Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Pomace
2.2. Chemicals
2.3. High-Pressure Homogenization Treatment of Tomato Pomace
2.4. Chemical Characterization of Raw Materials
2.5. Optimization of Bioactives Extraction
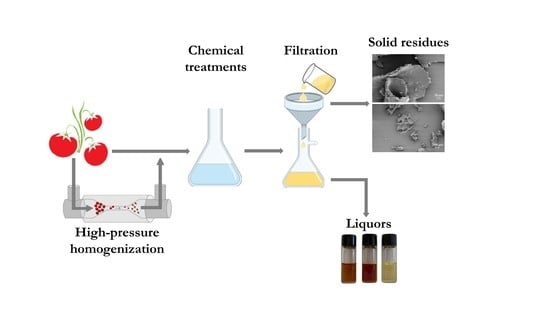
2.6. Isolation of Cellulose
2.7. Liquor Analysis
2.7.1. Total Phenolic Content
2.7.2. Reducing Activity
2.8. Cellulose Pulp Characterization
2.8.1. Structural Carbohydrate and Lignin Analysis
2.8.2. Fourier Transform Infrared Spectroscopy (FT-IR)
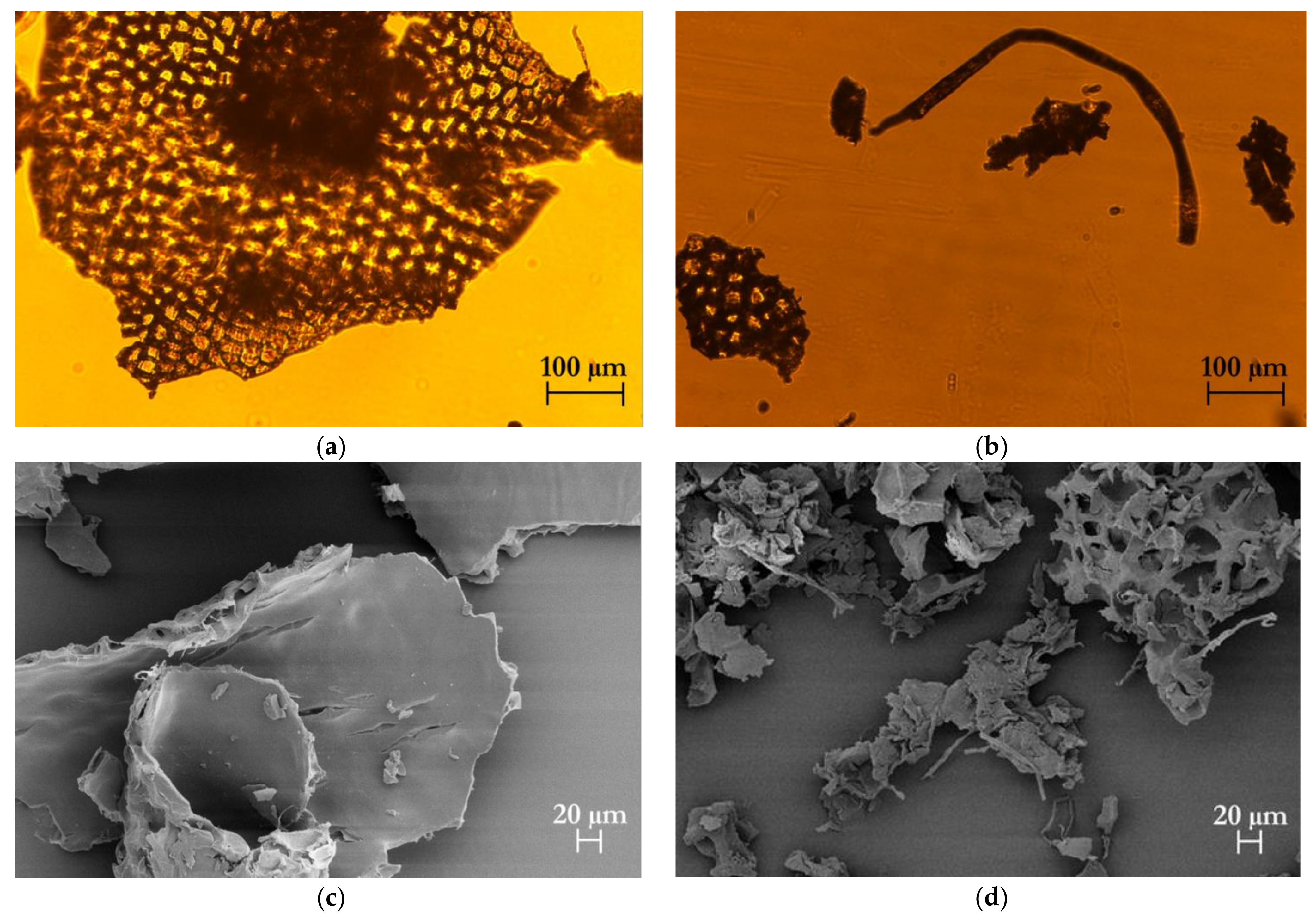
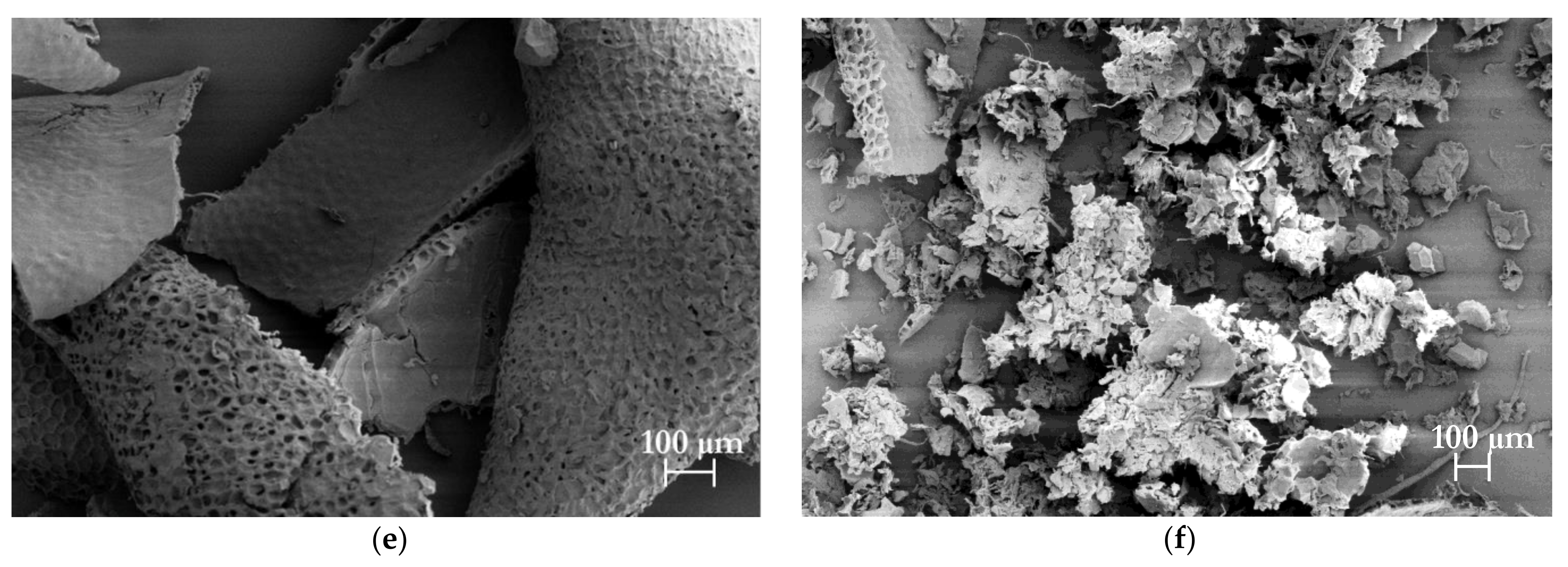
2.8.3. Morphological Analysis
2.8.4. Particle Size Distribution
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of TP and HPH-TP
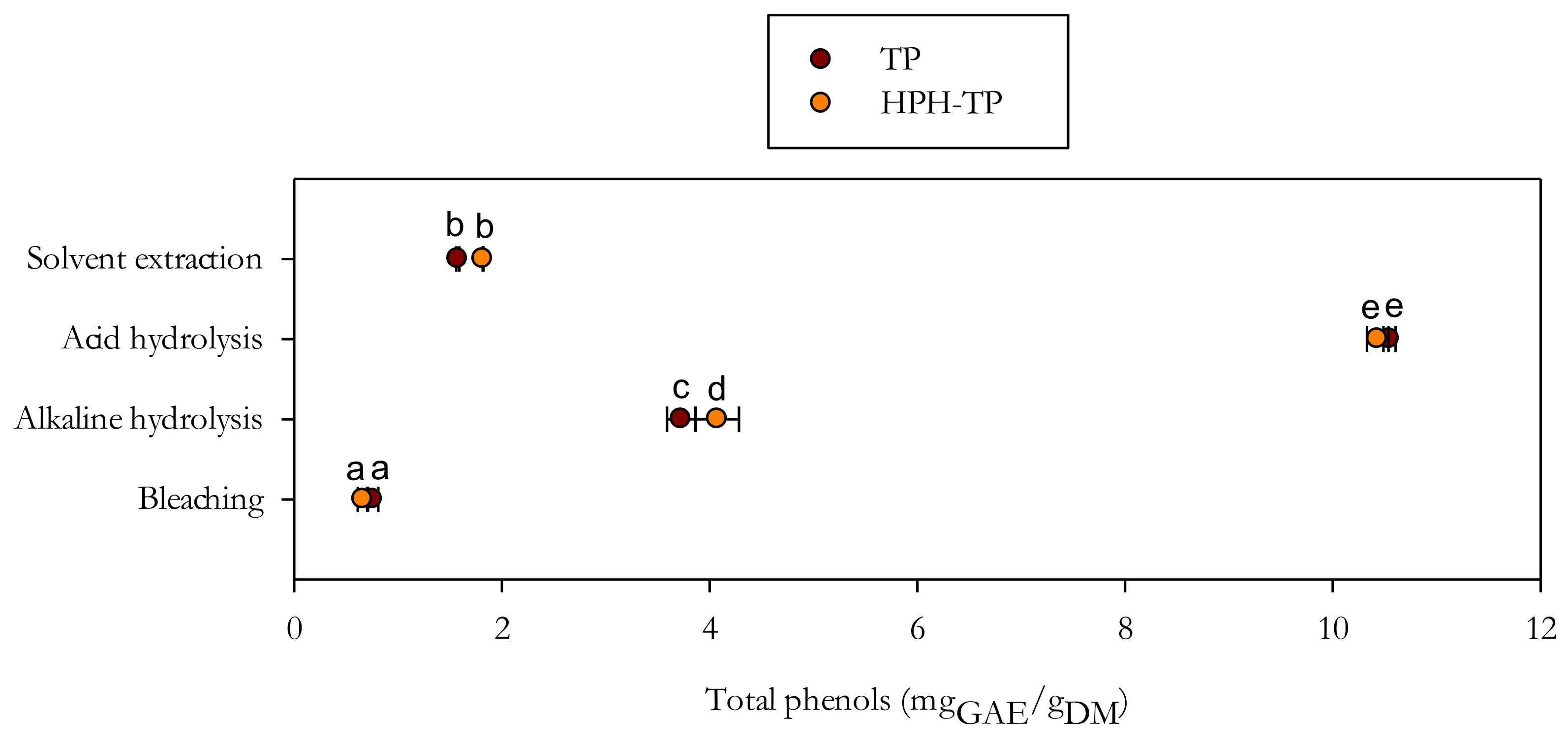
3.2. Optimization of Conventional Extraction Conditions
3.3. Cellulose Isolation
3.3.1. Biological Properties of Liquors
3.3.2. Chemical and Morphological Characteristics of Isolated Cellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, D.J.C.; Machado, A.V.; Vilarinho, M.C.L.G. Availability and Suitability of Agroindustrial Residues as Feedstock for Cellulose-Based Materials: Brazil Case Study. Waste Biomass Valorization 2019, 10, 2863–2878. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt Boix, A. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Pasquini, D.; de Morais Teixeira, E.; da Silva Curvelo, A.A.; Curvelo, A.A.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crops Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Vadivel, V.; Moncalvo, A.; Dordoni, R.; Spigno, G. Effects of an acid/alkaline treatment on the release of antioxidants and cellulose from different agro-food wastes. Waste Manag. 2017, 64, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Rosa, S.M.L.; Rehman, N.; De Miranda, M.I.G.; Nachtigall, S.M.B.; Bica, C.I.D. Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr. Polym. 2012, 87, 1131–1138. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Li, J.B.; Dong, H.L.; Xiu, H.J.; Zhang, M.Y.; Reddy, K.S.; Zhang, X.F.; Ji, Y. Extraction, separation and refining of microcrystalline cellulose from wheat straw using various pretreatments. Int. J. Agric. Biol. Eng. 2016, 9, 137. