Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges
Abstract
:1. Introduction
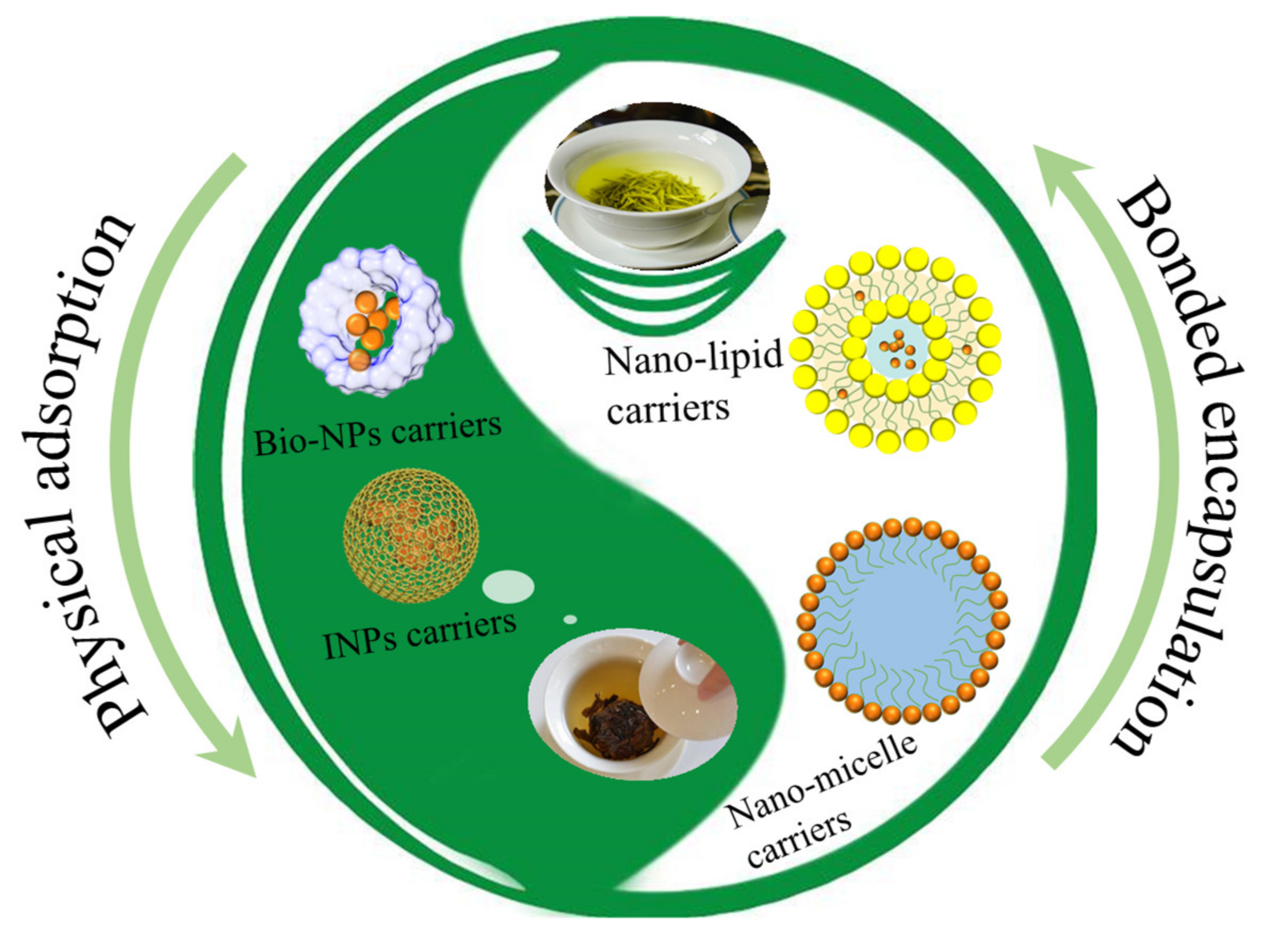
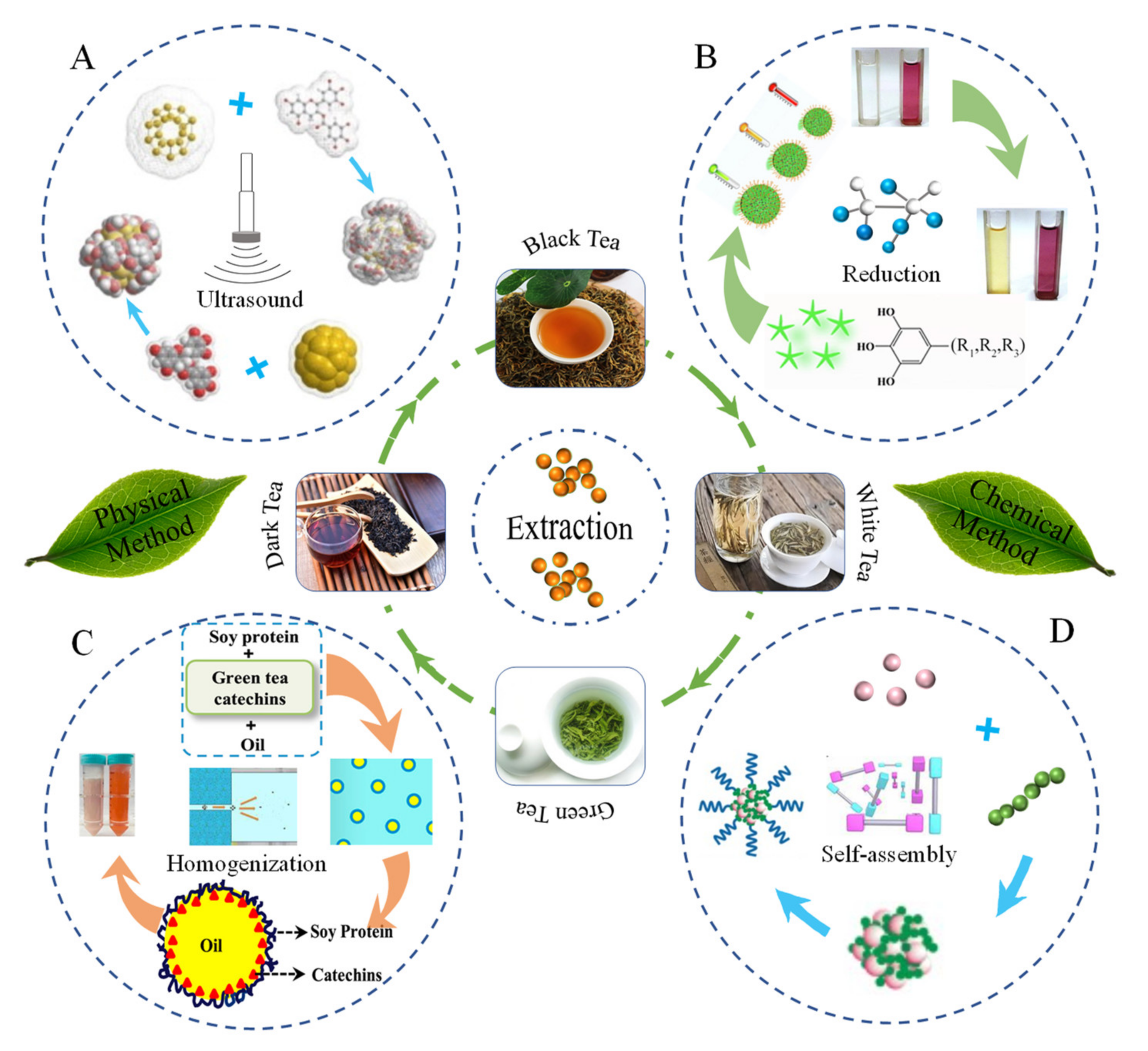
2. Preparation Methods of TPs Nanocarriers
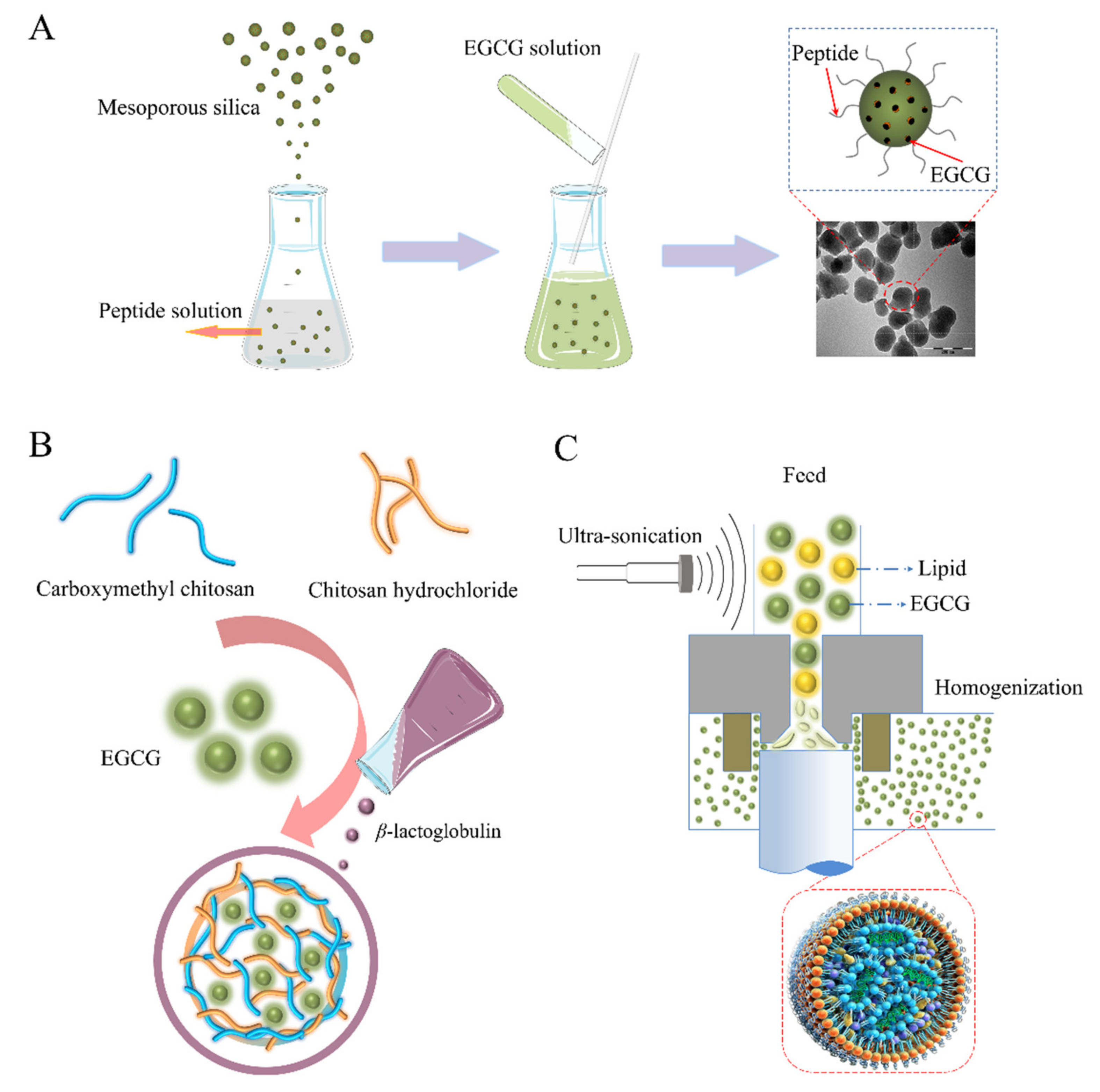
2.1. Nano-Particles
2.2. Nano-Emulsions
2.3. Nano-Micelles
2.4. Nano-Lipids
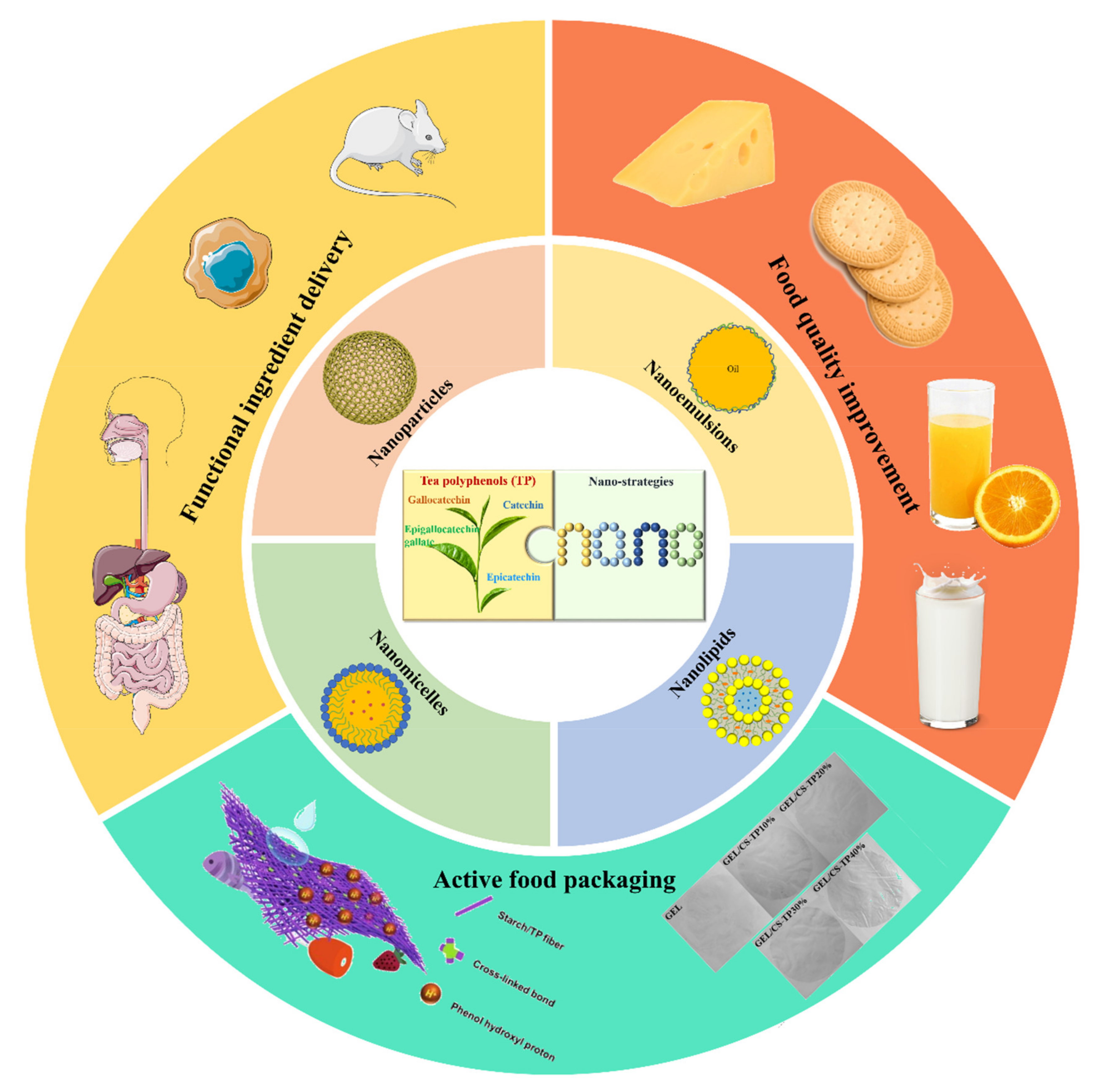
3. Applications of TPs Nanostrategies in the Food and Nutrition Sector
3.1. Functional Ingredient Delivery
3.2. Food Quality Improvement
3.3. Active Food Packaging
4. Challenges and Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural products and skeletal muscle health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef]
- Gong, X.; Ji, M.; Xu, J.; Zhang, C.; Li, M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020, 60, 2303–2326. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215s–217s. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (-)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.; Rsool, H.A. Black tea polyphenols: A mechanistic treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ye, X.; Jiang, C.; Shao, J.; Ma, C.; Wang, H. Separation of epigallocatechin gallate and epicatechin gallate from tea polyphenols by macroporous resin and crystallization. Anal. Methods 2021, 13, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, W.; Jia, T.; Liu, Y.; Yao, S. Ionic liquid beta-cyclodextrin-gelatin composite membrane for effective separation of tea polyphenols from green tea. Food Chem. 2020, 333, 127534. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Liu, C.; Tang, Q.; Zhan, L.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Theaflavin Promotes Mitochondrial Abundance and Glucose Absorption in Myotubes by Activating the CaMKK2-AMPK Signal Axis via Calcium-Ion Influx. J. Agric. Food Chem. 2021, 69, 8144–8159. [Google Scholar] [CrossRef] [PubMed]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Feng, M.; Pan, W.; Zheng, X.; Xie, X.; Hu, B.; Teng, C.; Wang, Y.; Liu, Z.; Wu, J.; et al. Inhibitory Effects of Six Types of Tea on Aging and High-Fat Diet-Related Amyloid Formation Activities. Antioxidants 2021, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M. Nanocontainers in food preservation: Techniques and uses. In Smart Nanocontainers; Phuong, N.T., Trong, O.D., Tuan, A.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–155. [Google Scholar]
- Milincic, D.D.; Popovic, D.A.; Levic, S.M.; Kostic, A.Z.; Tesic, Z.L.; Nedovic, V.A.; Pesic, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 629. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3. [Google Scholar] [CrossRef] [Green Version]
- Bing, H.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan–caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohydr. Polym. 2012, 89, 362–370. [Google Scholar]
- Homayouni, H.; Kavoosi, G.; Nassiri, S.M. Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT Food Sci. Technol. 2017, 77, 503–509. [Google Scholar] [CrossRef]
- Zuluaga, R.; Guerra, A.M.S.; Gomez, C.; Velásquez-Cock, J.A.; Rivera, N.H.C. The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1842–1854. [Google Scholar]
