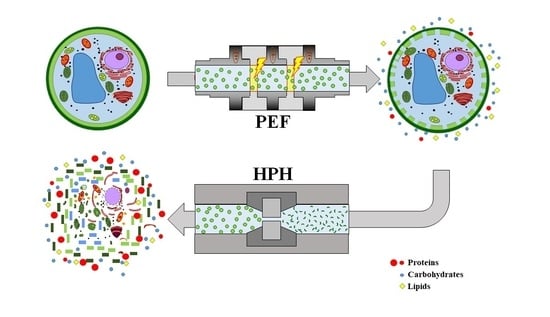
Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Cultivation
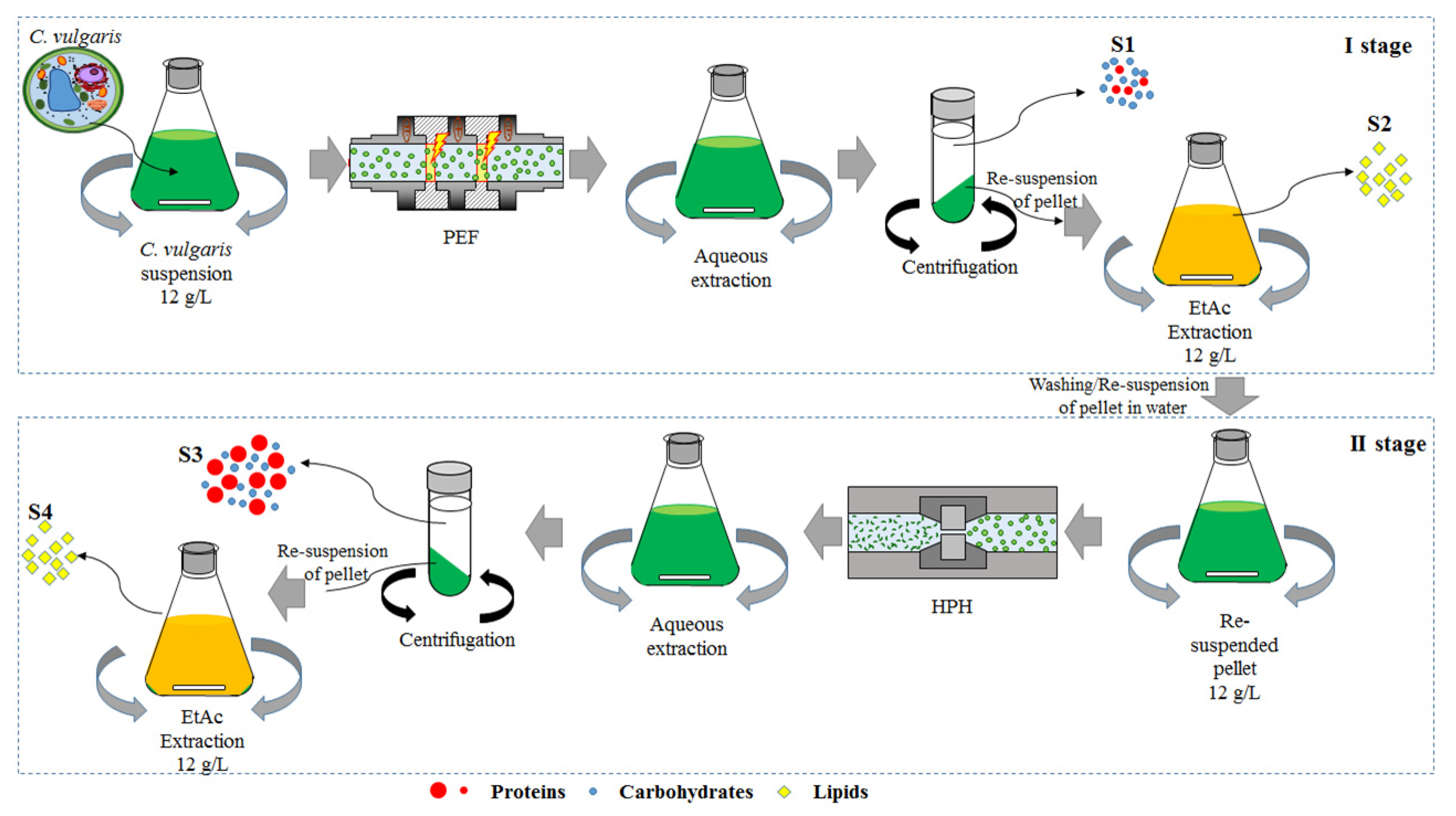
2.2. Cascade of Pulsed Electric Fields and High-Pressure Homogenization Treatments
2.3. Analytical Methods
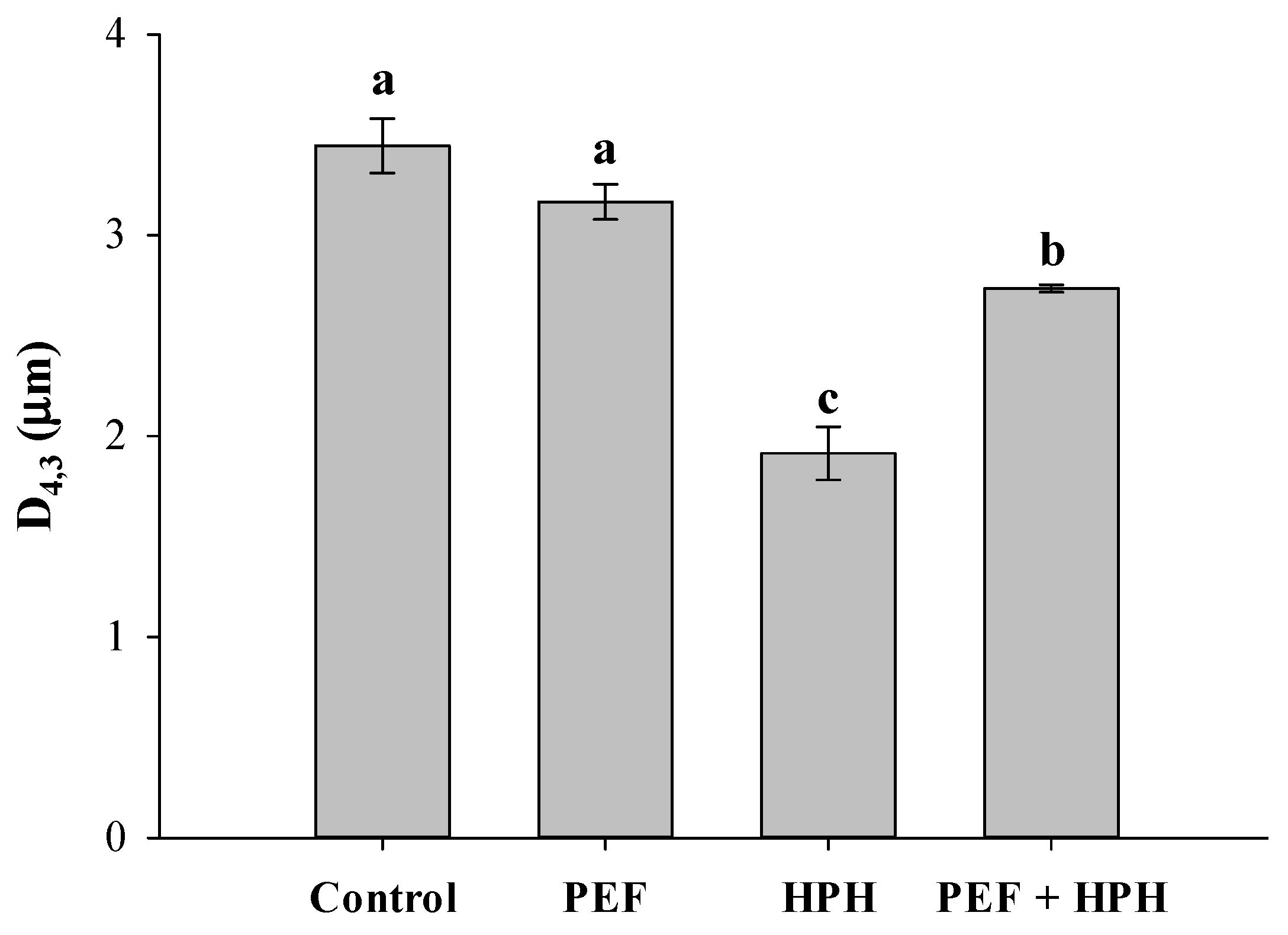
2.3.1. Particle Size Distribution (PSD)
2.3.2. Scanning Electron Microscopy (SEM)
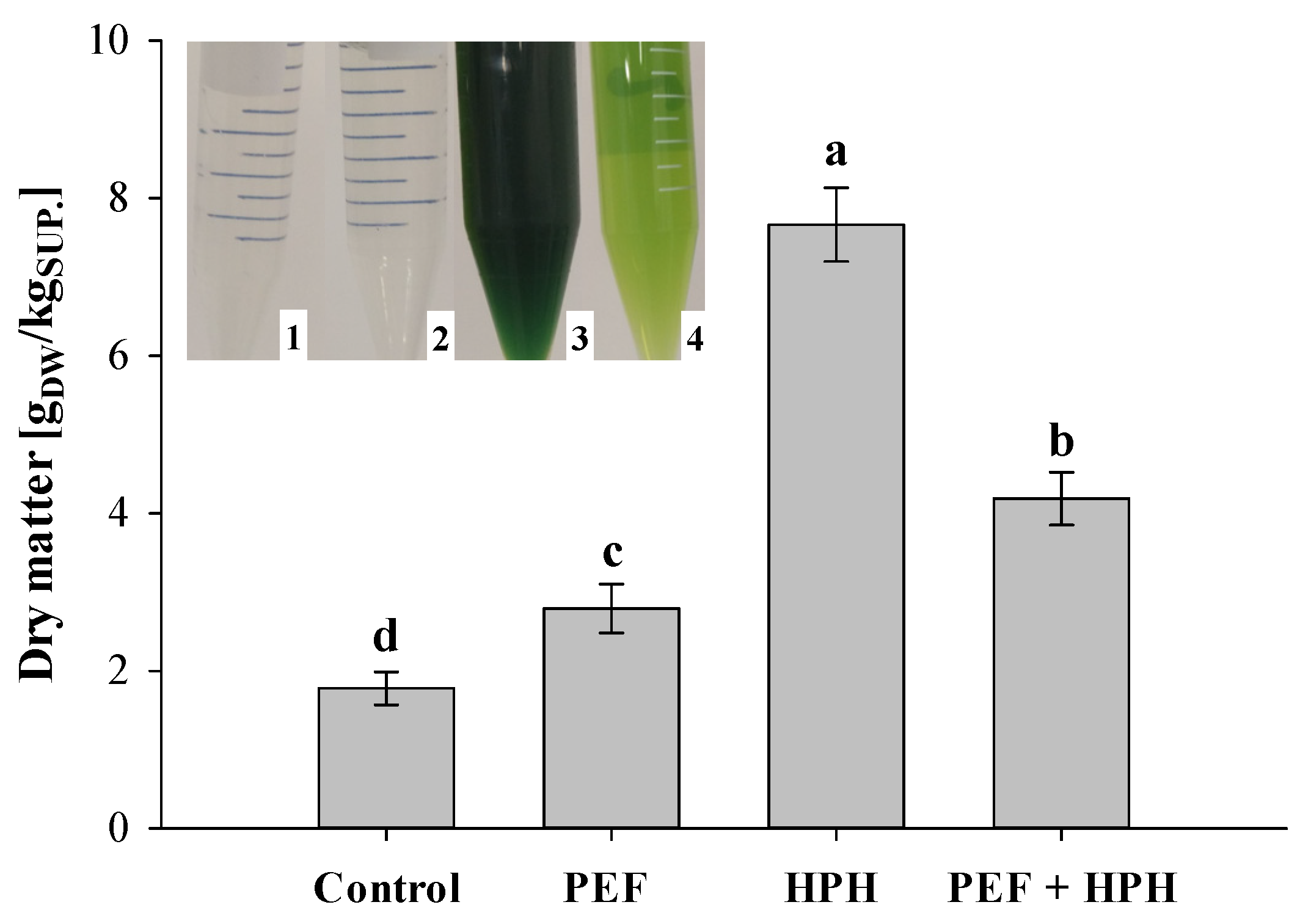
2.3.3. Dry Matter (DM) Content
2.3.4. Water-Soluble Proteins (WSP) and Carbohydrates (CH) Content of Aqueous Supernatants
2.3.5. Lipids (LIP) Content of Organic Supernatants
2.3.6. Ultraviolet-Visible (UV-Vis) Spectra Measurements
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Individual PEF, Individual HPH, or Cascade Treatments on Microalgal Morphological Aspects
3.2. Effect of Individual PEF, Individual HPH, or Cascade Treatment on the Recovery of Intracellular Compounds
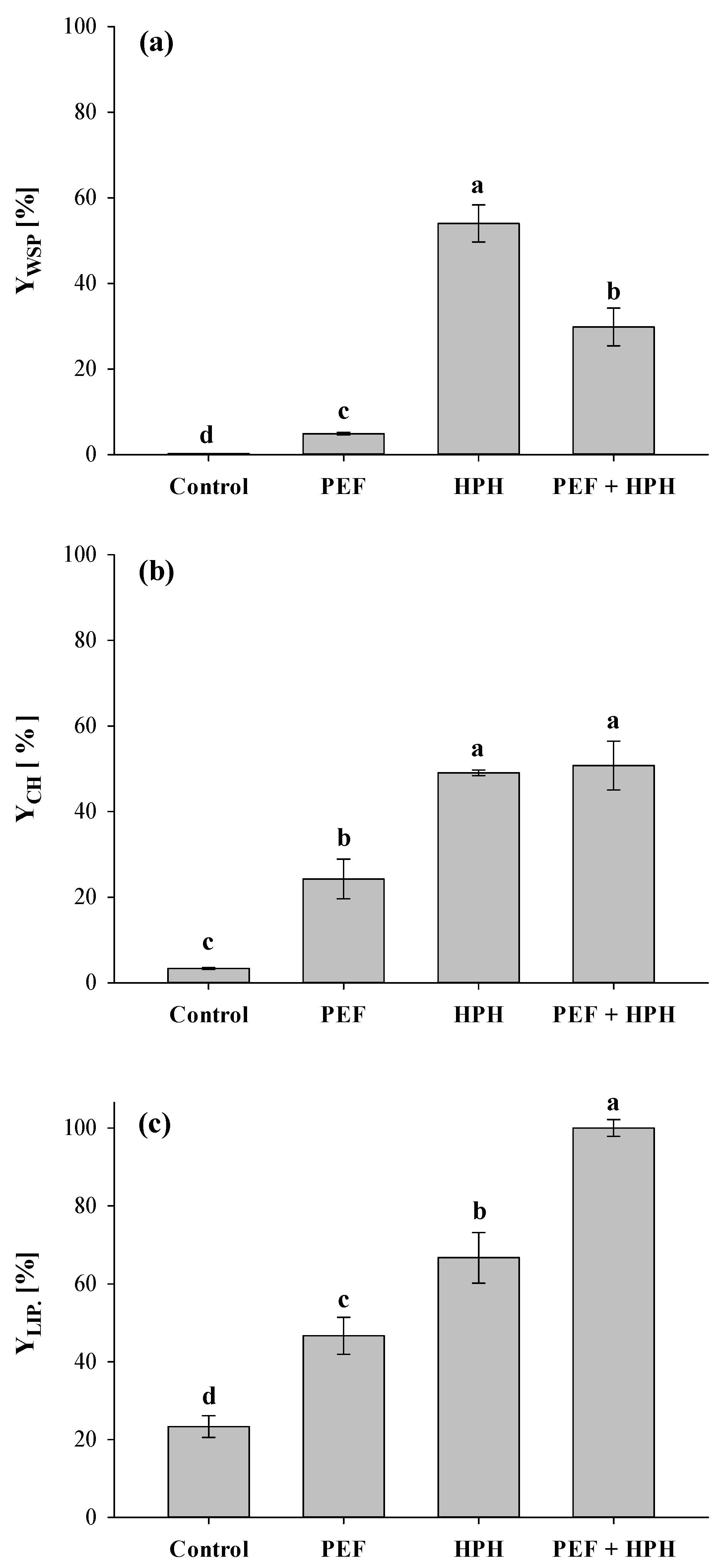
3.2.1. Extraction Yields
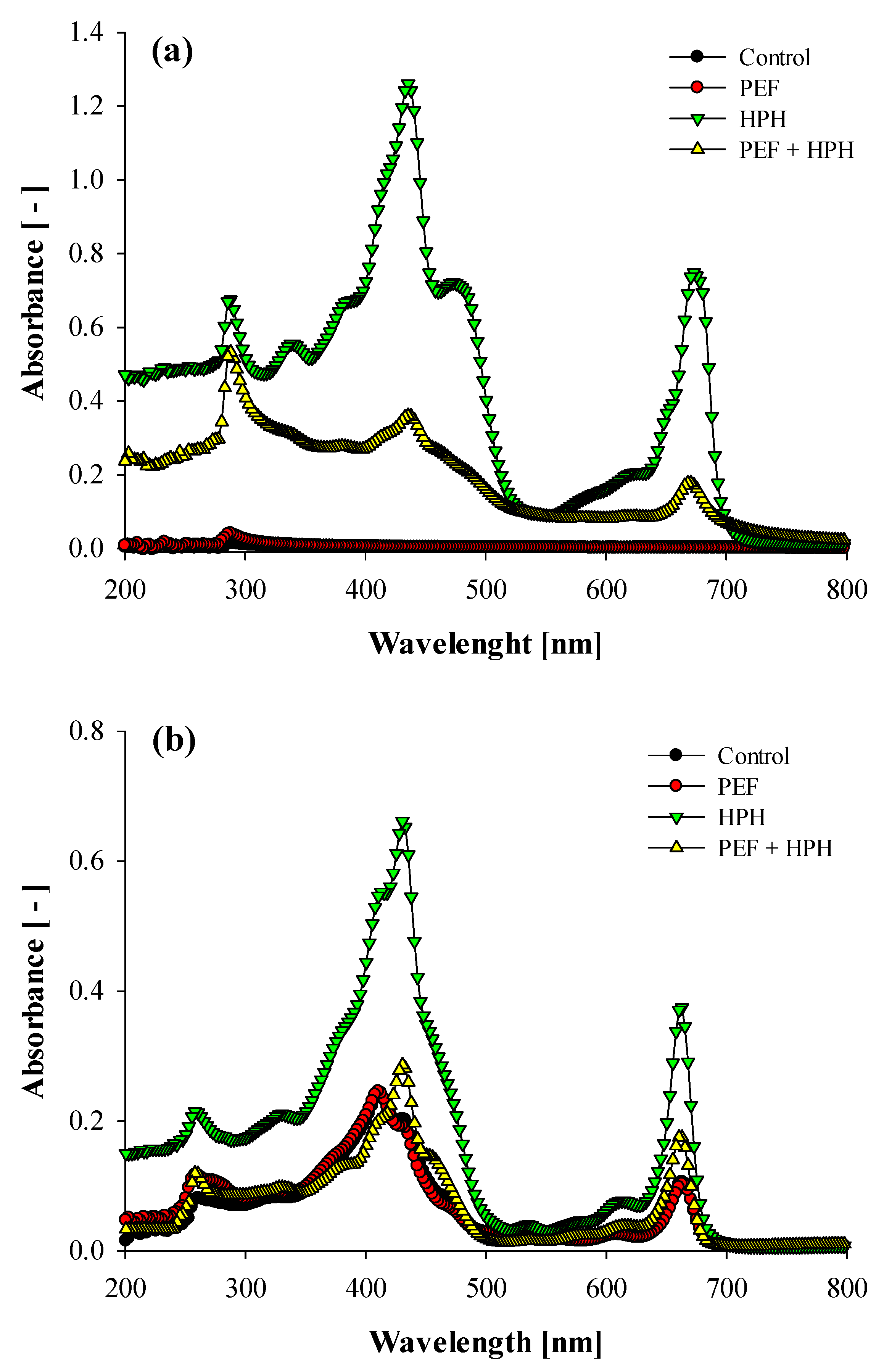
3.2.2. UV-Vis Absorption Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of Pulsed Electric Fields and High-Pressure Homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G.; Pataro, G. Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal Res. 2021, 57, 102341. [Google Scholar] [CrossRef]
