Metal-Organic Frameworks-Based Sensors for Food Safety
Abstract
:1. Introduction
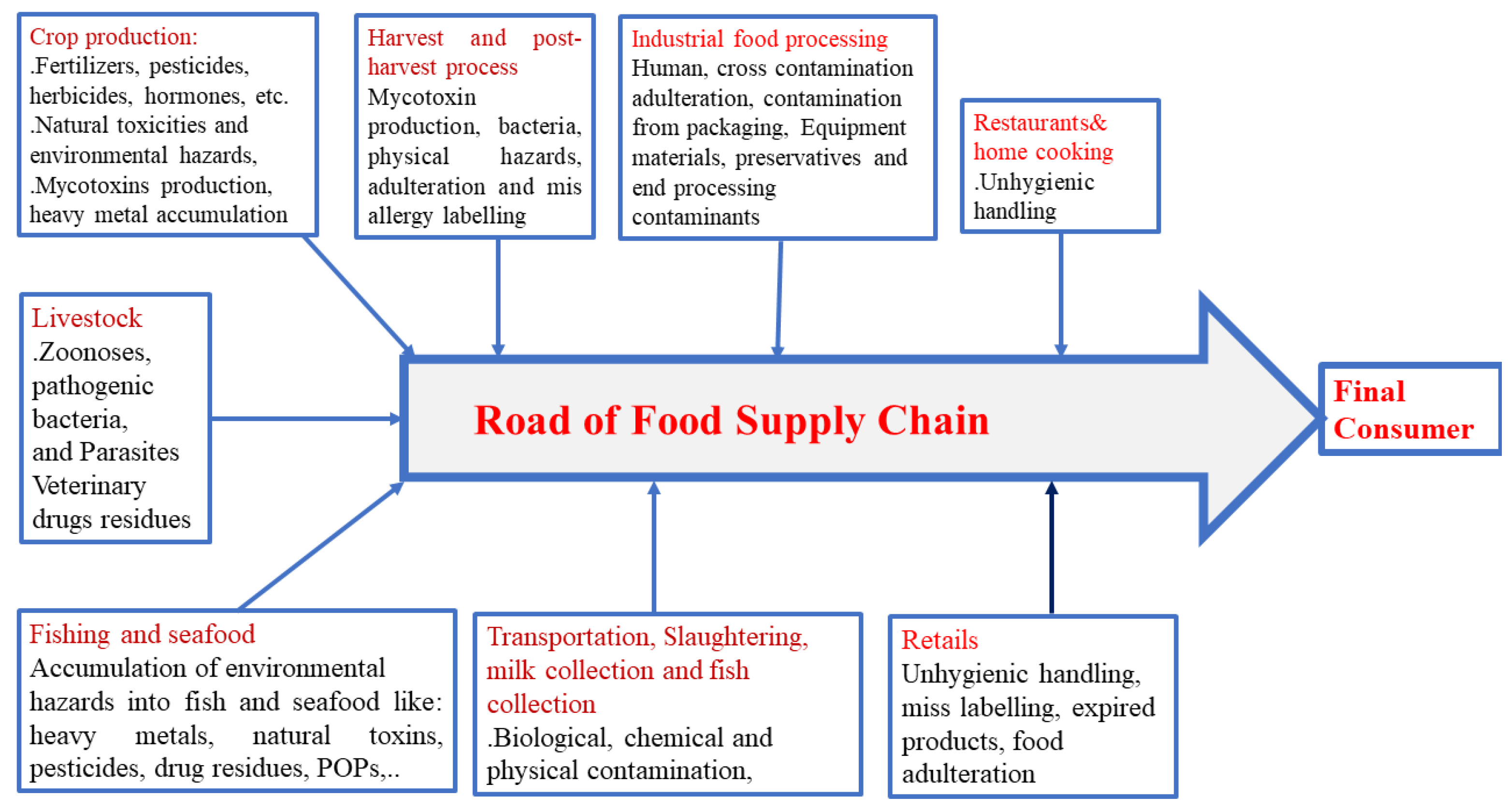
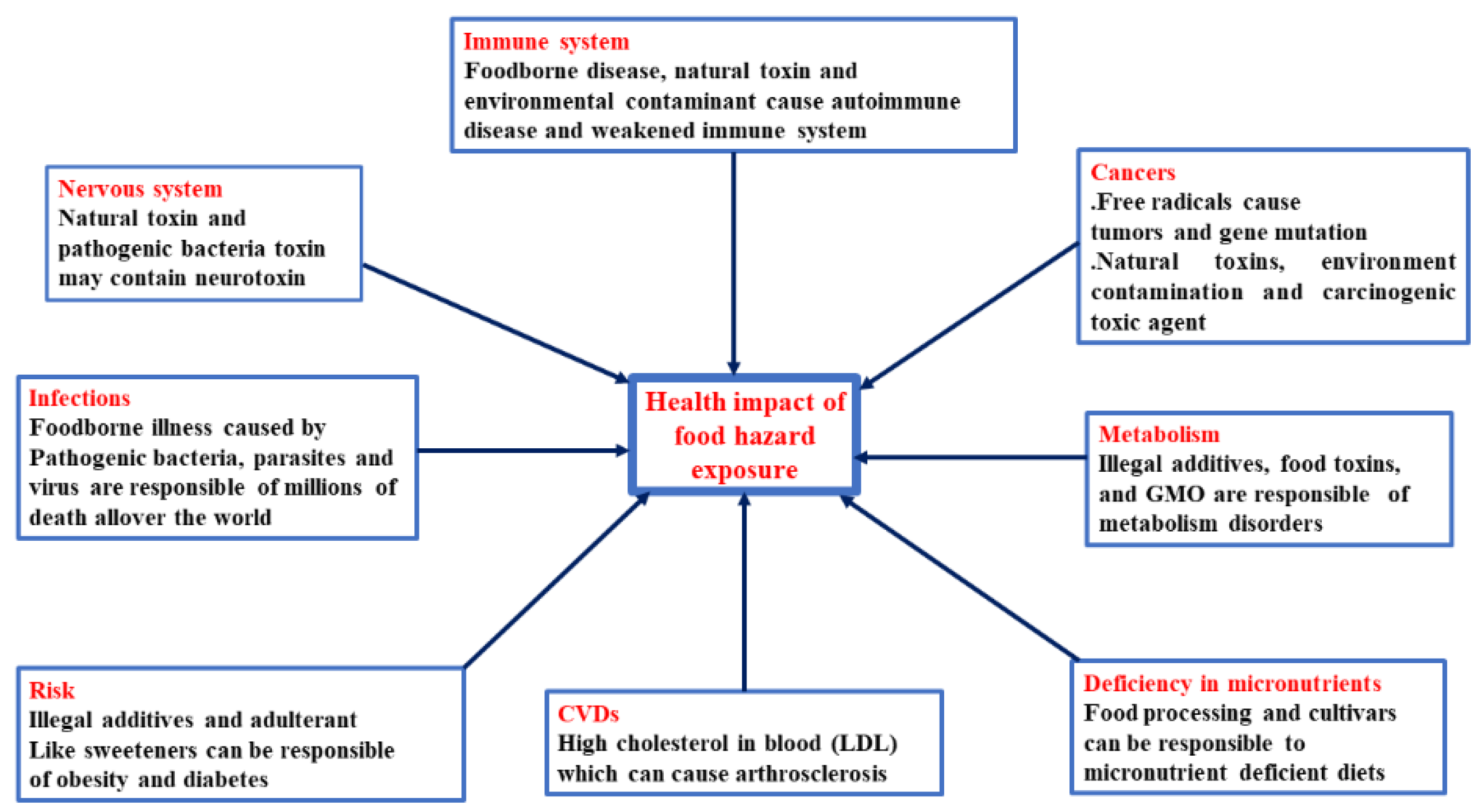
2. Food Exposition to Hazard and Food Contaminants
| Food Hazards | Harmful Health Effect | Source of Contamination | Most Contaminated Food | Control Majors | Reference |
|---|---|---|---|---|---|
| pathogenic bacteria | |||||
| Salmonella enterica serovars Thphimurium | Food toxin and typhoid fever | Fecal contamination, eating raw, or inadequately cooked food or contaminated water | dairy product, meat, eggs, vegetables and processed food, and untreated water | Frequent handwashing, consuming treated water, and well-cooked food served hot | [33] |
| Salmonella enterica serovars | Gastroenteritis and bloody diarrhea | Eating raw or inadequately cooked seafood or other contaminated food and water | Seafood and water | Eating cooked seafood and other foods and drinking treated water | [34] |
| Shigella dysenteriae | Epidemic bacillary dysentery and Shigellosis | Inadequate water and poor sanitation | Water and contaminated food | Frequent handwashing, drinking and using treated water | [35] |
| Escherichia coli O157:H7 | Produce shiga toxin which can damage lining of intestine | Contaminated water and raw food | Meat products, dairy products, juice, fruits and vegetables | Consuming well-cooked food served hot | [36] |
| Listeria monocytogenes | Listeriosis | Raw food and having the ability to resist low temperatures | Meat and meat products, dairy product, fruits and vegetables | Consuming cooked food and treated milk | [37] |
| Shigella sonnei | Shigellosis, bacteria dysentery, diarrhea, tenesmus, and toxic shock | Fecal contamination caused by unproper hygiene | Fresh fruit and vegetables, raw oysters, deli meats and unpasteurized milk | Good hygiene practice during food handling | [38] |
| Staphylococcus aureus | Food poisoning, skin infection, Animal infection, Bacteremia and Bone and joint infection | Close contamination caused by unproper hygiene | Milk and dairy products | Consuming pasteurized milk and milk products | [1] |
| campylobacter jejuni | Bacterial gastroenteritis, autoimmune neurological disorders like Guillain-Barre syndrome, Miller Fisher | Consumption of undercooked meat and meat products and other contaminated food | Meat products, especially poultry products | Consuming cooked meat | [39] |
| Name of Heavy Metal | |||||
| Pb2+ | Interfering with proper enzymes function, anemia, insomnia, irritability, memory loss, weight loss, hearing loss, loss of coordination, etc. | Environmental and water pollution | Water, beverages, fruits and vegetables, cereal products | Using and consuming tested water and food | [40] |
| Hg2+ | Neurotoxin, acrodynia, Hunter-Rusell syndrome, damaged brain, kidney, and lungs | Environmental and water pollution | Water, beverages, fruits and Vegetables, cereal products | Using and consuming tested water and food | [41] |
| K+ | Abnormal concentration causing kidney disease, heart disease, diabetes, anorexia, bulimia, blood high pressure, stroke, Addison’s and adrenaline gland disease | Environmental and water pollution | Water, beverages, fruits and vegetables, cereal products | Using and consuming tested water and food | [42] |
| As3+ | Causes cancer of the skin, lung, urinary bladder, liver, and kidney | Environmental and water pollution | Water, beverages, fruits and vegetables, cereal products | Using and consuming tested water and food | [43] |
| Cd2+ | Metal fume fever, pneumonitis and pulmonary edema | Environmental and water pollution | Cereal products, water, beverages, vegetables and fruits | Using and consuming tested water and food | [44] |
| Natural Toxin | |||||
| Staphylococcus aureus enterotoxin A | Gastrointestinal, severe allergic, auto immune response and toxic shock syndrome | Produced by Staphylococcus aureus | Milk and dairy products | Consume pasteurized milk and milk products | [45] |
| T-2 Toxin | Emesis, diarrhea, necrosis, cartilage damage, immunosuppression and apoptosis | Secondary metabolite of fusarium | Barley, wheat, maize, oats | Consumption of tested cereals product | [46] |
| Aflatoxin B1 (AFB1) | Cirrhosis, necrosis and carcinoma of liver | Secondary metabolites of Aspergillus flavus and Aspergillus parasiticus | Fruits, cereals, wine nuts, spices and soy products | Consumption of tested cereals food | [47] |
| Ochratoxin (OTA) | carcinogenic, hepatotoxic, teratogenic, nephrotoxic and immunotoxin | Secondary metabolites of Aspergillus ochraceus, penicillium verrucosum and penicillium nordicum | Wheat, corn, beans, wine, cereals and cereals products milk and milk products meat and meat products | Consumption of tested cereals food | [48] |
| Fumonisin B1 | Carcinogen to human, leukoencephalomalaciia to horses and pulmonary edema to swine | Produced by more than ten species of Fusarium. F. verticillioides and F. proliferatum produce high concentration | Cereals and cereals products soybean and soy product | Consumption of and feeding tested cereals food | [49] |
| Okadaic acid (OA) | Immunotoxic and tumor promotion, diarrhea | Produced by harmful algal blooms (HABs) | Seafood | test seafood before consumption | [50] |
| Tetrodotoxin (TTX) | Neurotoxin and carcinogenic toxin | Produced by harmful algal blooms (HABs) | Seafood and water | test seafood and water before consumption | [51] |
| Microcystin-LR (MC-LR) | Cause live damage | Produced by cy | Seafood and water | test seafood water before consumption | [52] |
| β-lactoglobulin | Allergen | Milk allergen | Milk and milk products | Test and food labeling | [53] |
| Ricin toxin | Deadly plant toxin via inhibition of protein synthesizes, ribosome inactivation, dysphagia, hematemesis, and hypovolemia | Produced by castor beans (Ricinus communis) | castor beans | Food testing | [54] |
| Abrin toxin | Deadly plant toxins through ribosome and proteins inactivation, | Produced by peas (Abrus precatorius) | Rosary peas (Abrus precatorius) | Food testing | [55] |
| Botulinum toxins | Paralysis, arrhythmia, heart attack and respiratory arrest | Nerve toxin produced by the bacterium clostridium (c. botulinum) | Dairy products, vegetables, fruits, seafood, canned foods | Consume cooked and treated foods | [56] |
| Dopamine | Severe Psychiatric disorder, depression, schizophrenia and euphoria | Milk and milk products, meat and meat product | Early testing | [57] | |
| Staphylococcus aureus enterotoxin C1 | Diarrhea, vomiting and abdominal pain | Produced by S. aureus | Milk and milk products, meat and meat product, fruits and vegetable | Early testing and good hygiene practice during food handling | [58] |
| Food adulteration | |||||
| Melamine | Kidney failure | Food adulteration | Milk and milk products, meat, and meat products | Early testing | [59] |
| Veterinary drugs and pesticides residues | |||||
| Kanamycin | Ototoxicity, nephrotoxicity, allergic reaction to the drugs, vomiting, diarrhea, blurring of vision, and malabsorption syndrome | Animal breeding are stable resistance to decomposition, and elimination from biological systems | Meat and meat products and dairy products and eggs | Usage of appropriate dose and food testing | [60] |
| Chloramphenicol | Aplastic anemia and bone marrow suppression | Veterinary antibiotic used in animal breeding | Meat and meat products and dairy products and eggs | Usage of appropriate dose and food testing | [61] |
| Ractopamine | Muscle tremors, tachycardia, headache, cardiovascular and nervous system | Feed additives which are stably resistant to decomposition and elimination from biological systems | Meat and meat products and dairy products and eggs | Usage of appropriate dose and food testing | [62] |