- Liang, J.; Yan, H.; Yang, H.J.; Kim, H.W.; Wan, X.; Lee, J.; Ko, S. Synthesis and controlled-release properties of chitosan/β-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016, 25, 1583–1590. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Duan, M.; Chen, X.; Li, J.; Zhao, T.; Fu, Y.; Julian McClements, D.; Huang, J.; Lin, H.; et al. TiO(2) nanoparticles negatively impact the bioavailability and antioxidant activity of tea polyphenols. Food Chem. 2022, 371, 131045. [Google Scholar] [CrossRef]
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Devel. Ther. 2016, 10, 3519–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, S.; Rani, R.; Kumar, S.; Dhingra, D.; Dilbaghi, N. Chitosan-Gellan Gum Bipolymeric Nanohydrogels—A Potential Nanocarrier for the Delivery of Epigallocatechin Gallate. BioNanoScience 2017, 7, 508–520. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-H.; Augustin, M.A. Nano-and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2018, 59, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ruan, C.; Sun, Y.; Gao, X.; Liang, J. Controlled release, and antioxidant activity of chitosan and beta-lactoglobulin complex nanoparticles loaded with epigallocatechin gallate. Colloids Surf. B Biointerfaces 2020, 188, 110802. [Google Scholar] [CrossRef]
- Sabaghi, M.; Hoseyni, S.Z.; Tavasoli, S.; Mozafari, M.R.; Katouzian, I. Strategies of confining green tea catechin compounds in nano-biopolymeric matrices: A review. Colloids Surf. B Biointerfaces 2021, 204, 111781. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Guidance, D. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Biotechnol. Law Rep. 2014, 30, 613–616. [Google Scholar]
- Ritter, A. FDA Issues Draft Guidance on Nanotechnology. Biopharm. Int. 2011, 94, 97–107. [Google Scholar]
- Wang, Y.H.; Zhang, R.; Qin, W.; Dai, J.W.; Zhang, Q.; Lee, K.J.; Liu, Y.W. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Cai, J.; Dong, Q.; Din, Z.-u.; Hu, Z.-Z.; Wang, G.-Z.; Ding, W.-P.; He, J.-R.; Cheng, S.-Y. Starch/tea polyphenols nanofibrous films for food packaging application: From facile construction to enhance mechanical, antioxidant and hydrophobic properties. Food Chem. 2021, 360, 129922. [Google Scholar] [CrossRef]
- Lu, Q.; Li, D.-C.; Jiang, J.-G. Preparation of a Tea Polyphenol Nanoliposome System, and Its Physicochemical Properties. J. Agric. Food Chem. 2011, 59, 13004–13011. [Google Scholar] [CrossRef]
- Tang, D.-W.; Yu, S.-H.; Ho, Y.-C.; Huang, B.-Q.; Tsai, G.-J.; Hsieh, H.-Y.; Sung, H.-W.; Mi, F.-L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Yin, C.; Cheng, L.; Zhang, X.; Wu, Z. Nanotechnology improves delivery efficiency and bioavailability of tea polyphenols. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Wei, X.-L.; Fang, Y.-P.; Gan, R.-Y.; Wang, M.; Ge, Y.-Y.; Zhang, D.; Cheng, L.-Z.; Corke, H. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 2019, 60, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Kim, T.-H.; Oh, J.-M.; Choy, J.-H. Emerging nanomaterials with advanced drug delivery functions; focused on methotrexate delivery. Coord. Chem. Rev. 2018, 359, 32–51. [Google Scholar] [CrossRef]
