Metabolomic Analysis by RP-LC-QTOF-MS
2.2.2. Quality Control (QC) Samples
2.2.3. Data Treatment
2.2.4. Statistical Analysis
2.2.5. Metabolite Identification
2.3. Integration of the Physicochemical and Sensory Properties
2.3.1. pH and Titratable Acidity
2.3.2. Total Dissolved Solids (TDS) Content
2.3.3. Color
2.3.4. Oxygen and Carbon Dioxide Contents
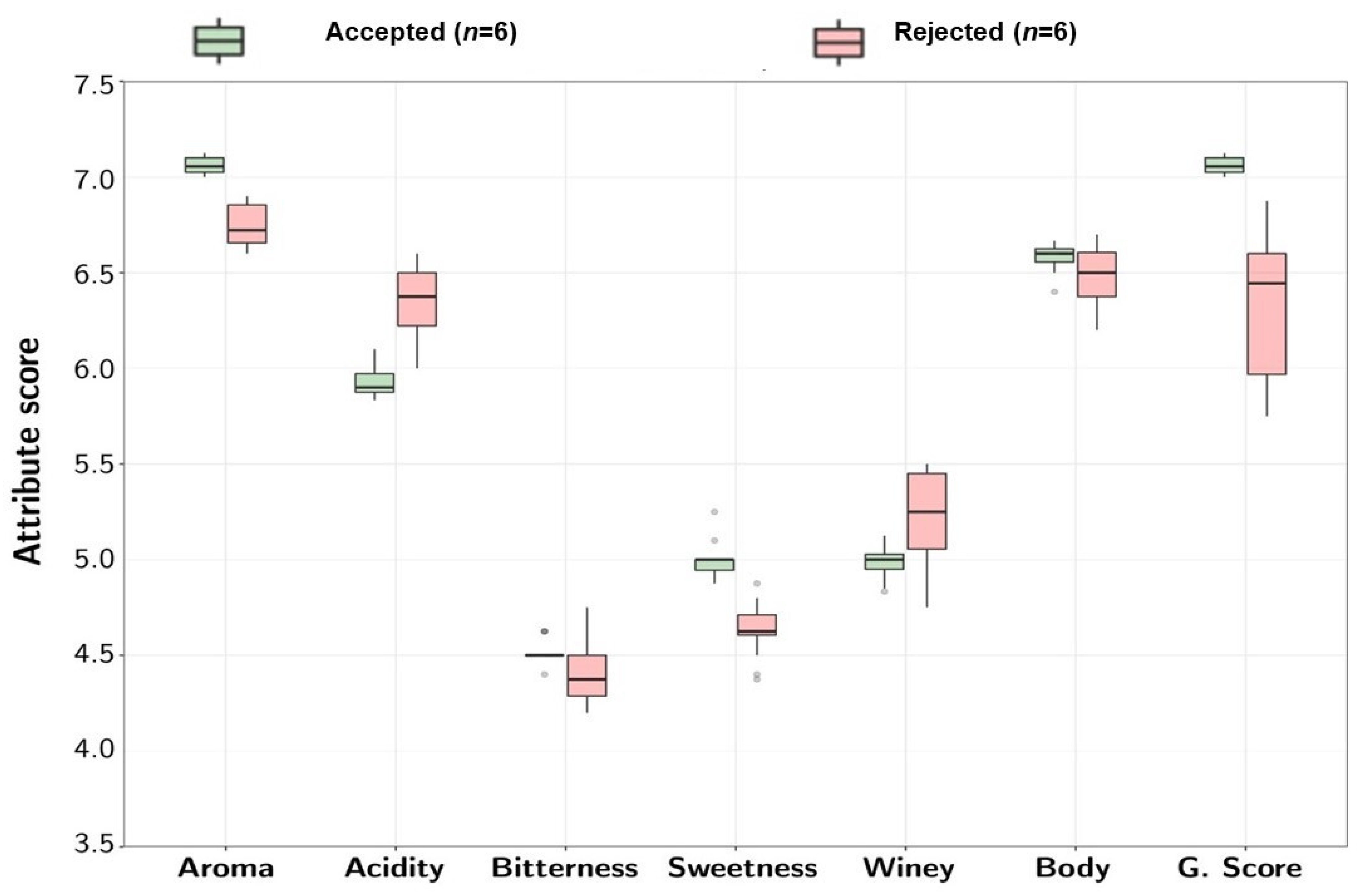
2.3.5. Sensory Analysis