- Zhang, R.; Lebovka, N.; Marchal, L.; Vorobiev, E.; Grimi, N. Comparison of aqueous extraction assisted by pulsed electric energy and ultrasonication: Efficiencies for different microalgal species. Algal Res. 2020, 47, 101857. [Google Scholar] [CrossRef]
- Rahman, K.M. Food and High Value Products from Microalgae: Market Opportunities and Challenges. In Microalgae Biotechnology for Food, Health and High Value Products, 1st ed.; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer Nature: Singapore, 2020; pp. 3–27. [Google Scholar]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Xu, G.; Fu, X. Improving the lipid extraction yield from Chlorella based on the controllable electroporation of cell membrane by pulsed electric field. Bioresour. Technol. 2021, 330, 124933. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.-J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Postma, P.R.; ‘t Lam, G.P.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G. Microalgal Biorefinery for Bulk and High-Value Products: Product Extraction Within Cell Disintegration. In Handbook of Electroporation, 1st ed.; Miklavcic, D., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 2205–2224. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 15, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.A.; Pellone, P.; Lubritto, C.; Ciniglia, C. Evaluation of Microalgae Antiviral Activity and Their Bioactive Compounds. Antibiotics 2021, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.M.; Delso, C.; Alvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Rashmi; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sust. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Papachristou, I.; Akaberi, S.; Silve, A.; Navarro-Lopez, R.; Wustner, R.; Leber, K.; Nazarova, N.; Muller, G.; Frey, W. Analysis of the lipid extraction performance in a cascade process for Scenedesmus almeriensis biorefinery. Biotechnol. Biofuels 2021, 14, 20. [Google Scholar] [CrossRef]
- Safi, C.; Cabas Rodriguez, L.; Mulder, W.J.; Engelen-Smit, N.; Spekking, W.; van den Broek, L.A.M.; Olivieri, G.; Sijtsma, L. Energy consumption and water-soluble protein release by cell wall disruption of Nannochloropsis gaditana. Bioresour. Technol. 2017, 239, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Bioref. 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsi, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Postma, P.R.; Pataro, G.; Capitoli, M.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G.; Ferrari, G. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatments. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds from Arthrospira Platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. [Google Scholar] [CrossRef]