- Hsieh, D.-S.; Wang, H.; Tan, S.-W.; Huang, Y.-H.; Tsai, C.-Y.; Yeh, M.-K.; Wu, C.-J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.S.; Lu, H.C.; Chen, C.C.; Wu, C.J.; Yeh, M.K. The preparation and characterization of gold-conjugated polyphenol nanoparticles as a novel delivery system. Int. J. Nanomed. 2012, 7, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; He, Y.; Zhou, G.; Li, X.; Feng, A.; Zheng, W. Target challenging-cancer drug delivery to gastric cancer tissues with a fucose graft epigallocatechin-3-gallate-gold particles nanocomposite approach. J. Photochem. Photobiol. B Biol. 2018, 183, 147–153. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, S.; Das, D.K.; Chakraborty, P.; Choudhury, S.; Gupta, P.; Adhikary, A.; Dey, S.; Chattopadhyay, S. Gold-conjugated green tea nanoparticles for enhanced anti-tumor activities and hepatoprotection—Synthesis, characterization and in vitro evaluation. J. Nutr. Biochem. 2015, 26, 1283–1297. [Google Scholar] [CrossRef]
- David, S.R.; Abdullah, K.; Shanmugam, R.; Thangavelu, L.; Das, S.K.; Rajabalaya, R. Green Synthesis, Characterization and In Vivo Evaluation of White Tea Silver Nanoparticles with 5-Fluorouracil on Colorectal Cancer. Bionanoscience 2021, 11, 1095–1107. [Google Scholar] [CrossRef]
- Barbasz, A.; Czyżowska, A.; Piergies, N.; Oćwieja, M. Design cytotoxicity: The effect of silver nanoparticles stabilized by selected antioxidants on melanoma cells. J. Appl. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liang, G.; Cheng, D.; Liu, Y.; Cheng, X.; Yang, P.; Wu, N.; Zhao, Y.; Wei, J. Controllable synthesis of iron-polyphenol colloidal nanoparticles with composition-dependent photothermal performance. J. Colloid Interface Sci. 2021, 593, 172–181. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Ding, J.; Yao, J.; Xue, J.J.; Li, R.; Bao, B.; Jiang, L.P.; Zhu, J.J.; He, Z.W. Tumor-Homing Cell-Penetrating Peptide Linked to Colloidal Mesoporous Silica Encapsulated (-)-Epigallocatechin-3-gallate as Drug Delivery System for Breast Cancer Therapy in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 18145–18155. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, H.; Li, K.; Ren, H.; Lei, J.; Huang, C. Development of Epigallocatechin-3-gallate-Encapsulated Nanohydroxyapatite/Mesoporous Silica for Therapeutic Management of Dentin Surface. ACS Appl. Mater. Interfaces 2017, 9, 25796–25807. [Google Scholar] [CrossRef]
- Yu, J.; Yi, L.; Guo, R.; Guo, J.; Huang, C. The Stability of Dentin Surface Biobarrier Consisting of Mesoporous Delivery System on Dentinal Tubule Occlusion and Streptococcus Mutans Biofilm Inhibition. Int. J. Nanomed. 2021, 16, 3041–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, K.; Lin, X.; Zhu, C. Multifunctional Ultra-Small Nanocomplexes Capping Mesoporous Silica Nanoparticles for Multimodal Imaging and Chemo-Photothermal Therapy. ChemNanoMat 2019, 5, 1115–1122. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, N.; Guan, P.; Chen, P.; Ding, S.; Hou, T.; Hu, X.; Wang, J.; Wang, C. Drug-based magnetic imprinted nanoparticles: Enhanced lysozyme amyloid fibrils cleansing and anti-amyloid fibrils toxicity. Int. J. Biol. Macromol. 2020, 153, 723–735. [Google Scholar] [CrossRef]