2.3.6. Parameter Identification and Classification Model
3. Results and Discussion
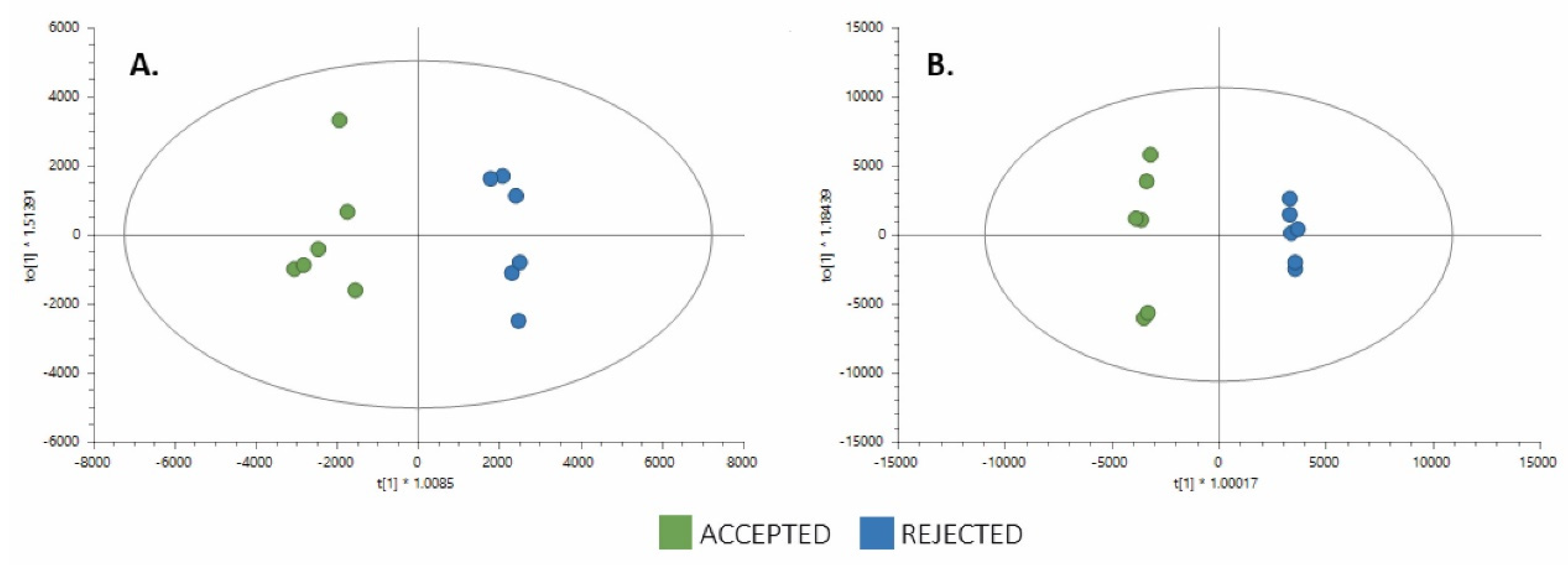
3.1. Untargeted Metabolomics by LC-QTOF-MS
3.2. Integration of Untargeted Metabolomics with Sensory and Physicochemical Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization. Coffee Market Report—June 2021. Available online: https://www.ico.org/Market-Report-20-21-e.asp (accessed on 12 July 2021).