- Martinez, J.M.; Luengo, E.; Saldana, G.; Alvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 10421047. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Ferrari, G. PEF-assisted supercritical CO2 extraction of pigments from microalgae Nannochloropsis oceanica in a continuous flow system. Chem. Eng. Trans. 2019, 74, 97–102. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.-P.; Chang, J.-S. Mild cell disruption methods for bio-functional proteins recovery from microalgae—Recent developments and future perspectives. Algal Res. 2017, 31, 506–516. [Google Scholar] [CrossRef]
- Silve, A.; Kian, C.B.; Papachristou, I.; Kubisch, C.; Nazarova, N.; Wustner, R.; Leber, K.; Strassner, R.; Frey, W. Incubation time after pulsed electric field treatment of microalgae enhances the efficiency of extraction processes and enables the reduction of specific treatment energy. Bioresous. Technol. 2018, 269, 179–187. [Google Scholar] [CrossRef] [Green Version]
- ‘T Lam, G.P.; Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermue, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Olivieri, G. Pulsed electric field for protein release of the microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2014, 184, 297–304. [Google Scholar] [CrossRef]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Yonathan, K.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2016, 224, 670–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shene, C.; Monsalve, M.T.; Vergara, D.; Lienqueo, M.E.; Rubilar, M. High pressure homogenization of Nannochloropsis oculata for the extraction of intracellular components: Effect of process conditions and culture age. Eur. J. Lipid Sci. Technol. 