- Ke, X.; Qin, N.; Zhang, T.; Ke, F.; Yan, X. Highly Augmented Antioxidant and Anticancer Effect of Biocompatible MIL-100(Fe)@SiO2-Immobilized Green Tea Catechin. J. Inorg. Organomet. Polym. Mater. 2019, 30, 935–942. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, M.; Qin, N.; Zhao, G.; Chu, J.; Wan, X. Synergistic antioxidant activity and anticancer effect of green tea catechin stabilized on nanoscale cyclodextrin-based metal–organic frameworks. J. Mater. Sci. 2019, 54, 10420–10429. [Google Scholar] [CrossRef]
- Shao, G.; Wang, S.; Zhao, H.; Zhao, G.; Yang, L.; Zhu, L.; Liu, H. Tunable arrangement of hydrogel and cyclodextrin-based metal organic frameworks suitable for drug encapsulation and release. Carbohydr. Polym. 2021, 69, 118915. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, W.; Zhen, T.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Preparation of debranched starch nanoparticles by ionic gelation for encapsulation of epigallocatechin gallate. Int. J. Biol. Macromol. 2020, 161, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.; Chen, Q.; Liu, Y.; Zeng, L.; Song, H.; Xu, Z.; Kang, Y.; Li, C.; Xiao, B. Green Fabrication of Ovalbumin Nanoparticles as Natural Polyphenol Carriers for Ulcerative Colitis Therapy. ACS Sustain. Chem. Eng. 2018, 6, 12658–12667. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Tsai, Y.J. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-H.; Wang, C.-C.; Lin, Y.-H.; Chen, B.-H. Preparation of Catechin Nanoemulsion from Oolong Tea Leaf Waste and Its Inhibition of Prostate Cancer Cells DU-145 and Tumors in Mice. Molecules 2021, 26, 3260. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2006, 24, 1–16. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for increased micronutrient bioavailability. Trends Food Sci. Technol. 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Haratifar, S.; Meckling, K.A.; Corredig, M. Antiproliferative activity of tea catechins associated with casein micelles, using HT29 colon cancer cells. J. Dairy Sci. 2014, 97, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekhosseini, P.; Alami, M.; Khomeiri, M.; Esteghlal, S.; Nekoei, A.R.; Hosseini, S.M.H. Development of casein-based nanoencapsulation systems for delivery of epigallocatechin gallate and folic acid. Food Sci. Nutr. 2019, 7, 519–527. [Google Scholar] [CrossRef]
- Sánchez-Giraldo, V.; Monsalve, Y.; Palacio, J.; Mendivil-Perez, M.; Sierra, L.; Velez-Pardo, C.; López, B.L.; Jiménez-Del-Rio, M. Role of a novel (-)-epigallocatechin-3-gallate delivery system on the prevention against oxidative stress damage in vitro and in vivo model of Parkinson’s disease. J. Drug Deliv. Sci. Technol. 2020, 55, 101466. [Google Scholar] [CrossRef]
- Vittorio, O.; Curcio, M.; Cojoc, M.; Goya, G.F.; Hampel, S.; Iemma, F.; Dubrovska, A.; Cirillo, G. Polyphenols delivery by polymeric materials: Challenges in cancer treatment. Drug Deliv. 2017, 24, 162–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of tea catechins with chitosan nanoparticles. Food Hydrocoll. 2018, 84, 561–570. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.; Zhu, J.-J. Stealth and Fully-Laden Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948. [Google Scholar] [CrossRef]