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Xiong, L.; Chen, X.; Wang, C.; Huang, C.; Chen, X. Isolation of Cellulose from Wheat Straw and Its Utilization for the Preparation of Carboxymethyl Cellulose. Fibers Polym. 2019, 20, 975–981. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A Green Approach Based on Reactive Extrusion to Produce Nanofibrillated Cellulose from Oat Hull. Waste Biomass Valorization 2021, 12, 1051–1060. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and characterization of okara nanocellulose fabricated using sonication or high-pressure homogenization treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McClements, D.J.; He, M.; Huang, Y.; Zhu, H.; Jiang, L.; Teng, F.; Li, Y. Okara nanocellulose fabricated using combined chemical and mechanical treatments: Structure and properties. J. Mol. Liq. 2021, 335, 116231. [Google Scholar] [CrossRef]
- Selim, I.Z.; Zikry, A.A.F.; Gaber, S.H. Physicochemical properties of prepared cellulose sulfates: II. From linen pulp bleached by the H2O2 method. Polym.-Plast. Technol. Eng. 2004, 43, 1387–1402. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient Extraction of Cellulose Nanocrystals through Hydrochloric Acid Hydrolysis Catalyzed by Inorganic Chlorides under Hydrothermal Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656–4664. [Google Scholar] [CrossRef]
- Higgins, F.J.; Ho, G.E. Hydrolysis of cellulose using HCl: A comparison between liquid phase and gaseous phase processes. Agric. Wastes 1982, 4, 97–116. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose biorefinery feedstock engineering. In Lignocellulose Biorefinery Engineering; Woodhead Publishing: Cambridge, UK, 2015; pp. 37–86. Available online: https://www.elsevier.com/books/lignocellulose-biorefinery-engineering/chen/978-0-08-100135-6 (accessed on 19 December 2021). [CrossRef]
- Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Sain, M. The use of biobased nanofibres in composites. In Biofiber Reinforcements in Composite Materials; Woodhead Publishing: Cambridge, UK, 2015; pp. 571–647. [Google Scholar] [CrossRef]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The effect of alkaline pretreatment methods on cellulose structure and accessibility. ChemSusChem 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 7. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Shakeri, A.; Misra, M.; Oksmand, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4, 626–639. [Google Scholar] [CrossRef]