- GlobalData. Global Iced/RTD Coffee Drinks 2019; Global Data: London, UK, 2019. [Google Scholar]
- Torga, G.N.; Spers, E.E. Chapter 2—Perspectives of global coffee demand. In Coffee Consumption and Industry Strategies in Brazil; Florêncio de Almeida, L., Spers, E.E., Eds.; Woodhead Publishing Series in Consumer Science and Strategic Marketing; Woodhead Publishing: Sawston, UK, 2019; pp. 21–49. ISBN 978-0-12-814721-4. [Google Scholar]
- Rocchetti, G.; Braceschi, G.P.; Odello, L.; Bertuzzi, T.; Trevisan, M.; Lucini, L. Identification of markers of sensory quality in ground coffee: An untargeted metabolomics approach. Metabolomics 2020, 16, 127. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Kerler, J.; Baggenstoss, J.; Moser, M.; Rytz, A.; Thomas, E.; Glabasnia, A.; Poisson, L.; Blank, I. Advanced Analytical Sensory Correlation—Towards a Better Molecular Understanding of Coffee Flavor. In Flavour Science; Academic Press: Cambridge, MA, USA, 2014; pp. 39–44. [Google Scholar]
- Villalón-López, N.; Serrano-Contreras, J.I.; Téllez-Medina, D.I.; Gerardo Zepeda, L. An 1H NMR-based metabolomic approach to compare the chemical profiling of retail samples of ground roasted and instant coffees. Food Res. Int. 2018, 106, 263–270. [Google Scholar] [CrossRef]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. CyTA-J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Arana, V.A.; Medina, J.; Alarcon, R.; Moreno, E.; Heintz, L.; Schäfer, H.; Wist, J. Coffee’s country of origin determined by NMR: The Colombian case. Food Chem. 2015, 175, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Polla, D.; Cagliani, L.R. Organic and conventional coffee differentiation by NMR spectroscopy. Food Control 2018, 94, 284–288. [Google Scholar] [CrossRef]
- Hoyos Ossa, D.E.; Gil-Solsona, R.; Peñuela, G.A.; Sancho, J.V.; Hernández, F.J. Assessment of protected designation of origin for Colombian coffees based on HRMS-based metabolomics. Food Chem. 2018, 250, 89–97. [Google Scholar] [CrossRef]
- Gamboa-Becerra, R.; Hernández-Hernández, M.C.; González-Ríos, Ó.; Suárez-Quiroz, M.L.; Gálvez-Ponce, E.; Ordaz-Ortiz, J.J.; Winkler, R. Metabolomic markers for the early selection of coffea canephora plants with desirable cup quality traits. Metabolites 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Privat, I.; Foucrier, S.; Prins, A.; Epalle, T.; Eychenne, M.; Kandalaft, L.; Caillet, V.; Lin, C.; Tanksley, S.; Foyer, C.; et al. Differential regulation of grain sucrose accumulation and metabolism in Coffea arabica (Arabica) and Coffea canephora (Robusta) revealed through gene expression and enzyme activity analysis. New Phytol. 2008, 178, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, J.M.; Aceves, C.M.; Aksenov, A.A.; Aleti, G.; Almaliti, J.; Bouslimani, A.; Brown, E.A.; Campeau, A.; Caraballo-Rodríguez, A.M.; Chaar, R.; et al. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in raw and processed foods and beverages. Food Chem. 2020, 302, 125290. [Google Scholar] [CrossRef]