- Kumar, M.; Tiwari, A.; Asdaq, S.M.B.; Nair, A.B.; Bhatt, S.; Shinu, P.; Al Mouslem, A.K.; Jacob, S.; Alamri, A.S.; Alsanie, W.F.; et al. Itraconazole loaded nano-structured lipid carrier for topical ocular delivery: Optimization and evaluation. Saudi J. Biol. Sci. 2022, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Vieira, A.C.; Chaves, L.L.; Nunes, C.; Neves, A.R.; Pinheiro, M.; Reis, S. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chem. 2017, 237, 803–810. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef]
- Manea, A.-M.; Vasile, B.S.; Meghea, A. Antioxidant and antimicrobial activities of green tea extract loaded into nanostructured lipid carriers. Comptes Rendus Chim. 2014, 17, 331–341. [Google Scholar] [CrossRef]
- Gao, J.; Mao, Y.; Xiang, C.; Cao, M.; Ren, G.; Wang, K.; Ma, X.; Wu, D.; Xie, H. Preparation of β-lactoglobulin/gum arabic complex nanoparticles for encapsulation and controlled release of EGCG in simulated gastrointestinal digestion model. Food Chem. 2021, 354, 129516. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tian, J.; Liu, Y.; Meng, D.; Blanchard, C.L.; Zhou, Z. One-step fabrication of phytoferritin-chitosan-epigallocatechin shell-core nanoparticles by thermal treatment. Food Hydrocoll. 2018, 80, 24–32. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Shashidhar, M.G.; Balaraman, M. Delivery of green tea catechins through Oil-in-Water (O/W) nanoemulsion and assessment of storage stability. J. Food Eng. 2017, 199, 65–76. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (-)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Shpigelman, A.; Cohen, Y.; Livney, Y.D. Thermally-induced β-lactoglobulin–EGCG nanovehicles: Loading, stability, sensory and digestive-release study. Food Hydrocoll. 2012, 29, 57–67. [Google Scholar] [CrossRef]
- Siraj, A.; Naqash, F.; Shah, M.A.; Fayaz, S.; Majid, D.; Dar, B.N. Nanoemulsions: Formation, stability and an account of dietary polyphenol encapsulation. Int. J. Food Sci. Technol. 2021, 56, 4193–4205. [Google Scholar] [CrossRef]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the Antioxidant Activity of Green Tea Extract through Encapsulation in Chitosan-Citrate Nanogel. J. Food Qual. 2020, 2020, 7935420. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Effect of liposomal encapsulation on the recovery and antioxidant properties of green tea catechins incorporated into a hard low-fat cheese following in vitro simulated gastrointestinal digestion. Food Bioprod. Processing 2016, 100, 238–245. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. A novel functional full-fat hard cheese containing liposomal nanoencapsulated green tea catechins: Manufacture and recovery following simulated digestion. Food Funct. 2016, 7, 3283–3294. [Google Scholar] [CrossRef]