2016, 118, 631–639. [Google Scholar] [CrossRef]
- Spiden, M.E.; Yap, B.H.J.; Hill, D.R.A.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Zhang, R.; Marchal, L.; Vorobiev, E.; Grimi, N. Effect of combined pulsed electric energy and high pressure homogenization on selective and energy efficient extraction of bio-molecules from microalga Parachlorella kessleri. LWT Food Sci. Technol. 2021, 141, 110901. [Google Scholar] [CrossRef]
- ‘T Lam, G.P.; van der Kolk, J.A.; Chordia, A.; Vermue, M.H.; Olivieri, G.; Eppink, M.H.M.; Wijffels, R.H. Mild and Selective Protein Release of Cell Wall Deficient Microalgae with Pulsed Electric Field. ACS Sustain. Chem. Eng. 2017, 5, 6046–6053. [Google Scholar] [CrossRef] [Green Version]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Zbinden, M.D.A.; Sturm, B.S.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef]
- Donsì, F.; Senatore, B.; Huang, Q.; Ferrari, G. Development of novel pea protein-based nanoemulsions for delivery of nutraceuticals. J. Agric. Food Chem. 2010, 58, 10653–10660. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1957, 28, 350–356. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbuhler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, W.; Min, M.; Du, Z.; Chen, P.; Ma, X.; Liu, Y.; Lei, H.; Shi, J.; Ruan, R. Development of an effective acidogenically digested swine manure-based algal system for improved wastewater treatment and biofuel and feed production. Appl. Energy 2013, 107, 255–263. [Google Scholar] [CrossRef]
- Kaferbock, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Han, S.-F.; Jin, W.; Yang, Q.; Abomohra, A.E.-F.; Zhou, X.; Tu, R.; Chen, C.; Xie, G.-Y.; Wang, Q. Application of pulsed electric field pretreatment for enhancing lipid extraction from Chlorella pyrenoidosa grown in wastewater. Renew. Energy 2019, 133, 233–239. [Google Scholar] [CrossRef]
- Canelli, G.; Martínez, P.M.; Hauser, B.M.; Kuster, I.; Rohfritsch, Z.; Dionisi, F.; Bolten, C.J.; Neutsch, L.; Mathys, A. Tailored enzymatic treatment of Chlorella vulgaris cell wall leads to effective disruption while preserving oxidative stability. LWT Food Sci. Technol. 2021, 143, 111157. [Google Scholar] [CrossRef]
- Alvarez, I.; Heinz, V. Hurdle technology and the preservation of food by pulsed electric fields. In Food Preservation by Pulsed Electric Fields—From Research to Application, 1st ed.; Lelieveld, H.L.M., Notermans, S., de Haan, S.W.H., Eds.; Woodhead Publishing: Abington, UK, 2007; pp. 165–177. [Google Scholar]