- Cavanna, D.; Righetti, L.; Elliott, C.; Suman, M. The scientific challenges in moving from targeted to non-targeted mass spectrometric methods for food fraud analysis: A proposed validation workflow to bring about a harmonized approach. Trends Food Sci. Technol. 2018, 80, 223–241. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Hyötyläinen, T.; Orešič, M. An Overview of metabolomics data analysis: Current tools and future perspectives. Compr. Anal. Chem. 2018, 82, 387–413. [Google Scholar] [CrossRef]
- Sébédio, J.L.; Malpuech-Brugère, C. Implementation of Foodomics in the Food Industry. In Innovation Strategies in the Food Industry: Tools for Implementation; Academic Press: Cambridge, MA, USA, 2016; pp. 251–269. ISBN 9780128037935. [Google Scholar]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 16–23. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Loftfield, E.; Wedekind, R.; Freedman, N.; Kambanis, C.; Scalbert, A.; Sinha, R. A metabolomic study of the variability of the chemical composition of commonly consumed coffee brews. Metabolites 2019, 9, 17. [Google Scholar] [CrossRef]
- Quintero, M.; Velásquez, S.; Zapata, J.; López, C.; Cisneros-Zevallos, L. Assessment of concentrated liquid coffee acceptance during storage: Sensory and physicochemical perspective. Molecules 2021, 26, 3545. [Google Scholar] [CrossRef]
- Gigl, M.; Frank, O.; Barz, J.; Gabler, A.; Hegmanns, C.; Hofmann, T. Identification and quantitation of reaction products from quinic acid, quinic acid lactone, and chlorogenic acid with strecker aldehydes in roasted coffee. J. Agric. Food Chem. 2021, 69, 1027–1038. [Google Scholar] [CrossRef]
- Schoenauer, S.; Schieberle, P. Structure–odor correlations in homologous series of mercapto furans and mercapto thiophenes synthesized by changing the structural motifs of the key coffee odorant furan-2-ylmethanethiol. J. Agric. Food Chem. 2018, 66, 4189–4199. [Google Scholar] [CrossRef]
- Charles-Bernard, M.; Kraehenbuehl, K.; Rytz, A.; Roberts, D.D. Interactions between volatile and nonvolatile coffee components. 1. Screening of nonvolatile components. J. Agric. Food Chem. 2005, 53, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Charles-Bernard, M.; Roberts, D.D.; Kraehenbuehl, K. Interactions between volatile and nonvolatile coffee components. 2. Mechanistic study focused on volatile thiols. J. Agric. Food Chem. 2005, 53, 4426–4433. [Google Scholar] [CrossRef]
- Frank, O.; Blumberg, S.; Kunert, C.; Zehentbauer, G.; Hofmann, T. Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J. Agric. Food Chem. 2007, 55, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Lagazio, C.; Manzocco, L.; Barnabà, M.; Cappuccio, R. Risks and pitfalls of sensory data analysis for shelf life prediction: Data simulation applied to the case of coffee. LWT-Food Sci. Technol. 2008, 41, 2070–2078. [Google Scholar] [CrossRef]