| Streptomycin (Str) | Nephrotoxicity, Ototoxicity, vomiting and rash | Veterinary medicine used in animal breeding | Meat and meat products and dairy products and eggs | Usage of appropriate dose and food testing | [63] |
| Tetracycline | Allergen, bacteria drugs resistance | Veterinary antibiotic used in animal breeding | Meat and meat products and dairy products and eggsMeat and meat products and dairy products and eggs | Usage of appropriate dose and food testing | [64] |
| Organophosphorus pesticides | Tumors, genital change, blood and nerve disorders, endocrine disruption, coma, and leukemia | Used in agricultural pest control | cereal products, beans, coffee, fruits and vegetables | Limitation of its utilization and food testing | [65] |
| Acetamiprid | Carcinogenic, mutagenic and neurotoxic | Used in agricultural pest control | cereal products, beans, coffee, fruits and vegetables | Limitation of its utilization and food testing | [66] |
| Malathion | Carcinogenic | Used in agricultural pest control and mosquito control | cereal products, beans, coffee, fruits and vegetables | Limitation of its utilization and food testing | [67] |
2.1. Pathogenic Bacteria
2.2. Heavy Metals
2.3. Illegal Food Additives
2.4. Mycotoxins in Food
2.5. Drug and Pesticide Residues
2.6. Persistent Organic Pollutants (POPs)
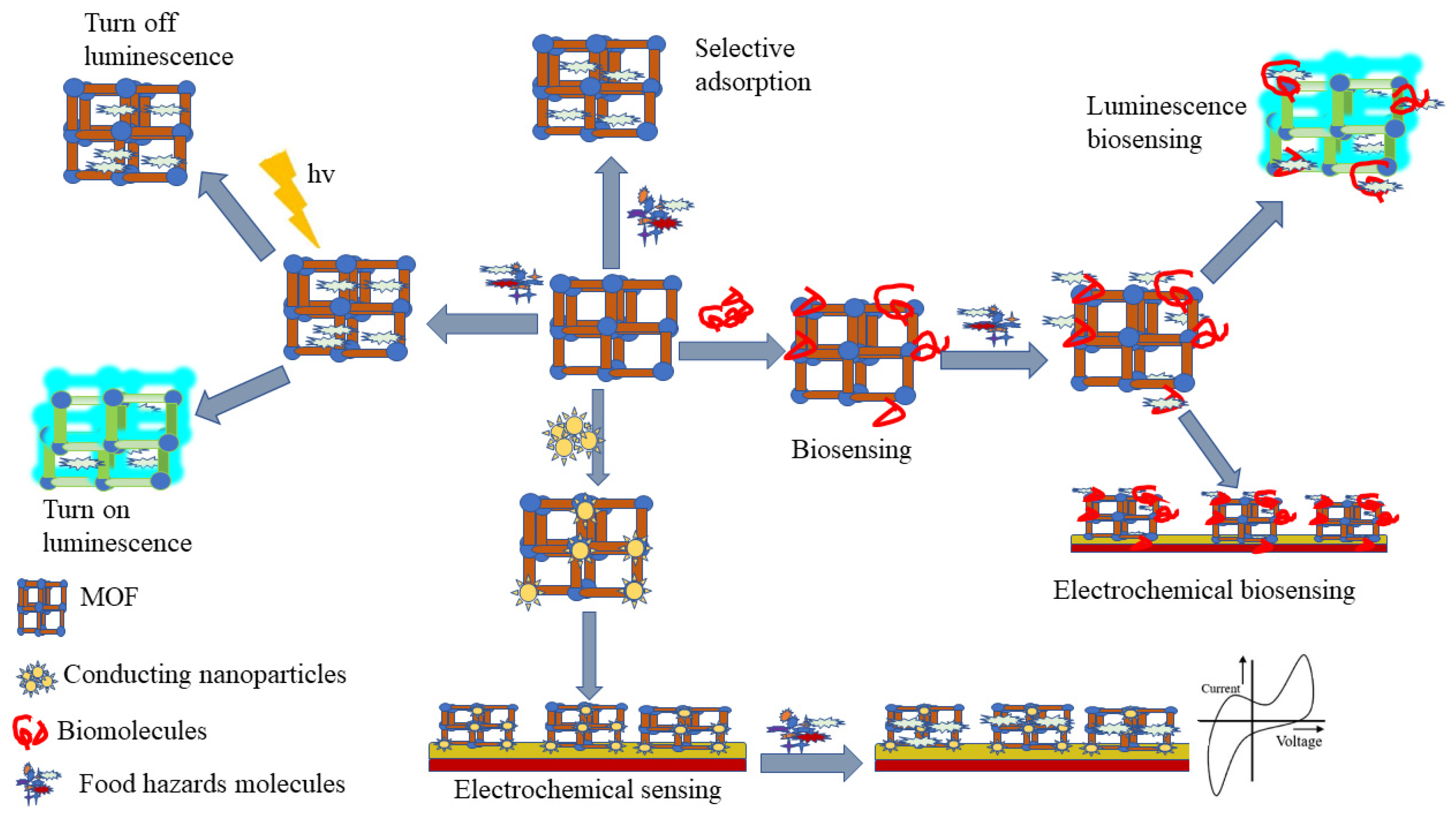
3. MOF-Based Sensors for Food Safety
3.1. MOF-Based Electrochemical-Sensing Method
3.2. MOF-Based Chemical Sensing Method
3.3. MOF-Based Biosensing Method
3.4. MOF-Based SERS Sensing Method
4. Use of MOF-Based Sensors for Food Safety Analysis
4.1. Detection of Pathogenic Bacteria
4.2. Detection of Heavy Metals
4.3. Detection of Illegal Food Additives
4.4. Detection of Natural Toxins in Food
4.5. Detection of Drug and Pesticide Residues
4.6. Persistent Organic Pollutants (POPs)
5. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef]
- Thompson, L.; Vipham, J.; Hok, L.; Ebner, P. Towards improving food safety in Cambodia: Current status and emerging opportunities. Glob. Food Secur. 2021, 31, 100572. [Google Scholar] [CrossRef]
- Liguori, J.; Trübswasser, U.; Pradeilles, R.; Le Port, A.; Landais, E.; Talsma, E.F.; Lundy, M.; Béné, C.; Bricas, N.; Laar, A.; et al. How do food safety concerns affect consumer behaviors and diets in low- and middle-income countries? A systematic review. Glob. Food Secur. 2022, 32, 100606. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Tilsala-Timisjärvi, A.; Virtanen, E. Rapid detection and identification methods for Listeria monocytogenes in the food chain—A review. Food Control 2015, 55, 103–114. [Google Scholar] [CrossRef]
- Kim, S.U.; Batule, B.S.; Mun, H.; Shim, W.-B.; Kim, M.-G. Ultrasensitive colorimetric detection of Salmonella enterica Typhimurium on lettuce leaves by HRPzyme-Integrated polymerase chain reaction. Food Control 2018, 84, 522–528. [Google Scholar] [CrossRef]
- Matumba, L.; Van Poucke, C.; Njumbe Ediage, E.; De Saeger, S. Keeping mycotoxins away from the food: Does the existence of regulations have any impact in Africa? Crit. Rev. Food Sci. Nutr. 2017, 57, 1584–1592. [Google Scholar] [CrossRef]
- Qu, J.H.; Liu, D.; Cheng, J.H.; Sun, D.W.; Ma, J.; Pu, H.; Zeng, X.A. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef]
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Xie, X.; Pu, H.; Sun, D.W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2800–2813. [Google Scholar] [CrossRef]
- Seiichi, K.; Kenichi, Y.; Kuwako, O. A proton conductive coordination polymer. I. [N,N′-Bis(2-hydroxyethyl)dithiooxamido]copper(II). Bull. Chem. Soc. Jpn. 1979, 52, 3296–3301. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-l.; Zhang, X.; Ma, Y.; Deng, Y.; Hu, R.; Yang, Y. Preparation of an OTA aptasensor based on a metal–organic framework. Anal. Methods 2018, 10, 3273–3279. [Google Scholar] [CrossRef]
- Chen, M.-L.; Chen, J.-H.; Ding, L.; Xu, Z.; Wen, L.; Wang, L.-B.; Cheng, Y.-H. Study of the detection of bisphenol A based on a nano-sized metal–organic framework crystal and an aptamer. Anal. Methods 2017, 9, 906–909. [Google Scholar] [CrossRef]
- Dong, S.; Peng, L.; Wei, W.; Huang, T. Three MOF-templated carbon nanocomposites for potential platforms of enzyme immobilization with improved electrochemical performance. ACS Appl. Mater. Interfaces 2018, 10, 14665–14672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, H.; Wei, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Metal-organic framework (MOF): A novel sensing platform for biomolecules. Chem. Commun. 2013, 49, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lan, L.; Yao, Y.; Zhao, F.; Ping, J. Recent progress in application of nanomaterial-enabled biosensors for ochratoxin A detection. TrAC Trends Anal. Chem. 2018, 102, 236–249. [Google Scholar] [CrossRef]
- Alhamoud, Y.; Yang, D.; Fiati Kenston, S.S.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Singha, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. Highly selective aqueous phase detection of azinphos-methyl pesticide in ppb level using a cage-connected 3D MOF. ChemistrySelect 2017, 2, 5760–5768. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Deep, A. Recent advancements in sensing techniques based on functional materials for organophosphate pesticides. Biosens. Bioelectron. 2015, 70, 469–481. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Basaleh, A.; Sheta, S. Manganese metal–organic framework: Chemical stability, photoluminescence studies, and biosensing application. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1726–1737. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Sharanyakanth, P.S.; Radhakrishnan, M. Synthesis of metal-organic frameworks (MOFs) and its application in food packaging: A critical review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Du, T.; Huang, L.; Wang, J.; Sun, J.; Zhang, W.; Wang, J. Luminescent metal-organic frameworks (LMOFs): An emerging sensing platform for food quality and safety control. Trends Food Sci. Technol. 2021, 111, 716–730. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–organic frameworks for food safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Recent applications of nanomaterials in food safety. TrAC Trends Anal. Chem. 2017, 96, 172–200. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; den Besten, H.M.W.; Böhnlein, C.; Gareis, M.; Zwietering, M.H.; Fusco, V. Microbial food safety in the 21st century: Emerging challenges and foodborne pathogenic bacteria. Trends Food Sci. Technol. 2018, 81, 155–158. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Y.; Wang, Y.; Fanning, S.; Cui, S.; Chen, Q.; Liu, G.; Chen, Q.; Zhou, G.; Yang, B.; et al. Serovar diversity and antimicrobial resistance of non-typhoidal Salmonella enterica recovered from retail chicken carcasses for sale in different regions of China. Food Control 2017, 81, 46–54. [Google Scholar] [CrossRef]
- Zhong, H.; Zhong, Y.; Deng, Q.; Zhou, Z.; Guan, X.; Yan, M.; Hu, T.; Luo, M. Virulence of thermolable haemolysi tlh, gastroenteritis related pathogenicity tdh and trh of the pathogens Vibrio Parahemolyticus in Viable but Non-Culturable (VBNC) state. Microb. Pathog. 2017, 111, 352–356. [Google Scholar] [CrossRef]
- Duan, N.; Ding, X.; Wu, S.; Xia, Y.; Ma, X.; Wang, Z.; Chen, J. In vitro selection of a DNA aptamer targeted against Shigella dysenteriae. J. Microbiol. Methods 2013, 94, 170–174. [Google Scholar] [CrossRef]
- Kakagianni, M.; Koutsoumanis, K.P. Assessment of Escherichia coli O157:H7 growth in ground beef in the Greek chill chain. Food Res. Int. 2019, 123, 590–600. [Google Scholar] [CrossRef]
- Duan, N.; Ding, X.; He, L.; Wu, S.; Wei, Y.; Wang, Z. Selection, identification and application of a DNA aptamer against Listeria monocytogenes. Food Control 2013, 33, 239–243. [Google Scholar] [CrossRef]