- Ruengdech, A.; Siripatrawan, U. Application of catechin nanoencapsulation with enhanced antioxidant activity in high pressure processed catechin-fortified coconut milk. LWT Food Sci. Technol. 2021, 140, 110594. [Google Scholar] [CrossRef]
- Feng, S.M.; Sun, Y.X.; Wang, P.; Sun, P.L.; Ritzoulis, C.; Shao, P. Co-encapsulation of resveratrol and epigallocatechin gallate in low methoxyl pectin-coated liposomes with great stability in orange juice. Int. J. Food Sci. Tech. 2020, 55, 1872–1880. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Hernandez-Rojas, M.; Tarancon, P.; Tenon, M.; Feuillere, N.; Ruiz, J.F.V.; Fiszman, S.; Lopez-Rubio, A. Impact of microencapsulation within electrosprayed proteins on the formulation of green tea extract-enriched biscuits. LWT Food Sci. Technol. 2017, 81, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. F 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef]
- Liu, F.; Yokoyama, W.; Zhong, F.; Li, Y. Preparation of gelatin films incorporated with tea polyphenol nanoparticles for enhancing controlled-release antioxidant properties. Abstr. Pap. Am. Chem. S 2016, 251, 3987–3995. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liang, X.; Wang, S.Y.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Niu, B.; Chen, H.J.; Sun, P.L. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Shi, Y.Y.; Cao, X.K.; Liu, Q.; Wang, H.; Kong, B.H. Preparation and functional properties of poly(vinyl alcohol)/ethyl cellulose/tea polyphenol electrospun nanofibrous films for active packaging material. Food Control. 2021, 130, 108331. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Yi, J.; Yokoyama, W.; Ma, J.; Zhong, F. Preparation of Gelatin Films Incorporated with Tea Polyphenol Nanoparticles for Enhancing Controlled-Release Antioxidant Properties. J. Agric. Food Chem. 2015, 63, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Xu, S.; Wang, Z. Antioxidant activity and properties of gelatin films incorporated with tea polyphenol-loaded chitosan nanoparticles. J. Sci. Food Agric. 2009, 89, 2692–2700. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Nano-Carriers | Tea Polyphenols | Site of Action | Effect | Ref. |
|---|---|---|---|---|
| Chitosan/β-lactoglobulin nanoparticles | Epigallocatechin gallate (EGCG) | Simulated gastrointestinal conditions | The release rate and degradation of EGCG-loaded nanoparticles (20 and 60 min, respectively) in simulated stomach conditions were slower than those of the control particles (5 and 30 min, respectively) | [25] |
| β-lactoglobulin/gum arabic complex nanoparticles | Epigallocatechin gallate (EGCG) | Simulated gastric and intestinal fluids | The accumulative EGCG release of β-lactoglobulin/gum arabic–EGCG complex nanoparticles was only 21% after 60 min digestion in simulated gastric juice and 86% after 120 min digestion in simulated intestinal juice | [82] |
| Phytoferritin–chitosan–epigallocatechin nanoparticles | Epigallocatechin (EGC) | Simulated gastric/intestinal tract | The nanoparticles prepared by the heat treatment further improved the retention ratio of EGC to 38.25 ± 1.8% | [83] |