- Jaeschke, D.P.; Domeneghini Mercali, G.; Ferreira Marczak, L.D.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Berliner, M.D. Proteins in Chlorella vulgaris. Microbios 1986, 46, 199–203. [Google Scholar]
- Coustets, M.; Al-Karablieh, N.; Thomsen, C.; Teissie, J. Flow Process for Electroextraction of Total Proteins from Microalgae. J. Membr. Bio. 2013, 246, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Parameswaran, P.; Li, A.; Baez, M.; Rittmann, B.E. Effects of pulsed electric field treatment on enhancing lipid recovery from the microalga. Scenedesmus. Bioresour. Technol. 2014, 173, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, D.; Abera, B.D.; Scognamiglio, M.; Donsì, F.; Ferrari, G.; Pataro, G. Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods 2022, 11, 471. https://doi.org/10.3390/foods11030471
Carullo D, Abera BD, Scognamiglio M, Donsì F, Ferrari G, Pataro G. Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods. 2022; 11(3):471. https://doi.org/10.3390/foods11030471
Chicago/Turabian StyleCarullo, Daniele, Biresaw Demelash Abera, Mariarosa Scognamiglio, Francesco Donsì, Giovanna Ferrari, and Gianpiero Pataro. 2022. "Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae" Foods 11, no. 3: 471. https://doi.org/10.3390/foods11030471
APA StyleCarullo, D., Abera, B. D., Scognamiglio, M., Donsì, F., Ferrari, G., & Pataro, G. (2022). Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods, 11(3), 471. https://doi.org/10.3390/foods11030471