- Gong, W.; Duan, N.; Wu, S.; Huang, Y.; Chen, X.; Wang, Z. Selection, identification, and application of dual DNA aptamers against Shigella sonnei. Anal. Methods 2015, 7, 3625–3631. [Google Scholar] [CrossRef]
- Suh, S.H.; Dwivedi, H.P.; Jaykus, L.-A. Development and evaluation of aptamer magnetic capture assay in conjunction with real-time PCR for detection of Campylobacter jejuni. LWT-Food Sci. Technol. 2014, 56, 256–260. [Google Scholar] [CrossRef]
- Wang, W.; Jin, Y.; Zhao, Y.; Yue, X.; Zhang, C. Single-labeled hairpin probe for highly specific and sensitive detection of lead(II) based on the fluorescence quenching of deoxyguanosine and G-quartet. Biosens. Bioelectron. 2013, 41, 137–142. [Google Scholar] [CrossRef]
- Han, S.; Zhou, X.; Tang, Y.; He, M.; Zhang, X.; Shi, H.; Xiang, Y. Practical, highly sensitive, and regenerable evanescent-wave biosensor for detection of Hg2+ and Pb2+ in water. Biosens. Bioelectron. 2016, 80, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jarczewska, M.; Górski, Ł.; Malinowska, E. Application of DNA aptamers as sensing layers for electrochemical detection of potassium ions. Sens. Actuators B Chem. 2016, 226, 37–43. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemifard, N.; Rezaei, B. A simple and sensitive fluorimetric aptasensor for the ultrasensitive detection of arsenic(III) based on cysteamine stabilized CdTe/ZnS quantum dots aggregation. Biosens. Bioelectron. 2016, 77, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, H.; Wang, J.; Xu, L.; Chen, H.; Pei, R. Selection and characterization of DNA aptamers for the development of light-up biosensor to detect Cd(II). Talanta 2016, 154, 498–503. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Xia, Y.; Wu, S.; Duan, N.; Ma, X.; Wang, Z. Selection, identification and application of a DNA aptamer against Staphylococcus aureus enterotoxin A. Anal. Methods 2014, 6, 690–697. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Zhu, C.; Jiang, Y.; Wang, Z. Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J. Agric. Food Chem. 2014, 62, 10368–10374. [Google Scholar] [CrossRef]
- Guo, X.; Wen, F.; Zheng, N.; Luo, Q.; Wang, H.; Wang, H.; Li, S.; Wang, J. Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron. 2014, 56, 340–344. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhou, Y.; Xing, Y.; Zhang, G.-M.; Zhang, Y.; Zhang, C.-H.; Lei, P.; Dong, C.; Deng, X.; He, Y.; et al. A label-free aptasensor based on Aptamer/NH2 Janus particles for ultrasensitive electrochemical detection of Ochratoxin A. Talanta 2019, 199, 310–316. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Ma, X.; Jia, F.; Guo, X.; Wang, Z. Impedimetric aptamer-based determination of the mold toxin fumonisin B1. Microchim. Acta 2015, 182, 1709–1714. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef]
- Fu, L.-L.; Zhao, X.-Y.; Ji, L.-D.; Xu, J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. In vitro selection of DNA aptamers targeting β-lactoglobulin and their integration in graphene-based biosensor for the detection of milk allergen. Biosens. Bioelectron. 2017, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Xiao, X.; Tao, J.; Wang, D.M.; Huang, C.Z.; Zhen, S.J. A graphene oxide-based strand displacement amplification platform for ricin detection using aptamer as recognition element. Biosens. Bioelectron. 2017, 91, 149–154. [Google Scholar] [CrossRef]
- Tang, J.; Yu, T.; Guo, L.; Xie, J.; Shao, N.; He, Z. In vitro selection of DNA aptamer against abrin toxin and aptamer-based abrin direct detection. Biosens. Bioelectron. 2007, 22, 2456–2463. [Google Scholar] [CrossRef]
- Bruno, J.G.; Richarte, A.M.; Carrillo, M.P.; Edge, A. An aptamer beacon responsive to botulinum toxins. Biosens. Bioelectron. 2012, 31, 240–243. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, X.; Fu, Y.; Li, Y. Rapid methods for detecting acrylamide in thermally processed foods: A review. Food Control 2015, 56, 135–146. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Duan, N.; Wu, S.; Wang, Z.; Wei, X.; Wang, Y. Selection and characterization of DNA aptamers against Staphylococcus aureus enterotoxin C1. Food Chem. 2015, 166, 623–629. [Google Scholar] [CrossRef]
- Dong, N.; Hu, Y.; Yang, K.; Liu, J. Development of aptamer-modified SERS nanosensor and oligonucleotide chip to quantitatively detect melamine in milk with high sensitivity. Sens. Actuators B Chem. 2016, 228, 85–93. [Google Scholar] [CrossRef]
- Sharma, A.; Istamboulie, G.; Hayat, A.; Catanante, G.; Bhand, S.; Marty, J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B Chem. 2017, 245, 507–515. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, K.; Okoth, O.K.; Zhang, J. A label-free photoelectrochemical aptasensor based on nitrogen-doped graphene quantum dots for chloramphenicol determination. Biosens. Bioelectron. 2015, 74, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Gong, W.; Wu, S.; Wang, Z. An ssDNA library immobilized SELEX technique for selection of an aptamer against ractopamine. Anal. Chim. Acta 2017, 961, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem. 2016, 190, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. A novel M-shape electrochemical aptasensor for ultrasensitive detection of tetracyclines. Biosens. Bioelectron. 2016, 85, 509–514. [Google Scholar] [CrossRef]
- Tang, T.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: A universal strategy for ratiometric detection of organophosphorus pesticides. Talanta 2016, 146, 55–61. [Google Scholar] [CrossRef]
- Lin, B.; Yu, Y.; Li, R.; Cao, Y.; Guo, M. Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sens. Actuators B Chem. 2016, 229, 100–109. [Google Scholar] [CrossRef]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–449. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Dehydrated foods: Are they microbiologically safe? Crit. Rev. Food Sci. Nutr. 2019, 59, 2734–2745. [Google Scholar] [CrossRef]
- Flynn, K.; Villarreal, B.P.; Barranco, A.; Belc, N.; Björnsdóttir, B.; Fusco, V.; Rainieri, S.; Smaradóttir, S.E.; Smeu, I.; Teixeira, P.; et al. An introduction to current food safety needs. Trends Food Sci. Technol. 2019, 84, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J.J. Potential impacts of climate change on agriculture and food safety within the island of Ireland. Trends Food Sci. Technol. 2015, 44, 1–10. [Google Scholar] [CrossRef]
- Tirado, M.C.; Clarke, R.; Jaykus, L.A.; McQuatters-Gollop, A.; Frank, J.M. Climate change and food safety: A review. Food Res. Int. 2010, 43, 1745–1765. [Google Scholar] [CrossRef]
- D’Souza, R.M.; Becker, N.G.; Hall, G.; Moodie, K.B.A. Does ambient temperature affect foodborne disease? Epidemiology 2004, 15, 86–92. [Google Scholar] [CrossRef] [PubMed]
- van der Spiegel, M.; van der Fels-Klerx, H.J.; Marvin, H.J.P. Effects of climate change on food safety hazards in the dairy production chain. Food Res. Int. 2012, 46, 201–208. [Google Scholar] [CrossRef]
- Abdel-Rahman, G.N.; Ahmed, M.B.M.; Sabry, B.A.; Ali, S.S.M. Heavy metals content in some non-alcoholic beverages (carbonated drinks, flavored yogurt drinks, and juice drinks) of the Egyptian markets. Toxicol. Rep. 2019, 6, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Asgari Lajayer, B.; Najafi, N.; Moghiseh, E.; Mosaferi, M.; Hadian, J. Micronutrient and heavy metal concentrations in basil plant cultivated on irradiated and non-irradiated sewage sludge-treated soil and evaluation of human health risk. Regul. Toxicol. Pharm. 2019, 104, 141–150. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Buha, A.; Wallace, D.; Matovic, V.; Schweitzer, A.; Oluic, B.; Micic, D.; Djordjevic, V. Cadmium exposure as a putative risk factor for the development of pancreatic cancer: Three different lines of evidence. Biomed. Res. Int. 2017, 2017, 1981837. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Boadi, N.; Mensah, J.; Twumasi, S.; Badu, M.; Osei, I. Levels of selected heavy metals in canned tomato paste sold in Ghana. Food Addit. Contam. Part B 2012, 5, 50–54. [Google Scholar] [CrossRef] [PubMed]