- Shin, H.K.; Pyo Jeun, J.; Bin Kim, H.; Hyun Kang, P. Isolation of cellulose fibers from kenaf using electron beam. Radiat. Phys. Chem. 2012, 81, 936–940. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Fitriana, N.E.; Suwanto, A.; Jatmiko, T.H.; Mursiti, S.; Prasetyo, D.J. Cellulose extraction from sugar palm (Arenga pinnata) fibre by alkaline and peroxide treatments. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 12053. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Yang, F.; Tang, C.; Huang, Q. Effect of H2O2 bleaching treatment on the properties of finished transparent wood. Polymers 2019, 11, 776. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT-Food Sci. Technol. 2017, 85, 517–523. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Squillace, P.; Adani, F.; Scaglia, B. Supercritical CO2 extraction of tomato pomace: Evaluation of the solubility of lycopene in tomato oil as limiting factor of the process performance. Food Chem. 2020, 315, 126224. [Google Scholar] [CrossRef]
- Zuknik, M.H.; Nik Norulaini, N.A.; Mohd Omar, A.K. Supercritical carbon dioxide extraction of lycopene: A review. J. Food Eng. 2012, 112, 253–262. [Google Scholar] [CrossRef]
- Achmon, Y.; Claypool, J.T.; Pace, S.; Simmons, B.A.; Singer, S.W.; Simmons, C.W. Assessment of biogas production and microbial ecology in a high solid anaerobic digestion of major California food processing residues. Bioresour. Technol. Rep. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Chiariotti, A.; Rossi, E.; Scalella, R.; La Mantia, M.C.; Monti, F.; Santangelo, E. Anaerobic codigestion of tomato pomace (Peels plus seeds) with buffalo sludge improves methane production. In Proceedings of the European Biomass Conference and Exhibition Proceedings, Online platform, 26–29 April 2021. [Google Scholar]
- Del Valle, M.; Cámara, M.; Torija, M.E. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 89, 1232–1236. [Google Scholar] [CrossRef]