- Sopelana, P.; Pérez-Martínez, M.; López-Galilea, I.; de Peña, M.P.; Cid, C. Effect of ultra-high temperature (UHT) treatment on coffee brew stability. Food Res. Int. 2013, 50, 682–690. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Shelf-life testing of coffee and related products: Uncertainties, pitfalls, and perspectives. Food Eng. Rev. 2009, 1, 159–168. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Application of multivariate analysis to the effects of additives on chemical and sensory quality of stored coffee brew. J. Agric. Food Chem. 2008, 56, 11845–11853. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Peterson, D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chem. 2019, 301, 125250. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Tello, E.; Peterson, D.G. Identification of compounds that negatively impact coffee flavor quality using untargeted Liquid Chromatography/Mass Spectrometry Analysis. J. Agric. Food Chem. 2020, 68, 10424–10431. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Effects of refrigeration and oxygen on the coffee brew composition. Eur. Food Res. Technol. 2008, 227, 1633–1640. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Fragmentation pathways during Maillard-induced carbohydrate degradation. J. Agric. Food Chem. 2013, 61, 10198–10208. [Google Scholar] [CrossRef]


| Compound | Formula | Mass | RT (min) | Mass Error (ppm) | Adduct | CVforQC (%) a | DET | CON | Rejected vs. Accepted Samples | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Change (%) b | VIP c | p Value d | |||||||||
| Cytosine | C4H5N3O | 111.0433 | 1.59 | 7 | M+H | 3.3 | ESI+ | Putative | −55 | 1.5155 | 0.0086 |
| Methylfuran-carboxylic acid | C6H6O3 | 126.0317 | 4.45 | 5 | M+H-H2O | 4.3 | ESI+ | Putative | −173 | 2.3476 | 0.0338 * |
| Octene-diynoic acid | C8H6O2 | 134.0368 | 7.01 | 4 | M+H-H2O | 1.8 | ESI+ | Putative | 29 | 1.5942 | 0.0649 |
| Hydroxy-methylbenzaldehyde | C8H8O2 | 136.0524 | 11.33 | 5 | M-H | 2.8 | ESI− | Putative | 61 | 1.6083 | 0.0275 * |
| Methoxybenzaldehyde | C8H8O2 | 136.0524 | 6.29 | 2 | M-H | 2.9 | ESI− | Putative | 17 | 1.7607 | 0.0275 * |
| Vinylcatechol | C8H8O2 | 136.0524 | 9.52 | 4 | M-H | 3.7 | ESI− | Putative | 49 | 1.8414 | 0.0086 |
| Hydroxyphenethylamine | C8H11NO | 137.0841 | 11.52 | 4 | M+H-H2O | 6.3 | ESI+ | MSMS | 82 | 1.0769 | 0.0151 |
| Benzofurancarboxaldehyde | C9H6O2 | 146.0368 | 8.27 | 4 | M+H | 1.4 | ESI+ | Putative | 29 | 1.4441 | 0.0411 |
| Cinnamic acid | C9H8O2 | 148.0524 | 8.95 | 5 | M+H | 1.6 | ESI+ | Putative | 44 | 1.2417 | 0.0151 |
| (Furanyl)-tetrahydropyridine | C9H11NO | 149.0841 | 1.57 | 5 | M+H | 3.4 | ESI+ | Putative | −97 | 1.5258 | 0.0510 * |
| Ethylnicotinate | C8H9NO2 | 151.0633 | 2.26 | 3 | M+H | 13.7 | ESI+ | Putative | −130 | 1.6802 | 0.0086 |
| Furfurylpyrrolidine | C9H13NO | 151.0997 | 6.29 | 4 | M+H-H2O | 5.8 | ESI+ | Putative | 93 | 1.8865 | 0.0338 * |
| Methoxybenzoic acid | C8H8O3 | 152.0473 | 8.42 | 3 | M+H-H2O | 2.5 | ESI+ | Putative | 109 | 1.6151 | 0.0510 * |
| Hydroxycoumarin | C9H6O3 | 162.0317 | 11.84 | 3 | M-H | 8.5 | ESI− | MSMS | −92 | 3.4280 | 0.0275 * |
| Pyridoxal | C8H9NO3 | 167.0582 | 1.51 | 5 | M+H | 3.4 | ESI+ | Putative | 90 | 1.6302 | 0.0338 * |
| Carboline | C11H8N2 | 168.0687 | 10.30 | 5 | M+H | 1.3 | ESI+ | Putative | −29 | 1.1236 | 0.0411 |