- De Toni, L.; Tisato, F.; Seraglia, R.; Roverso, M.; Gandin, V.; Marzano, C.; Padrini, R.; Foresta, C. Phthalates and heavy metals as endocrine disruptors in food: A study on pre-packed coffee products. Toxicol. Rep. 2017, 4, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Antoine, J.M.R.; Fung, L.A.H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Ismail, A.; Omar, H.; Hussin, M.Z. Exposure of the endangered Milky stork population to cadmium and lead via food and water intake in Kuala Gula Bird Sanctuary, Perak, Malaysia. Toxicol. Rep. 2017, 4, 502–506. [Google Scholar] [CrossRef]
- Achary, M.S.; Satpathy, K.K.; Panigrahi, S.; Mohanty, A.K.; Padhi, R.K.; Biswas, S.; Prabhu, R.K.; Vijayalakshmi, S.; Panigrahy, R.C. Concentration of heavy metals in the food chain components of the nearshore coastal waters of Kalpakkam, southeast coast of India. Food Control 2017, 72, 232–243. [Google Scholar] [CrossRef]
- Adel, M.; Oliveri Conti, G.; Dadar, M.; Mahjoub, M.; Copat, C.; Ferrante, M. Heavy metal concentrations in edible muscle of whitecheek shark, Carcharhinus dussumieri (elasmobranchii, chondrichthyes) from the Persian Gulf: A food safety issue. Food Chem. Toxicol. 2016, 97, 135–140. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Rahman, M.; Sultana, S.; Babu, S.M.O.F.; Sarker, M.S.I. Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar. Pollut. Bull. 2019, 145, 436–447. [Google Scholar] [CrossRef]
- Bansal, S.; Singh, A.; Mangal, M.; Mangal, A.K.; Kumar, S. Food adulteration: Sources, health risks, and detection methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1174–1189. [Google Scholar] [CrossRef]
- Guo, J.; Liu, W.; Lan, X.; Chen, H.; Xiao, Z. Development and evaluation of an immunochromatographic strip for rapid screening of sildenafil-type compounds as illegal additives in functional foods. Food Addit. Contam. Part A 2016, 33, 1095–1104. [Google Scholar] [CrossRef]
- Stadler, R.H. Introduction to the volume: Food adulteration & contamination. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 317–319. [Google Scholar] [CrossRef]
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E.; Kozłowska-Wojciechowska, M. Drug adulteration of food supplements: A threat to public health in the European Union? Regul. Toxicol. Pharmacol. 2018, 97, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, J. Economically motivated food fraud and adulteration in China: An analysis based on 1553 media reports. Food Control 2016, 67, 192–198. [Google Scholar] [CrossRef]
- Peng, G.-J.; Chang, M.-H.; Fang, M.; Liao, C.-D.; Tsai, C.-F.; Tseng, S.-H.; Kao, Y.-M.; Chou, H.-K.; Cheng, H.-F. Incidents of major food adulteration in Taiwan between 2011 and 2015. Food Control 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of agronomic programmes with different susceptibility to deoxynivalenol risk on emerging contamination in winter wheat. Eur. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Demaegdt, H.; Daminet, B.; Evrard, A.; Scippo, M.L.; Muller, M.; Pussemier, L.; Callebaut, A.; Vandermeiren, K. Endocrine activity of mycotoxins and mycotoxin mixtures. Food Chem. Toxicol. 2016, 96, 107–116. [Google Scholar] [CrossRef]
- Amaya-Gonzalez, S.; de-los-Santos-Alvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castanon, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [Green Version]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef]
- Benkerroum, N. Mycotoxins in dairy products: A review. Int. Dairy J. 2016, 62, 63–75. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef]
- Guo, L.; Feng, J.; Fang, Z.; Xu, J.; Lu, X. Application of microfluidic “lab-on-a-chip” for the detection of mycotoxins in foods. Trends Food Sci. Technol. 2015, 46, 252–263. [Google Scholar] [CrossRef]
- Aldars-García, L.; Ramos, A.J.; Sanchis, V.; Marín, S. Modeling postharvest mycotoxins in foods: Recent research. Curr. Opin. Food Sci. 2016, 11, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.; Reddy, K.R. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Alkon, A.; Nouredini, S.; Swartz, A.; Sutherland, A.M.; Stephens, M.; Davidson, N.A.; Rose, R. Integrated pest management intervention in child care centers improves knowledge, pest control, and practices. J. Pediatric Health Care 2016, 30, e27–e41. [Google Scholar] [CrossRef] [PubMed]
- Sundlof, S.F. Veterinary drugs residues: Veterinary drugs–general. In Encyclopedia of Food Safety; Academic Press: Cambridge, MA, USA, 2014; pp. 35–38. [Google Scholar] [CrossRef]
- Mohammad Danesh, N.; Ramezani, M.; Sarreshtehdar Emrani, A.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor based on arch-shape structure of aptamer-complimentary strand conjugate and exonuclease I for sensitive detection of streptomycin. Biosens. Bioelectron. 2016, 75, 123–128. [Google Scholar] [CrossRef]
- Chandra Yadav, I.; Devi, N.L.; Li, J.; Zhang, G. Examining the role of total organic carbon and black carbon in the fate of legacy persistent organic pollutants (POPs) in indoor dust from Nepal: Implication on human health. Ecotoxicol. Environ. Saf. 2019, 175, 225–235. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Singh, K.; Chan, H.M. Persistent organic pollutants and diabetes among Inuit in the Canadian Arctic. Environ. Int. 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Govaerts, A.; Verhaert, V.; Covaci, A.; Jaspers, V.L.B.; Berg, O.K.; Addo-Bediako, A.; Jooste, A.; Bervoets, L. Distribution and bioaccumulation of POPs and mercury in the Ga-Selati River (South Africa) and the rivers Gudbrandsdalslågen and Rena (Norway). Environ. Int. 2018, 121, 1319–1330. [Google Scholar] [CrossRef]
- Imbeault, P.; Ravanelli, N.; Chevrier, J. Can POPs be substantially popped out through sweat? Environ. Int. 2018, 111, 131–132. [Google Scholar] [CrossRef]
- Malisch, R.; Kotz, A. Dioxins and PCBs in feed and food—Review from European perspective. Sci. Total Environ. 2014, 491–492, 2–10. [Google Scholar] [CrossRef]
- Bányiová, K.; Černá, M.; Mikeš, O.; Komprdová, K.; Sharma, A.; Gyalpo, T.; Čupr, P.; Scheringer, M. Long-term time trends in human intake of POPs in the Czech Republic indicate a need for continuous monitoring. Environ. Int. 2017, 108, 1–10. [Google Scholar] [CrossRef]
- Lind, P.M.; Penell, J.; Salihovic, S.; van Bavel, B.; Lind, L. Circulating levels of p,p′-DDE are related to prevalent hypertension in the elderly. Environ. Res. 2014, 129, 27–31. [Google Scholar] [CrossRef]
- Aerts, R.; Van Overmeire, I.; Colles, A.; Andjelković, M.; Malarvannan, G.; Poma, G.; Den Hond, E.; Van de Mieroop, E.; Dewolf, M.-C.; Charlet, F.; et al. Determinants of persistent organic pollutant (POP) concentrations in human breast milk of a cross-sectional sample of primiparous mothers in Belgium. Environ. Int. 2019, 131, 104979. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Xu, W.-Q.; He, S.; Liu, S.-J.; Liu, X.-H.; Qiu, Y.-X.; Liu, W.-T.; Liu, X.-J.; Jiang, L.-C.; Jiang, J.-J. Post-synthetic modification of a metal-organic framework based on 5-aminoisophthalic acid for mercury sorption. Inorg. Chem. Commun. 2019, 108, 107515. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Elsabawy, K.M.; Fallatah, A.M. Microwave assisted synthesis and molecular structure visualization of ultrahigh surface area Ni-6,6′-dibromo-indigo coordinated polymeric MOFs stabilized via hydrogen bonding. Inorg. Chem. Commun. 2018, 92, 78–83. [Google Scholar] [CrossRef]