- Hafid, H.S.; Omar, F.N.; Zhu, J.; Wakisaka, M. Enhanced crystallinity and thermal properties of cellulose from rice husk using acid hydrolysis treatment. Carbohydr. Polym. 2021, 260, 117789. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, G.; Zhang, P.; Fan, S.; Li, F. High-pressure homogenization pretreatment of four different lignocellulosic biomass for enhancing enzymatic digestibility. Bioresour. Technol. 2015, 181, 270–274. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Tappi Standard: TAPPI T-264 cm-07; Preparation of Wood for Chemical Analysis; IHS Markit: London, UK, 2007; Available online: https://tappi.micronexx.com/CD/TESTMETHODS/T264.pdf (accessed on 15 October 2021).
- Tappi Standard: TAPPI T-222 om-22; Acid-Insoluble Lignin in Wood and Pulp; IHS Markit: London, UK, 2006; Available online: https://www.tappi.org/content/SARG/T222.pdf (accessed on 15 October 2021).
- Slinkard, K.; Singleton, V. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2010, 1617, 1–16. [Google Scholar]
- Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W Pickering emulsions stabilized with cellulose nanofibrils produced through different mechanical treatments. Foods 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Mesa, J.; Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, E.; Betoret, N. High homogenization pressures to improve food quality, functionality and sustainability. Molecules 2020, 25, 3305. [Google Scholar] [CrossRef]
- Donsì, F.; Velikov, K.P. Mechanical cell disruption of mustard bran suspensions for improved dispersion properties and protein release. Food Funct. 2020, 11, 6273–6284. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Jurić, M.; Ferrari, G.; Režek Jambrak, A.; Donsì, F. Lycopene-rich cream obtained via high-pressure homogenisation of tomato processing residues in a water–oil mixture. Int. J. Food Sci. Technol. 2021, 56, 4907–4914. [Google Scholar] [CrossRef]
- Fu, T.-K.; Wang, Y.-H.; Li, J.-H.; Wei, X.-Y.; Wang, F. Effect Of High Pressure Homogenization (HPH) On The Rheological Properties Of Pineapple Leaf Cellulose/[BMIM]Cl Solution. In Proceedings of the International Conference on Mechanics Design, Manufacturing and Automation (MDM), Porto, Portugal, 13–16 June 2016. [Google Scholar] [CrossRef][Green Version]