| Isovalerylalanine | C8H15NO3 | 173.1052 | 2.60 | 16 | M+Na | 3.1 | ESI+ | Putative | −101 | 1.1844 | 0.0151 |
| Methyl-quinolin-diol | C10H9NO2 | 175.0633 | 8.24 | 4 | M+H | 2.1 | ESI+ | Putative | −78 | 2.3966 | 0.0510 * |
| (Cyclohexylmethyl)pyrazine | C11H16N2 | 176.1313 | 3.53 | 1 | M+K | 1.8 | ESI+ | Putative | 52 | 1.0421 | 0.0649 |
| [2H-Pyrrol-(3H)-ylidenemethyl]-furanmethanol | C10H11NO2 | 177.0790 | 6.94 | 3 | M+H-H2O | 1.4 | ESI+ | Putative | −65 | 1.2231 | 0.0151 |
| Dimethyl-(1-pyrrolidinyl)-cyclopenten-one | C11H17NO | 179.1310 | 8.88 | 5 | M+H | 3.6 | ESI+ | Putative | −130 | 1.0109 | 0.0510 * |
| Caffeic acid | C9H8O4 | 180.0423 | 8.90 | 3 | M+H-H2O | 8.0 | ESI+/− | Identified | 37 | 1.0605 | 0.1320 |
| Hydroxy-(hydroxyphenyl)propenoicacid | C9H8O4 | 180.0423 | 14.69 | 3 | M+H-H2O | 1.9 | ESI+ | Putative | −27 | 1.5147 | 0.1320 |
| Indole-propionic acid | C11H11NO2 | 189.0790 | 11.14 | 4 | M+H | 4.9 | ESI+ | Putative | −102 | 1.3162 | 0.0338 * |
| Quinic acid | C7H12O6 | 192.0634 | 6.09 | 1 | M-H | 1.7 | ESI− | MSMS | 16 | 1.5511 | 0.0086 |
| Ferulic acid | C10H10O4 | 194.0579 | 10.57 | 3 | M+H-H2O | 6.0 | ESI+ | Putative | 161 | 1.5558 | 0.0338 * |
| (Furan+B63:B75yl)-hexahydro-7H-cyclopenta[b]pyridinone | C12H13NO2 | 203.0946 | 5.51 | 3 | M+H | 5.9 | ESI+ | Putative | −173 | 1.6442 | 0.0338 * |
| Methylsalicyluric acid | C10H11NO4 | 209.0688 | 1.33 | 3 | M+H | 5.2 | ESI+ | Putative | 1918 | 2.5034 | 0.0338 * |
| 1-Isothiocyanato-8-(methylthio)octane | C10H19NS2 | 217.0959 | 3.64 | 10 | M+Na | 5.5 | ESI+ | Putative | 1011 | 1.6326 | 0.0338 * |
| 1-Arabinofuranosylcytosine | C9H13N3O5 | 243.0895 | 4.40 | 20 | M+H | 9.1 | ESI+ | Putative | 199 | 1.0998 | 0.0086 |
| 243,0895@8,74 | - | 243.0895 | 8.74 | - | M+H | 4.8 | ESI+ | Putative | −95 | 1.0254 | 0.0510 * |
| Hydroxy-(hydroxy-methyl-hexenyl)benzofuran | C15H18O3 | 246.1256 | 5.01 | 2 | M+Na | 2.8 | ESI+ | Putative | −328 | 2.5216 | 0.0338 * |
| N-Phenylacetylasparticacid | C12H13NO5 | 251.0794 | 3.12 | 2 | M+H-H2O | 5.2 | ESI+ | MSMS | −254 | 2.2707 | 0.0338 * |
| N-Pyruvoyl-methoxy-hydroxyanthranilate | C11H11NO6 | 253.0586 | 1.36 | 3 | M+H | 3.2 | ESI+ | Putative | 1860 | 1.9896 | 0.0338 * |
| N,N’-Diphenyl-phenylenediamine | C18H16N2 | 260.1313 | 1.67 | 10 | M+Cl | 3.6 | ESI− | Putative | 25 | 1.0798 | 0.0086 |
| 268,1064@5,01 * | - | 268.1064 | 5.01 | - | M+H | 5.6 | ESI+ | Putative | −328 | 2.5216 | 0.0338 * |
| Evoxanthidine | C15H11NO4 | 269.0688 | 4.40 | 7 | M+H | 3.5 | ESI+ | Putative | 207 | 1.2801 | 0.0338 * |
| Trichostachine | C16H17NO3 | 271.1208 | 7.32 | 3 | M+H | 10.9 | ESI+ | Putative | 218 | 1.4652 | 0.0338 * |
| Deaminofusarochromanone | C15H19NO4 | 277.1314 | 5.13 | 3 | M+H | 5.7 | ESI+ | MSMS | 68 | 1.2220 | 0.0151 |
| Eriodictyol | C15H12O6 | 288.0634 | 11.34 | 1 | M-H | 3.9 | ESI− | MSMS | 34 | 1.0096 | 0.0275 * |
| 317,0903@3,49 | - | 295.1088 | 3.49 | - | - | 1.6 | ESI+ | MSMS | 94 | 1.1685 | 0.0510 * |
| ent-Hydroxybuphanisine | C17H19NO4 | 301.1314 | 6.93 | 3 | M+H | 4.4 | ESI+ | Putative | 205 | 1.9999 | 0.0338 * |