- Kogikoski, S.; Paschoalino, W.J.; Cantelli, L.; Silva, W.; Kubota, L.T. Electrochemical sensing based on DNA nanotechnology. TrAC Trends Anal. Chem. 2019, 118, 597–605. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, H.; Shen, Y.; Su, X.; Xu, Z.; Sun, Y.; Zou, X. Attomolar determination of coumaphos by electrochemical displacement immunoassay coupled with oligonucleotide sensing. Anal. Chem. 2012, 84, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Zapp, E.; Brondani, D.; Vieira, I.C.; Scheeren, C.W.; Dupont, J.; Barbosa, A.M.J.; Ferreira, V.S. Biomonitoring of methomyl pesticide by laccase inhibition on sensor containing platinum nanoparticles in ionic liquid phase supported in montmorillonite. Sens. Actuators B Chem. 2011, 155, 331–339. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Vellingiri, K.; Samaddar, P.; Kumar, P.; Deep, A.; Kumar, N. Hybrid porous thin films: Opportunities and challenges for sensing applications. Biosens. Bioelectron. 2018, 104, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Electrochemical acetylcholinesterase biosensor based on ZnO nanocuboids modified platinum electrode for the detection of carbosulfan in rice. Biosens. Bioelectron. 2016, 77, 1070–1077. [Google Scholar] [CrossRef]
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dincă, M. High charge mobility in a tetrathiafulvalene-based microporous metal–organic framework. J. Am. Chem. Soc. 2012, 134, 12932–12935. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Wang, P.; Zhao, Y.; Sun, W.-Y. Fabrication of desired metal–organic frameworks via postsynthetic exchange and sequential linker installation. Cryst. Growth Des. 2019, 19, 1454–1470. [Google Scholar] [CrossRef]
- Valizadeh, H.; Tashkhourian, J.; Abbaspour, A. A carbon paste electrode modified with a metal-organic framework of type MIL-101(Fe) for voltammetric determination of citric acid. Microchim. Acta 2019, 186, 455. [Google Scholar] [CrossRef]
- Minh, T.T.; Phong, N.H.; Van Duc, H.; Khieu, D.Q. Microwave synthesis and voltammetric simultaneous determination of paracetamol and caffeine using an MOF-199-based electrode. J. Mater. Sci. 2018, 53, 2453–2471. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Q.; Yu, L.; Hu, X. Photoelectrochemical detection of the herbicide clethodim by using the modified metal-organic framework amino-MIL-125(Ti)/TiO2. Microchim. Acta 2015, 182, 1885–1892. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Wang, L.; Zhuang, Q.; Ni, Y. Electrochemical determination of 2,4,6-trinitrophenol using a hybrid film composed of a copper-based metal organic framework and electroreduced graphene oxide. Microchim. Acta 2018, 185, 315. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, X.; Zhao, R.; Zheng, Z.; Yuan, Q.; Dong, Z.; Gan, W. Preparation of reduced graphite oxide loaded with cobalt(II) and nitrogen co-doped carbon polyhedrons from a metal-organic framework (type ZIF-67), and its application to electrochemical determination of metronidazole. Microchim. Acta 2019, 186, 623. [Google Scholar] [CrossRef] [PubMed]
- Asadi, F.; Azizi, S.N.; Ghasemi, S. Preparation of Ag nanoparticles on nano cobalt-based metal organic framework (ZIF-67) as catalyst support for electrochemical determination of hydrazine. J. Mater. Sci. Mater. Electron. 2019, 30, 5410–5420. [Google Scholar] [CrossRef]
- Wei, L.; Huang, X.; Zheng, L.; Wang, J.; Ya, Y.; Yan, F. Electrochemical sensor for the sensitive determination of parathion based on the synergistic effect of ZIF-8 and ionic liquid. Ionics 2019, 25, 5013–5021. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Su, D.; Liu, Y.; Hu, X. Photoelectrochemical determination of malathion by using CuO modified with a metal-organic framework of type Cu-BTC. Microchim. Acta 2019, 186, 481. [Google Scholar] [CrossRef]
- Sun, D.; Deng, Q.; Long, J. Highly sensitive electrochemical sensor for estradiol based on the signal amplification strategy of Cu-BDC frameworks. J. Solid State Electrochem. 2018, 22, 487–493. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Pan, Z.; Ye, B.; Xu, M. Determination of malachite green in fish by a modified MOF-based electrochemical sensor. Food Anal. Methods 2019, 12, 1246–1254. [Google Scholar] [CrossRef]
- Tang, J.; Jiang, S.; Liu, Y.; Zheng, S.; Bai, L.; Guo, J.; Wang, J. Electrochemical determination of dopamine and uric acid using a glassy carbon electrode modified with a composite consisting of a Co(II)-based metalorganic framework (ZIF-67) and graphene oxide. Microchim. Acta 2018, 185, 486. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Yu, G.; Lin, H.; Liang, C.; Zhang, T.; Zhang, F.; Qu, F. The incorporation of bismuth(III) into metal-organic frameworks for electrochemical detection of trace cadmium(II) and lead(II). Microchim. Acta 2019, 186, 451. [Google Scholar] [CrossRef] [PubMed]
- Ezhil Vilian, A.T.; Dinesh, B.; Muruganantham, R.; Choe, S.R.; Kang, S.-M.; Huh, Y.S.; Han, Y.-K. A screen printed carbon electrode modified with an amino-functionalized metal organic framework of type MIL-101(Cr) and with palladium nanoparticles for voltammetric sensing of nitrite. Microchim. Acta 2017, 184, 4793–4801. [Google Scholar] [CrossRef]
- Yu, G.; Song, X.; Zheng, S.; Zhao, Q.; Yan, D.; Zhao, J. A facile and sensitive tetrabromobisphenol-A sensor based on biomimetic catalysis of a metal–organic framework: PCN-222(Fe). Anal. Methods 2018, 10, 4275–4281. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Wu, L.; Wu, L.; Fu, L.; Gao, Y.; Chen, J. Response characteristics of bisphenols on a metal–organic framework-based tyrosinase nanosensor. ACS Appl. Mater. Interfaces 2016, 8, 16533–16539. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Zhang, R.; Shi, H.; Wang, N.; Li, J.; Ma, F.; Zhang, D. Cu-based metal–organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sens. Actuators B Chem. 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, R.; Yuan, Q.; Wang, F. Highly sensitive electrochemical sensor for chloramphenicol based on MOF derived exfoliated porous carbon. Talanta 2017, 167, 39–43. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B Chem. 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Yang, L.; Yan, S.; Wang, M.; Cheng, D.; Chen, Q.; Dong, Y.; Liu, P.; Cai, W.; et al. The Cu-MOF-199/single-walled carbon nanotubes modified electrode for simultaneous determination of hydroquinone and catechol with extended linear ranges and lower detection limits. Anal. Chim. Acta 2015, 899, 57–65. [Google Scholar] [CrossRef]
- Xu, J.; Xia, J.; Zhang, F.; Wang, Z. An electrochemical sensor based on metal-organic framework-derived porous carbon with high degree of graphitization for electroanalysis of various substances. Electrochim. Acta 2017, 251, 71–80. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Chen, L. An ultrasensitive electrochemical bisphenol A sensor based on hierarchical Ce-metal-organic framework modified with cetyltrimethylammonium bromide. Sens. Actuators B Chem. 2018, 261, 425–433. [Google Scholar] [CrossRef]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal–organic frameworks supported surface–imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lu, J.; Yin, H.; Qiao, X.; Xu, Z. A biomimetic sensor with signal enhancement of ferriferrous oxide-reduced graphene oxide nanocomposites for ultratrace levels quantification of methamidophos or omethoate in vegetables. Food Anal. Methods 2017, 10, 910–920. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Diamantis, S.A.; Margariti, A.; Pournara, A.D.; Papaefstathiou, G.S.; Manos, M.J.; Lazarides, T. Luminescent metal–organic frameworks as chemical sensors: Common pitfalls and proposed best practices. Inorg. Chem. Front. 2018, 5, 1493–1511. [Google Scholar] [CrossRef]
- Liu, B. Metal–organic framework-based devices: Separation and sensors. J. Mater. Chem. 2012, 22, 10094–10101. [Google Scholar] [CrossRef]