- Yan, Z.; Yi, C.; Liu, T.; Yang, J.; Ma, H.; Sha, L.; Guo, D.; Zhao, H.; Zhang, X.; Wang, W. Effect of Lignin containing Highly Fibrillated Cellulose on the Adsorption Behavior of an Organic Dye. BioResources 2021, 16, 6560–6576. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef]
- Arslan, Y.; Eken-Saraçoĝlu, N. Effects of pretreatment methods for hazelnut shell hydrolysate fermentation with Pichia Stipitis to ethanol. Bioresour. Technol. 2010, 101, 7860–8664. [Google Scholar] [CrossRef]
- Tian, C.; Zheng, L.; Miao, Q.; Nash, C.; Cao, C.; Ni, Y. Improvement in the Fock test for determining the reactivity of dissolving pulp. Tappi J. 2013, 12, 21–26. [Google Scholar] [CrossRef]
- Grönqvist, S.; Hakala, T.K.; Kamppuri, T.; Vehviläinen, M.; Hänninen, T.; Liitiä, T.; Maloney, T.; Suurnäkki, A. Fibre porosity development of dissolving pulp during mechanical and enzymatic processing. Cellulose 2014, 21, 3667–3676. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Duan, C.; Zhang, H.; Ni, Y. Mechanical pretreatment improving hemicelluloses removal from cellulosic fibers during cold caustic extraction. Bioresour. Technol. 2015, 192, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Moreno-Osorio, L.; Villalobos-Carvajal, R.; Pérez-Won, M. Protein Changes Caused by High Hydrostatic Pressure (HHP): A Study Using Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2015, 7, 222–230. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Ferrari, G. Potato starch hydrogels produced by high hydrostatic pressure (HHP): A first approach. Polymers 2019, 11, 1673. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef] [PubMed]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.H.; Yan, Z.M.; Wang, P.L.; Alkhatib, A.; Zhu, J.Y.; Zou, H.C.; Sun, D.Y.; Zhu, X.D.; Gao, F.; Shi, W.T.; et al. Effect of high pressure homogenization treatment on the rheological properties of citrus peel fiber/corn oil emulsion. J. Sci. Food Agric. 2020, 100, 3658–3665. [Google Scholar] [CrossRef]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [CrossRef]