| 291,1111@7,49 | - | 309.1212 | 7.49 | - | M+H | 4.2 | ESI+ | Putative | −189 | 1.1515 | 0.0411 |
| O-p-Coumaroyl-D-glucose | C15H18O8 | 326.1002 | 5.74 | 1 | M-H-H2O | 2.6 | ESI− | Putative | 192 | 1.0547 | 0.0275 * |
| Guaiacin | C20H24O4 | 328.1675 | 17.17 | 2 | M+H | 6.5 | ESI+ | Putative | −197 | 2.3579 | 0.0338 * |
| 331.215@13.47 | C20H29NO3 | 331.2147 | 13.47 | 3 | M+H | 3.2 | ESI+ | Putative | −102 | 1.1645 | 0.0338 * |
| 5-O-Caffeoylshikimicacid//4-Caffeoyl-1,5-quinolactone * | C16H16O8 | 336.0845 | 12.84 | 1 | M-H | 6 | ESI− | Putative | −100 | 2.4478 | 0.0275 * |
| Caffeoyl-quinolactone | C16H16O8 | 336.0845 | 11.32 | 3 | M+H | 5.6 | ESI+/− | Putative | −422 | 4.4402 | 0.0338 * |
| O-Caffeoylshikimicacid | C16H16O8 | 336.0845 | 11.84 | 0 | M-H | 2.1 | ESI− | Putative | −87 | 5.6218 | 0.0275 * |
| Feruloyl-quinolactone * | C17H18O8 | 350.1002 | 13.86 | 1 | M-H | 5.8 | ESI− | Putative | −82 | 3.3243 | 0.0275 * |
| Chlorogenic acid | C16H18O9 | 354.0951 | 8.42 | 1 | M-H | 2.7 | ESI− | Identified | 21 | 2.4680 | 0.0086 |
| Cryptochlorogenic acid | C16H18O9 | 354.0951 | 9.75 | 2 | M-H-H2O | 2.1 | ESI− | Identified | −77 | 2.9124 | 0.0275 * |
| Neochlorogenic acid | C16H18O9 | 354.0951 | 6.29 | 3 | M+H | 5.0 | ESI+/− | Identified | 106 | 1.1735 | 0.0086 |
| O-beta-D-Glucopyranuronosyl-D-mannose | C12H20O12 | 356.0955 | 1.38 | 1 | M-H-H2O | 2.8 | ESI− | Putative | −23 | 1.1169 | 0.0150 |
| N-Caffeoyltryptophan | C20H18N2O5 | 366.4000 | 1.37 | 1 | M-H | 4.2 | ESI− | Putative | −29 | 1.5959 | 0.0275 * |
| Piperundecalidine | C23H29NO3 | 367.2147 | 18.79 | 7 | M+H | 6.9 | ESI+ | Putative | −276 | 1.3874 | 0.0338 * |
| O-Feruloylquinicacid | C17H20O9 | 368.1107 | 8.64 | 0 | M-H | 3.7 | ESI− | Putative | 30 | 1.2937 | 0.0275 * |
| Pentamethoxyflavanone | C20H22O7 | 374.1366 | 18.72 | 1 | M+FA-H | 3.7 | ESI− | Putative | −48 | 1.0967 | 0.0275 * |
| 412.1014@8.70 * | C31H12N2 | 412.1000 | 8.70 | 1 | M-H | 3.6 | ESI− | Putative | −73 | 1.1022 | 0.0275 * |
| 439.1852@7.47//ValHisTyr | C20H27N5O5 | 417.2012 | 7.47 | 6 | M+Na | 14.3 | ESI+ | Putative | −543 | 1.8897 | 0.0338 * |
| ValHisTyr | C20H27N5O5 | 417.2012 | 5.94 | 6 | M+Na | 9.2 | ESI+ | Putative | −499 | 2.0702 | 0.0338 * |
| 447,1498@4,01 | - | 447.1498 | 4.01 | - | M+H | 5.0 | ESI+ | Putative | −30 | 1.0433 | 0.4848 |
| (+)-CatechinC-glucoside | C21H24O11 | 452.1319 | 16.70 | 2 | M-H-H2O | 3.9 | ESI− | Putative | −39 | 1.0612 | 0.0275 * |
| 481,138@11,29 | - | 459.1529 | 11.29 | - | M+H | 7.2 | ESI+ | Putative | 14895 | 1.2221 | 0.0338 * |
| N-[(Dihydropterinyl)methyl]-(beta-D-ribofuranosyl)anilinephosphate | C18H23N6O8P | 482.1315 | 3.91 | 8 | M-H-H2O | 2.0 | ESI− | Putative | −31 | 1.2776 | 0.0275 * |
| 482,2526@15,50 | - | 482.2526 | 15.50 | - | M-H | 2.7 | ESI− | Putative | −26 | 1.4000 | 0.0275 * |
| 498,3072@18,79 | - | 498.3072 | 18.79 | - | - | 2.1 | ESI+ | Putative | −245 | 2.7344 | 0.0338 * |
| Aconine | C25H41NO9 | 499.2781 | 12.24 | 3 | M+H | 2.5 | ESI+ | Putative | 21 | 1.3575 | 0.2402 |
| Galactomannan | C18H32O16 | 504.1690 | 1.59 | 1 | M+H-H2O | 2.9 | ESI+ | MSMS | −108 | 1.0085 | 0.1796 |