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and sensing applications of metal–organic framework composites. TrAC Trends Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, L.; Han, L.; Zhang, Z.; Zhao, H. Synthesis, structure and characterization of two solvatochromic metal–organic frameworks for chemical-sensing applications. CrystEngComm 2018, 20, 2237–2240. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal-organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Li, L.; Shen, S.; Su, J.; Ai, W.; Bai, Y.; Liu, H. Facile one-step solvothermal synthesis of a luminescent europium metal-organic framework for rapid and selective sensing of uranyl ions. Anal. Bioanal. Chem. 2019, 411, 4213–4220. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, L.; Gao, L.X.; Zhu, P.P.; Chen, Q.; Tan, K.J. A highly fluorescent lanthanide metal-organic framework as dual-mode visual sensor for berberine hydrochloride and tetracycline. Anal. Bioanal. Chem. 2019, 411, 5963–5973. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Bermejo-Gómez, A.; Martín-Matute, B.; Zou, X. A water-stable lanthanide metal-organic framework for fluorimetric detection of ferric ions and tryptophan. Microchim. Acta 2017, 184, 3363–3371. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, B.; Cheng, J.; Wang, R.; Zhang, S.; Dong, S.; Wei, S.; Wang, P.; Li, J.-R. Determination and removal of clenbuterol with a stable fluorescent zirconium(IV)-based metal organic framework. Microchim. Acta 2019, 186, 454. [Google Scholar] [CrossRef]
- Gao, T.; Dong, B.-X.; Sun, Y.; Liu, W.-L.; Teng, Y.-L. Fabrication of a water-stable luminescent MOF with an open Lewis basic triazolyl group for the high-performance sensing of acetone and Fe3+ ions. J. Mater. Sci. 2019, 54, 10644–10655. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Zhang, K.; Wang, Y.; Long, W.-W.; Sa, R.-J.; Liu, T.-F.; Lü, J. Fluorescent metal–organic framework (MOF) as a highly sensitive and quickly responsive chemical sensor for the detection of antibiotics in simulated wastewater. Inorg. Chem. 2018, 57, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. Hydrolytically stable luminescent cationic metal organic framework for highly sensitive and selective sensing of chromate anions in natural water systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.; Mehta, J.; Kim, K.-H.; Deep, A. Highly sensitive detection of dipicolinic acid with a water-dispersible terbium-metal organic framework. Biosens. Bioelectron. 2016, 86, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Zheng, Y. Rapid and facile ratiometric detection of an anthrax biomarker by regulating energy transfer process in bio-metal-organic framework. Biosens. Bioelectron. 2016, 85, 287–293. [Google Scholar] [CrossRef]
- Tian, D.; Liu, X.-J.; Feng, R.; Xu, J.-L.; Xu, J.; Chen, R.-Y.; Huang, L.; Bu, X.-H. Microporous luminescent metal–organic framework for a sensitive and selective fluorescence sensing of toxic mycotoxin in moldy sugarcane. ACS Appl. Mater. Interfaces 2018, 10, 5618–5625. [Google Scholar] [CrossRef]
- Han, M.-L.; Wen, G.-X.; Dong, W.-W.; Zhou, Z.-H.; Wu, Y.-P.; Zhao, J.; Li, D.-S.; Ma, L.-F.; Bu, X. A heterometallic sodium–europium-cluster-based metal–organic framework as a versatile and water-stable chemosensor for antibiotics and explosives. J. Mater. Chem. C 2017, 5, 8469–8474. [Google Scholar] [CrossRef]
- Xu, L.; Pan, M.; Fang, G.; Wang, S. Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuators B Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Zheng, M.; Qin, M.; Wei, W. Dual-emission of Eu based metal-organic frameworks hybrids with carbon dots for ratiometric fluorescent detection of Cr(VI). Talanta 2019, 191, 519–525. [Google Scholar] [CrossRef]
- Hao, J.; Liu, F.; Liu, N.; Zeng, M.; Song, Y.; Wang, L. Ratiometric fluorescent detection of Cu2+ with carbon dots chelated Eu-based metal-organic frameworks. Sens. Actuators B Chem. 2017, 245, 641–647. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Wang, X.; Li, W.; Qi, P.; Wang, Z.; Wang, X.; Gunasekaran, S.; Wang, Q. Sensitive detection of pesticides by a highly luminescent metal-organic framework. Sens. Actuators B Chem. 2018, 260, 339–345. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Shahtaheri, S.J. Biomimetic electrochemical sensor based on molecularly imprinted polymer for dicloran pesticide determination in biological and environmental samples. J. Iran. Chem. Soc. 2016, 13, 2077–2084. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal–Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: A new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Tan, H. Integrated antibody with catalytic metal–organic framework for colorimetric immunoassay. ACS Appl. Mater. Interfaces 2018, 10, 25113–25120. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Mohanta, G.C.; Kumar, P.; Sharma, A.L.; Kim, K.-H.; Deep, A. Immunosensing of atrazine with antibody-functionalized Cu-MOF conducting thin films. ACS Appl. Mater. Interfaces 2015, 7, 26124–26130. [Google Scholar] [CrossRef]
- Hintz, H.; Wuttke, S. Postsynthetic modification of an amino-tagged MOF using peptide coupling reagents: A comparative study. Chem. Commun. 2014, 50, 11472–11475. [Google Scholar] [CrossRef] [Green Version]
- Ikezoe, Y.; Fang, J.; Wasik, T.L.; Shi, M.; Uemura, T.; Kitagawa, S.; Matsui, H. Peptide–metal organic framework swimmers that direct the motion toward chemical targets. Nano Lett. 2015, 15, 4019–4023. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, J.-W.; Huang, G.; Du, Z.-Y.; Jiang, H.-L. An amine-functionalized metal–organic framework as a sensing platform for DNA detection. Chem. Commun. 2014, 50, 12069–12072. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Hidalgo Crespo, T.; Permyakova, A.; Sicard, C.; Serre, C.; Chaussé, A.; Steunou, N.; Legrand, L. Design of metal organic framework–enzyme based bioelectrodes as a novel and highly sensitive biosensing platform. J. Mater. Chem. B 2015, 3, 8983–8992. [Google Scholar] [CrossRef] [PubMed]
- Kempahanumakkagari, S.; Kumar, V.; Samaddar, P.; Kumar, P.; Ramakrishnappa, T.; Kim, K.-H. Biomolecule-embedded metal-organic frameworks as an innovative sensing platform. Biotechnol. Adv. 2018, 36, 467–481. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Nayak, M.K.; Deep, A. Bacteriophage conjugated IRMOF-3 as a novel opto-sensor for S. arlettae. New J. Chem. 2016, 40, 8068–8073. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.; Wang, B.; Guo, Y.; Dong, X.; Zhao, J. An amino-modified metal-organic framework (type UiO-66-NH2) loaded with cadmium(II) and lead(II) ions for simultaneous electrochemical immunosensing of triazophos and thiacloprid. Microchim. Acta 2019, 186, 101. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tu, X.; Ma, X.; Fang, Q.; Liu, G.; Dai, R.; Qu, F.; Yu, Y.; Lu, L.; Huang, X. A CuO-CeO2 composite prepared by calcination of a bimetallic metal-organic framework for use in an enzyme-free electrochemical inhibition assay for malathion. Microchim. Acta 2019, 186, 567. [Google Scholar] [CrossRef] [PubMed]
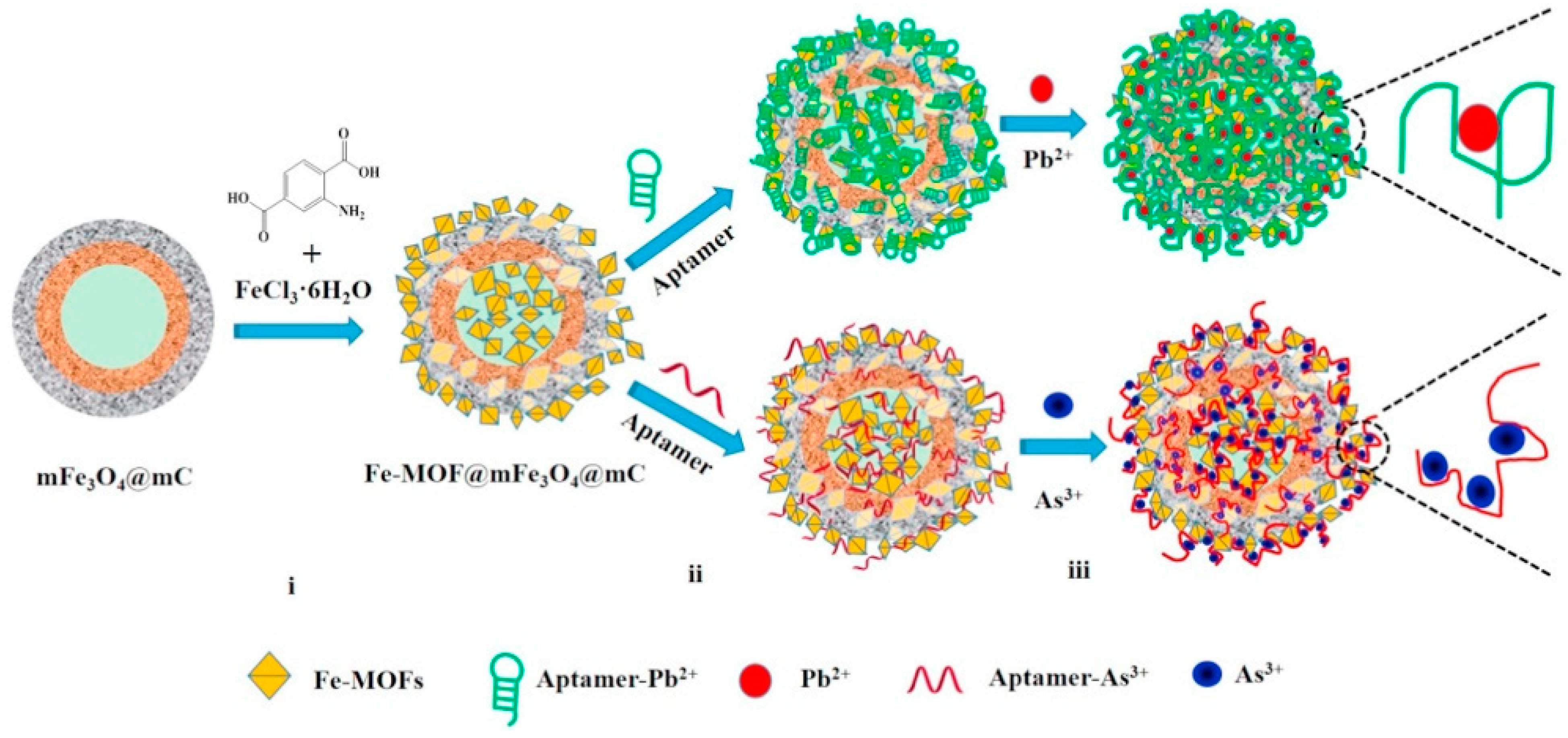
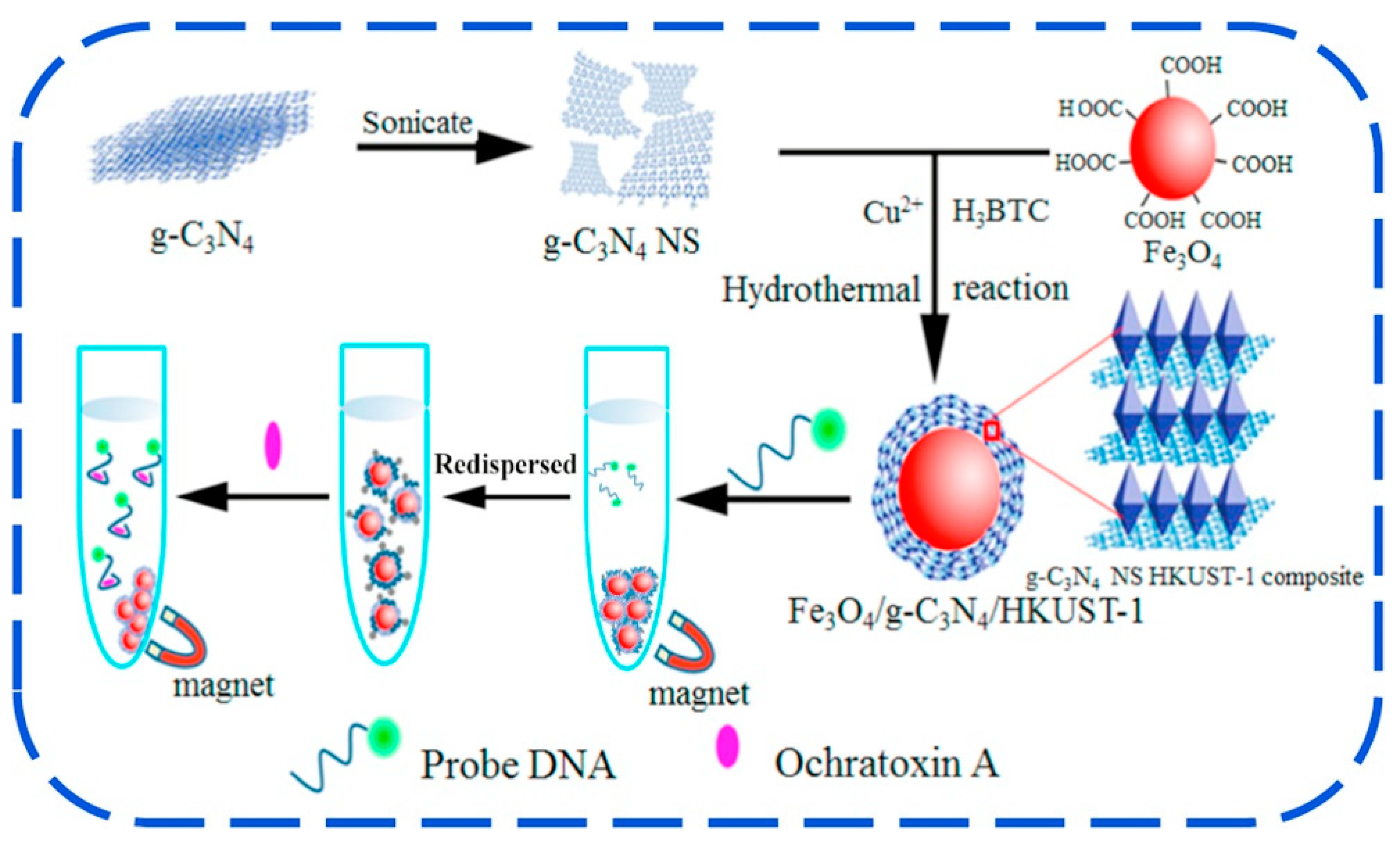
- Hu, S.; Ouyang, W.; Guo, L.; Lin, Z.; Jiang, X.; Qiu, B.; Chen, G. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens. Bioelectron. 2017, 92, 718–723. [Google Scholar] [CrossRef]