- Zhu, X.; Lundberg, B.; Cheng, Y.; Shan, L.; Xing, J.; Peng, P.; Chen, P.; Huang, X.; Li, D.; Ruan, R. Effect of high-pressure homogenization on the flow properties of citrus peel fibers. J. Food Process Eng. 2018, 41, e12659. [Google Scholar] [CrossRef]






| Run Number | Type of Solvent | Solvent Concentration (% v/v) |
|---|---|---|
| 1 | Acetone | 31 |
| 2 | Acetone | 80 |
| 3 | Acetone | 100 |
| 4 | Ethanol | 20 |
| 5 | Ethanol | 64.8 |
| 6 | Ethanol | 58.8 |
| 7 | Ethanol | 100 |
| 8 | Ethanol | 65.6 |
| 9 | Methanol | 20 |
| 10 | Methanol | 100 |
| 11 | Methanol | 57.2 |
| Component | TP | HPH-TP |
|---|---|---|
| Moisture content (%) | 80.70 ± 0.83 b | 6.94 ± 0.52 a |
| Ash content (%DM) | 3.47 ± 0.61 a | 3.26 ± 0.01 a |
| Protein (%DM) | 14.06 ± 1.04 b | 11.79 ± 0.61 a |
| Fat (%DM) | 1.20 ± 0.14 a | 1.43 ± 0.18 a |
| Extractives (%DM) | 8.24 ± 0.42 a | 14.66 ± 0.97 b |
| Cellulose (%DM) | 32.09 ± 2.24 a | 41.02 ± 1.79 b |
| Hemicellulose (%DM) | 26.62 ± 1.57 b | 6.27 ± 1.33 a |
| Lignin (%DM) | 30.54 ± 1.71 b | 24.90 ± 1.97 a |
| Component | TP | HPH-TP |
|---|---|---|
| Cellulose content (g/100 gFibre residue) | 29.33 ± 1.52 a | 37.72 ± 0.79 b |
| Hemicellulose content (g/100 gFibre residue) | 18.74 ± 0.52 a | 4.62 ± 0.65 b |
| Acetic acid content (g/100 gFibre residue) | 0.10 ± 0.02 a | 0.11 ± 0.02 a |
| Acid soluble lignin (g/100 gFibre residue) | 0.22 ± 0.01 a | 0.18 ± 0.01 a |
| Acid insoluble lignin (g/100 gFibre residue) | 0.52 ± 0.03 a | 0.48 ± 0.02 a |
| Component | TP | HPH-TP |
|---|---|---|
| d(0,1) | 21.6 | 55.7 |
| d(0,5) | 553.6 | 210.5 |
| d(0,9) | 1154.0 | 594.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Ferrari, G.; Donsì, F. Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods 2022, 11, 266. https://doi.org/10.3390/foods11030266
Pirozzi A, Ferrari G, Donsì F. Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods. 2022; 11(3):266. https://doi.org/10.3390/foods11030266
Chicago/Turabian StylePirozzi, Annachiara, Giovanna Ferrari, and Francesco Donsì. 2022. "Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization" Foods 11, no. 3: 266. https://doi.org/10.3390/foods11030266
APA StylePirozzi, A., Ferrari, G., & Donsì, F. (2022). Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods, 11(3), 266. https://doi.org/10.3390/foods11030266