| Cafamarine | C26H36O10 | 508.2308 | 17.19 | 0 | M+FA-H | 5.3 | ESI− | Putative | −38 | 1.6678 | 0.0275 * |
| Dicaffeoylquinicacid | C25H24O12 | 516.1268 | 7.92 | 2 | M+H | 6.3 | ESI+ | MSMS | 65 | 1.2450 | 0.0338 * |
| Di-O-caffeoylquinicacid//Dicaffeoylquinicacid | C25H24O12 | 516.1268 | 8.91 | 3 | M+H | 4.6 | ESI+ | Putative | 46 | 1.0344 | 0.1320 |
| 528,1487@8,70 | - | 528.1487 | 8.70 | - | M-H | 4.2 | ESI− | Putative | −35 | 1.2142 | 0.0275 * |
| PC(22:1) | C30H58NO8P | 591.3900 | 18.76 | 4 | M+Na | 10.9 | ESI+ | Putative | −73 | 1.3013 | 0.0510 * |
| Kaempferiderhamnoside-(succinylglucoside) | C32H36O18 | 708.1902 | 7.97 | 2 | M+H-H2O | 5.9 | ESI+ | Putative | 84 | 1.3239 | 0.0086 |
| 726,4535@18,99 | - | 726.4535 | 18.99 | - | M-H | 3.4 | ESI− | Putative | −26 | 2.2514 | 0.0275 * |
| PS(39:7) | C45H74NO10P | 819.5050 | 18.79 | 7 | M+Na | 5.3 | ESI+ | Putative | −96 | 1.2246 | 0.0151 |
| PC(DiMe(9,3)/MonoMe(11,3)) | C45H79NO10P | 824.5442 | 17.98 | 6 | M+H | 2.6 | ESI+ | Putative | −115 | 1.0803 | 0.0510 * |
| PI(33:0) | C42H81O13P | 825.5461 | 18.06 | 3 | M+H | 4 | ESI+ | Putative | −132 | 1.1234 | 0.0338 * |
| Acceptance Group | ||
|---|---|---|
| Parameter | Accepted Samples | Rejected Samples |
| Physicochemical | ||
| Titratable acidity | 59.93 ± 4.69 a | 72.19 ± 3.25 b |
| Concentration [°Bx] | 35.96 ± 0.56 a | 35.65 ± 0.26 a |
| pH | 4.79 ± 0.16 a | 4.76 ± 0.10 a |
| Chroma * | 27.92 ± 1.99 b | 25.09 ± 1.94 a |
| a* | 20.47 ± 1.30 b | 16.80 ± 1.39 a |
| b* | 18.96 ± 1.86 a | 18.63 ± 1.42 a |
| L* | 12.59 ± 1.14 a | 13.05 ± 0.78 a |
| Hue | 0.75 ± 0.04 a | 0.84 ± 0.02 b |
| Oxygen and carbon dioxide | ||
| O2—HS | 7.63 ± 7.79 b | 0.30 ± 0.02 a |
| CO2—HS | 29.37 ± 16.89 a | 40.48 ± 5.35 b |
| O2—Dissolved | 0.24 ± 0.09 a | 0.27 ± 0.06 a |
| O2—Saturation | 3.08 ± 0.97 a | 3.76 ± 0.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero, M.; Santander, M.J.; Velásquez, S.; Zapata, J.; Cala, M.P. Exploring Chemical Markers Related to the Acceptance and Sensory Profiles of Concentrated Liquid Coffees: An Untargeted Metabolomics Approach. Foods 2022, 11, 473. https://doi.org/10.3390/foods11030473
Quintero M, Santander MJ, Velásquez S, Zapata J, Cala MP. Exploring Chemical Markers Related to the Acceptance and Sensory Profiles of Concentrated Liquid Coffees: An Untargeted Metabolomics Approach. Foods. 2022; 11(3):473. https://doi.org/10.3390/foods11030473
Chicago/Turabian StyleQuintero, Mónica, Maria José Santander, Sebastián Velásquez, Julián Zapata, and Mónica P. Cala. 2022. "Exploring Chemical Markers Related to the Acceptance and Sensory Profiles of Concentrated Liquid Coffees: An Untargeted Metabolomics Approach" Foods 11, no. 3: 473. https://doi.org/10.3390/foods11030473
APA StyleQuintero, M., Santander, M. J., Velásquez, S., Zapata, J., & Cala, M. P. (2022). Exploring Chemical Markers Related to the Acceptance and Sensory Profiles of Concentrated Liquid Coffees: An Untargeted Metabolomics Approach. Foods, 11(3), 473. https://doi.org/10.3390/foods11030473