- Luan, Q.; Gan, N.; Cao, Y.; Li, T. Mimicking an enzyme-based colorimetric aptasensor for antibiotic residue detection in milk combining magnetic loop-DNA probes and CHA-assisted target recycling amplification. J. Agric. Food Chem. 2017, 65, 5731–5740. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, L.; Wu, Y.-X.; Zhang, K.; Cao, Y.; Zhou, Y.; Wu, D.; Hu, F.; Gan, N. A two dimensional metal–organic framework nanosheets-based fluorescence resonance energy transfer aptasensor with circular strand-replacement DNA polymerization target-triggered amplification strategy for homogenous detection of antibiotics. Anal. Chim. Acta 2018, 1020, 1–8. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.-H.; Deep, A. MOF–bacteriophage biosensor for highly sensitive and specific detection of staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 33589–33598. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce-MOF@COF hybrid nanostructure: Label-free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Li, T.; Wang, Y.; Xu, Q.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection using Y-shaped DNA-based metal ions encoded probes with NMOF substrate and CSRP target-triggered amplification strategy. Anal. Chim. Acta 2017, 968, 30–39. [Google Scholar] [CrossRef]
- Feng, D.; Tan, X.; Wu, Y.; Ai, C.; Luo, Y.; Chen, Q.; Han, H. Electrochemiluminecence nanogears aptasensor based on MIL-53(Fe)@CdS for multiplexed detection of kanamycin and neomycin. Biosens. Bioelectron. 2019, 129, 100–106. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dong, X. Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sens. Actuators B Chem. 2019, 294, 192–198. [Google Scholar] [CrossRef]
- Luan, Q.; Xiong, X.; Gan, N.; Cao, Y.; Li, T.; Wu, D.; Dong, Y.; Hu, F. A multiple signal amplified colorimetric aptasensor for antibiotics measurement using DNAzyme labeled Fe-MIL-88-Pt as novel peroxidase mimic tags and CSDP target-triggered cycles. Talanta 2018, 187, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Xia, F.; Tian, D.; Chen, P.; Liu, J.; Gu, J.; Zhou, C. Ultrasensitive “signal-on” electrochemical aptasensor for assay of acetamiprid residues based on copper-centered metal-organic frameworks. Anal. Chim. Acta 2019, 1050, 51–59. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Duan, F.; Hu, B.; He, L.; Wang, M.; Zhou, N.; Jia, Q.; Zhang, Z. Bimetallic cerium/copper organic framework-derived cerium and copper oxides embedded by mesoporous carbon: Label-free aptasensor for ultrasensitive tobramycin detection. Anal. Chim. Acta 2019, 1047, 150–162. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Paul, A.K.; Bharadwaj, L.M. Bioconjugation of MOF-5 for molecular sensing. J. Porous Mater. 2014, 21, 99–104. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Bharadwaj, L.M.; Paul, A.K.; Deep, A. Luminescent nanocrystal metal organic framework based biosensor for molecular recognition. Inorg. Chem. Commun. 2014, 43, 114–117. [Google Scholar] [CrossRef]
- Deep, A.; Bhardwaj, S.K.; Paul, A.K.; Kim, K.-H.; Kumar, P. Surface assembly of nano-metal organic framework on amine functionalized indium tin oxide substrate for impedimetric sensing of parathion. Biosens. Bioelectron. 2015, 65, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zeng, L.; Han, L.; Li, T.; Zheng, C.; Wei, M.; Liu, A. Ultrasensitive electrochemical sensor for p-nitrophenyl organophosphates based on ordered mesoporous carbons at low potential without deoxygenization. Anal. Chim. Acta 2014, 822, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tao, Z.; Yu, Y.; Ma, X.; Xia, Y.; Wang, L.; Wang, Z. A visual detection method for Salmonella Typhimurium based on aptamer recognition and nanogold labeling. Food Control 2014, 37, 188–192. [Google Scholar] [CrossRef]
- Suh, S.H.; Dwivedi, H.P.; Choi, S.J.; Jaykus, L.A. Selection and characterization of DNA aptamers specific for Listeria species. Anal. Biochem. 2014, 459, 39–45. [Google Scholar] [CrossRef]
- Li, Q.; Gong, S.; Zhang, H.; Huang, F.; Zhang, L.; Li, S. Tailored necklace-like Ag@ZIF-8 core/shell heterostructure nanowires for high-performance plasmonic SERS detection. Chem. Eng. J. 2019, 371, 26–33. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, J.; Wang, D.; Li, G. Fabrication of gold nanoparticle-embedded metal–organic framework for highly sensitive surface-enhanced raman scattering detection. Anal. Chem. 2014, 86, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; White, I.; DeVoe, D.L. Nanoparticle-functionalized porous polymer monolith detection elements for surface-enhanced raman scattering. Anal. Chem. 2011, 83, 2119–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435. [Google Scholar] [CrossRef]
- Qian, K.; Liu, H.; Yang, L.; Liu, J. Functionalized shell-isolated nanoparticle-enhanced Raman spectroscopy for selective detection of trinitrotoluene. Analyst 2012, 137, 4644–4646. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Barlow, S.M.; Boobis, A.R.; Bridges, J.; Cockburn, A.; Dekant, W.; Hepburn, P.; Houben, G.F.; König, J.; Nauta, M.J.; Schuermans, J.; et al. The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. Technol. 2015, 46, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Xu, X.; Xia, Y.; Wang, Z. SERS aptasensor for Salmonella typhimurium detection based on spiny gold nanoparticles. Food Control 2018, 84, 232–237. [Google Scholar] [CrossRef]
- Du, P.Y.; Gu, W.; Liu, X. Multifunctional three-dimensional europium metal-organic framework for luminescence sensing of benzaldehyde and Cu2+ and selective capture of dye molecules. Inorg Chem. 2016, 55, 7826–7828. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.K.; Bhatt, D.; Tuteja, S.K.; Kim, K.-H.; Deep, A. Highly sensitive optical biosensing of Staphylococcus aureus with an antibody/metal–organic framework bioconjugate. Anal. Methods 2019, 11, 917–923. [Google Scholar] [CrossRef]
- Singh, A.; Poshtiban, S.; Evoy, S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, A.; Zourob, M.; Allain, B.; Marquette, C.A.; Lawrence, M.F.; Mandeville, R. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal. Chem. 2008, 80, 9475–9482. [Google Scholar] [CrossRef]
- Shabani, A.; Marquette, C.A.; Mandeville, R.; Lawrence, M.F. Magnetically-assisted impedimetric detection of bacteria using phage-modified carbon microarrays. Talanta 2013, 116, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.-B.; Hosseini, S.R.; Ojani, R.; Mandegarzad, S. MOF-derived Cu/nanoporous carbon composite and its application for electro-catalysis of hydrogen evolution reaction. Energy 2015, 90, 1075–1081. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q.; Yun, H. (Metal-organic framework)-polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J. Power Source 2016, 316, 176–182. [Google Scholar] [CrossRef] [Green Version]
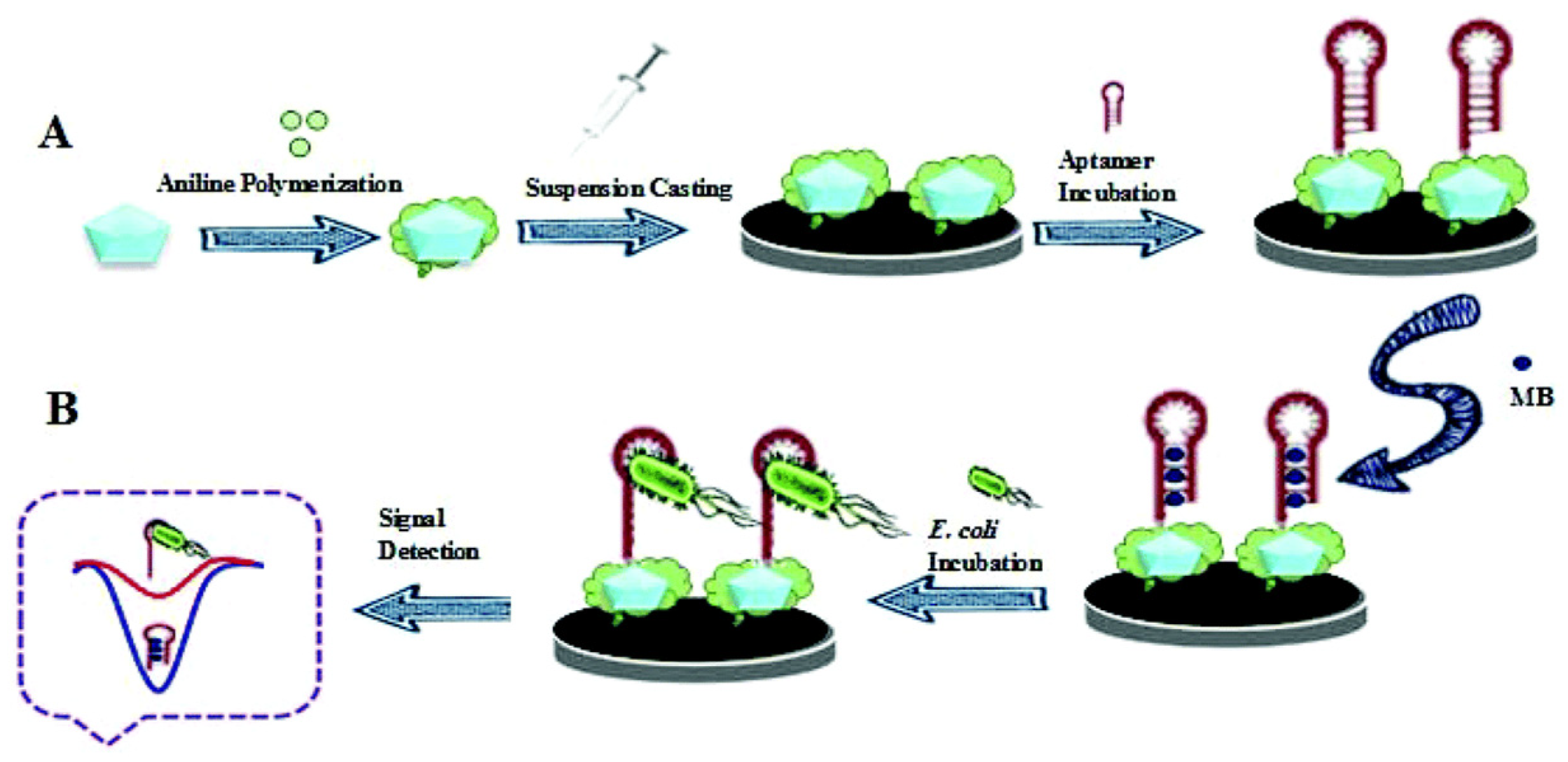
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal-organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157:H7. Analyst 2018, 143, 3191–3201. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, X.; Li, K.; Liu, R.; Peng, D.; He, L.; Wang, M.; Zhang, H.; Zhou, L. One-step fabrication of electrochemical biosensor based on DNA-modified three-dimensional reduced graphene oxide and chitosan nanocomposite for highly sensitive detection of Hg(II). Sens. Actuators B Chem. 2016, 225, 453–462. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wu, H. Reusable DNA-functionalized-graphene for ultrasensitive mercury (II) detection and removal. Biosens. Bioelectron. 2017, 87, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, H.; Guo, Z.; Wang, Y.; Ma, H.; Ren, X.; Du, B.; Wei, Q. A “turn-off” fluorescent biosensor for the detection of mercury (II) based on graphite carbon nitride. Talanta 2017, 162, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, T.; Zhang, J.; Cui, Y.; Li, B.; Yang, Y.; Qian, G. A manganese-based metal-organic framework electrochemical sensor for highly sensitive cadmium ions detection. J. Solid State Chem. 2019, 275, 38–42. [Google Scholar] [CrossRef]