| Debranched starch nanoparticles | Epigallocatechin gallate (EGCG) | Simulated gastric and intestinal fluids | After 600 min, the cumulative release rate of EGCG loaded nanoparticles in simulated intestinal fluid was about 63%, and the EGCG release rate in simulated intestinal fluid was slightly higher than that in simulated gastric fluid | [62] |
| Nanoemulsion prepared with corn oil and polysorbate-80 | Epigallocatechin gallate (EGCG) | Simulated saliva, gastric, and small intestinal fluid | The absorbed EGCG content of the nanoemulsion was significantly increased by 28.6% compared with that of TP solution | [51] |
| Nanoemulsion prepared with sunflower oil and Tween 80 | Green tea catechins | Mimicked gastric condition | 55.13 ± 1.26% of total polyphenol, 48.61 ± 0.78% of total catechins, and 46.94 ± 0.88% of EGCG were released from green tea emulsion during the first 20 h | [84] |
| Folic acid-functionalized nanolipid carriers | Epigallocatechin-3-gallate (EGCG) | Simulated gastric and intestinal fluids | After 3 h, the release of EGCG in simulated gastric juice was very low, about 13% and 9% of the initial amount of functional and nonfunctional nanolipid carriers. After 21 h, EGCG was released steadily in simulated intestinal fluid, with maximum cumulative release of functional and nonfunctional nanolipid carriers of 48% and 34%, respectively | [78] |
| Solid lipid nanoparticles | Epigallocatechin-3-gallate (EGCG) | Simulated gastric and intestinal fluids | Improved the stability of EGCG under intestinal conditions at pH 6.8 | [85] |
| Nano-Carriers | Tea Polyphenols | Food | Effect | Ref. |
|---|---|---|---|---|
| Soy lecithin liposomes | Catechin and epigallocatechin gallate | Hard low-fat cheese | Increased antioxidant properties of low-fat cheese. The treated cheese samples with liposome-encapsulated catechin or EGCG had higher FRAP (ferric reducing antioxidant power) values | [89] |
| Soy lecithin liposomes | Green tea catechins | Full-fat hard cheese | Increased antioxidant activity of full-fat hard cheese. The Total phenolic content (TPC), ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC) antioxidant activity of whole fat cheese treated with catechin after 90 days of ripening were higher than those of the control group | [90] |
| Nanoencapsulation | Catechin | Coconut milk | Enhanced the antioxidant activity and prolonged the shelf life of coconut milk. Coconut milk better retained antioxidant activity during 21 days of storage | [91] |
| Low-methoxy pectin-coated nanoliposomes | Epigallocatechin gallate | Orange juice | Maintained good stability after pasteurization and had a stronger antioxidant activity. The liposomes still showed sustained release effect for about 20 days in orange juice, and the appearance of the orange juice did not change | [92] |
| Protein microcapsule | Green tea catechins | Biscuits | Microcapsule can protect catechins during a thermal treatment (180 °C), but it did not improve the antioxidant capacity of biscuit dough | [93] |
| Nano-Carriers | Tea Polyphenols | Film Matrix | Effect | Ref. |
|---|---|---|---|---|
| Chitosan nanoparticles | Tea polyphenol (TP) (Catechins content ≥90%) | Gelatin films | Introduced antioxidant properties into the gelatin films and increased the compactness of films | [98] |
| Nanocapsule | Epigallocatechin gallate (EGCG) | Chitosan films | Increased the DPPH scavenging activity, the mechanical properties, and light barrier properties of films | [99] |
| Chitosan nanoparticles | Tea polyphenol (TP) (Catechins content ≥90%) | Gelatin films | Increased the antioxidant properties of films and control oil oxidation over a long term (6 weeks) | [100] |
| Chitosan nanoparticles | Tea polyphenol (TP) | Gelatin films | Exhibited a high ability of free radical-scavenging and prevented soybean oil oxidation for more than 14 days. Gelatin/chitosan–tea polyphenol 30% composite films showed the most delayed release of TP and had the highest DPPH radical scavenging activity of 80.50 ± 4.67% (p < 0.05) after 14 days | [36] |
| Starch nanofibers | Tea polyphenol (TP) (with a polyphenol content ≥99%) | Starch nanofibrous films | Introduced antioxidant activity into the films and increased the mechanical properties and hydrophobicity of films. The antioxidant activity of films gradually increased with increased TP content | [37] |
| Polylactic acid/tea polyphenol (PLA/TP) nanofibers | Tea polyphenol (TP) | Polylactic acid/tea polyphenol (PLA/TP) composite nanofibers films | Exhibited antioxidant activity and antimicrobial activities against Escherichia coli and Staphylococcus aureus. The scavenging ability of DPPH free radical was 95.07 ± 10.55% and the antibacterial activities of PLA/TP-3:1 composite fiber against Escherichia coli and Staphylococcus aureus were 92.26 ± 5.93% and 94.58 ± 6.53%, respectively | [101] |
| Pullulan-carboxymethylcellulose sodium-tea polyphenol (PUL-CMC-TP) nanofibers | Tea polyphenol (TP) | Pullulan-carboxymethylcellulose sodium-tea polyphenol (PUL-CMC-TP) nanofibers films | Reduced the weight loss and tissue softening properties of strawberries during storage and effectively prolonged their storage period | [102] |
| Poly(vinyl alcohol)/ethyl cellulose/tea polyphenol (PVA/EC-TP) nanofibers | Tea polyphenol (TP) | Poly(vinyl alcohol)/ethyl cellulose/tea polyphenol electrospun nanofibrous films | Exhibited good antioxidant and antimicrobial activity and maintain the quality of pork and prolong its shelf life by 3 days | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Li, Z.; Fan, W.; Zhong, X.; Peng, M.; Liu, Z. Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges. Foods 2022, 11, 387. https://doi.org/10.3390/foods11030387
Niu L, Li Z, Fan W, Zhong X, Peng M, Liu Z. Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges. Foods. 2022; 11(3):387. https://doi.org/10.3390/foods11030387
Chicago/Turabian StyleNiu, Li, Ziqiang Li, Wei Fan, Xiaohong Zhong, Miao Peng, and Zhonghua Liu. 2022. "Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges" Foods 11, no. 3: 387. https://doi.org/10.3390/foods11030387
APA StyleNiu, L., Li, Z., Fan, W., Zhong, X., Peng, M., & Liu, Z. (2022). Nano-Strategies for Enhancing the Bioavailability of Tea Polyphenols: Preparation, Applications, and Challenges. Foods, 11(3), 387. https://doi.org/10.3390/foods11030387