- Liu, B.-H.; Liu, D.-X.; Yang, K.-Q.; Dong, S.-J.; Li, W.; Wang, Y.-J. A new cluster-based metal-organic framework with triazine backbones for selective luminescent detection of mercury(II) ion. Inorg. Chem. Commun. 2018, 90, 61–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.Y.; He, L.; Zhang, X.; Liu, C.S. Fe(III)-based metal-organic framework-derived core-shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, H.; Zhu, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G.; Chen, Z.-N. Metal–organic frameworks-based biosensor for sequence-specific recognition of double-stranded DNA. Analyst 2013, 138, 3490–3493. [Google Scholar] [CrossRef]
- Wang, D.; Ke, Y.; Guo, D.; Guo, H.; Chen, J.; Weng, W. Facile fabrication of cauliflower-like MIL-100(Cr) and its simultaneous determination of Cd2+, Pb2+, Cu2+ and Hg2+ from aqueous solution. Sens. Actuators B Chem. 2015, 216, 504–510. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef]
- Wan, Y.; Zou, D.; Cui, Y.; Yang, Y.; Qian, G. A Zn based anionic metal-organic framework for trace Hg2+ ion detection. J. Solid State Chem. 2018, 266, 70–73. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Cagliani, L.R.; Tarantilis, P.A.; Polissiou, M.G.; Consonni, R. Sudan dyes in adulterated saffron (Crocus sativus L.): Identification and quantification by 1H NMR. Food Chem. 2017, 217, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Siddiquee, S. A review of recent advances in melamine detection techniques. J. Food Compos. Anal. 2015, 43, 25–38. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S. Analytical and advanced methods-based determination of melamine in food products. In Nanobiosensors; Academic Press: Cambridge, MA, USA, 2017; pp. 339–390. [Google Scholar] [CrossRef]
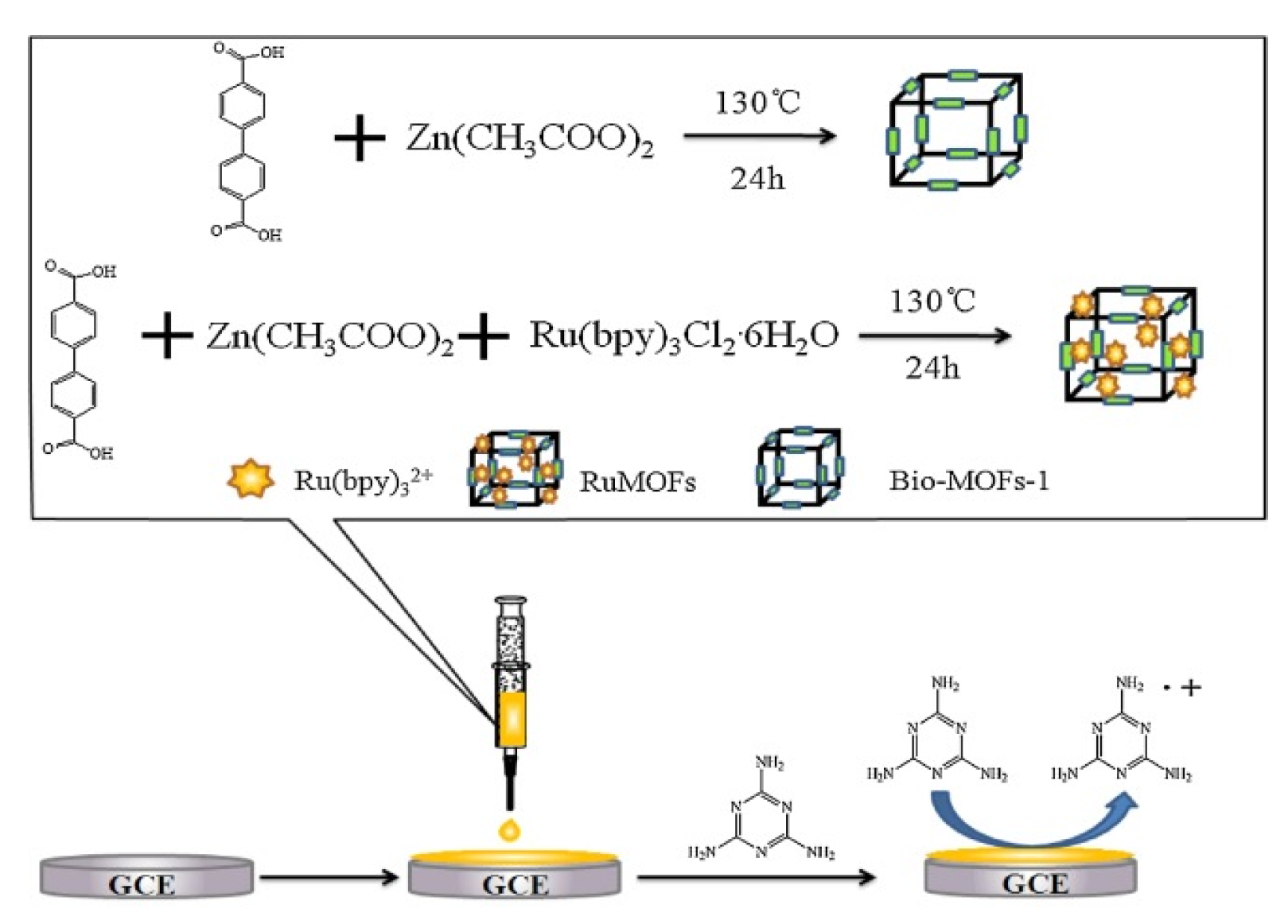
- Feng, D.; Wu, Y.; Tan, X.; Chen, Q.; Yan, J.; Liu, M.; Ai, C.; Luo, Y.; Du, F.; Liu, S.; et al. Sensitive detection of melamine by an electrochemiluminescence sensor based on tris(bipyridine)ruthenium(II)-functionalized metal-organic frameworks. Sens. Actuators B Chem. 2018, 265, 378–386. [Google Scholar] [CrossRef]
- Li, Q.; Xu, P.; Gao, W.; Ma, S.; Zhang, G.; Cao, R.; Cho, J.; Wang, H.-L.; Wu, G. Graphene/graphene-tube nanocomposites templated from cage-containing metal-organic frameworks for oxygen reduction in Li–O2 batteries. Adv. Mater. 2014, 26, 1378–1386. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Liu, S.-X.; Liang, D.-D.; Shao, K.-Z.; Ren, Y.-H.; Su, Z.-M. Highly stable crystalline catalysts based on a microporous metal−organic framework and polyoxometalates. J. Am. Chem. Soc. 2009, 131, 1883–1888. [Google Scholar] [CrossRef]
- Saha, S.; Das, G.; Thote, J.; Banerjee, R. Photocatalytic metal–organic framework from CdS quantum dot incubated luminescent metallohydrogel. J. Am. Chem. Soc. 2014, 136, 14845–14851. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, Y.; Wang, J.; Zheng, B.; Yuan, H.; Xiao, D. Anodic electrogenerated chemiluminescence behavior of graphite-like carbon nitride and its sensing for rutin. Anal. Chem. 2013, 85, 2601–2605. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, H.; Zhang, Q.; Xie, J.; Tian, Y.; Wang, J.; Xie, Y. Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 2014, 26, 4438–4443. [Google Scholar] [CrossRef]
- Ping, J.; Zhou, Y.; Wu, Y.; Papper, V.; Boujday, S.; Marks, R.S.; Steele, T.W.J. Recent advances in aptasensors based on graphene and graphene-like nanomaterials. Biosens. Bioelectron. 2015, 64, 373–385. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Ding, W.; Li, G. Multilayer interparticle linking hybrid MOF-199 for noninvasive enrichment and analysis of plant hormone ethylene. Anal. Chem. 2014, 86, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective detection of mycotoxins by a highly luminescent metal-organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Q.; Hu, Z.; Deibert, B.J.; Emge, T.J.; Teat, S.J.; Banerjee, D.; Mussman, B.; Rudd, N.D.; Li, J. Solution processable MOF yellow phosphor with exceptionally high quantum efficiency. J. Am. Chem. Soc. 2014, 136, 16724–16727. [Google Scholar] [CrossRef]
- Bhandari, G.; Zomer, P.; Atreya, K.; Mol, H.G.J.; Yang, X.; Geissen, V. Pesticide residues in Nepalese vegetables and potential health risks. Environ. Res. 2019, 172, 511–521. [Google Scholar] [CrossRef]
- Alister, C.; Araya, M.; Becerra, K.; Volosky, C.; Saavedra, J.; Kogan, M. Industrial prune processing and its effect on pesticide residue concentrations. Food Chem. 2018, 268, 264–270. [Google Scholar] [CrossRef]
- Bacanli, M.; Basaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Burns, L.E.; Borts, D.J. Rapid untargeted screening for drug residues in animal tissues with liquid microjunction surface sampling probe mass spectrometry. Anal. Chim. Acta 2019, 1063, 75–81. [Google Scholar] [CrossRef]
- Lozano, A.; Hernando, M.D.; Ucles, S.; Hakme, E.; Fernandez-Alba, A.R. Identification and measurement of veterinary drug residues in beehive products. Food Chem. 2019, 274, 61–70. [Google Scholar] [CrossRef]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total Environ. 2018, 640–641, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Parrilla Vázquez, P.; Ferrer, C.; Martínez Bueno, M.J.; Fernández-Alba, A.R. Pesticide residues in spices and herbs: Sample preparation methods and determination by chromatographic techniques. TrAC Trends Anal. Chem. 2019, 115, 13–22. [Google Scholar] [CrossRef]
- Liu, Q.; Ning, D.; Li, W.J.; Du, X.M.; Wang, Q.; Li, Y.; Ruan, W.J. Metal-organic framework-based fluorescent sensing of tetracycline-type antibiotics applicable to environmental and food analysis. Analyst 2019, 144, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, N.-Y.; Ruan, Q.; Lu, D.-Q.; Yang, Y.-H.; Hu, R. A label-free and sensitive photoluminescence sensing platform based on long persistent luminescence nanoparticles for the determination of antibiotics and 2,4,6-trinitrophenol. RSC Adv. 2018, 8, 5714–5720. [Google Scholar] [CrossRef] [Green Version]
- Vikrant, K.; Tsang, D.C.W.; Raza, N.; Giri, B.S.; Kukkar, D.; Kim, K.H. Potential utility of metal-organic framework-based platform for sensing pesticides. ACS Appl. Mater. Interfaces 2018, 10, 8797–8817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Cao, X.; Hong, S.; Jiang, Z.; She, Y.; Wang, S.; Zhang, C.; Li, H.; Jin, F.; Jin, M.; Wang, J. SERS-active metal–organic frameworks with embedded gold nanoparticles. Analyst 2017, 142, 2640–2647. [Google Scholar] [CrossRef]
- Khan, P.M.; Baderna, D.; Lombardo, A.; Roy, K.; Benfenati, E. Chemometric modeling to predict air half-life of persistent organic pollutants (POPs). J. Hazard. Mater. 2020, 382, 121035. [Google Scholar] [CrossRef]
- McComb, J.; Mills, I.G.; Muller, M.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Human blood-based exposure levels of persistent organic pollutant (POP) mixtures antagonise androgen receptor transactivation and translocation. Environ. Int. 2019, 132, 105083. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Wang, L.; Fan, G.; Xu, X.; Chen, D.; Wang, L.; Shi, W.; Cheng, P. Detection of polychlorinated benzenes (persistent organic pollutants) by a luminescent sensor based on a lanthanide metal–organic framework. J. Mater. Chem. A 2017, 5, 5541–5549. [Google Scholar] [CrossRef]
| MOF Synthesis Process | Application in Detection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target Food Hazards | MOF | Modification | Metal/Metal Cluster Source | Ligand Source | Solvent | Time (h) | Temperature (°C) | Real Samples | Linear Range | DL | Reference |
| Citric acid | MIL-101(Fe) | CPE | FeCl3·6H2O | Terephthalic acid | DMF | 20 | 110 | beverage | 5 × 10−6– 100 × 10−6 M | 44 × 10−6 M | [133] |
| Paracetamol(PC) and caffeine(C) | MOF-199 (HKUST-1) | GCE | Cu(NO3)2·3H2O | H3BTC | Ethanol, water and DMF | 8 | 180 | pharmaceutical tablets | C: 1.2 μM | [134] | |
| PC:1.3 μM | |||||||||||
| Clethodim | MIL-125(Ti)/TiO2 | GCE | TBT | H2BDC-NH2 | DMF and methanol | 20 | 150 | Soil | 0.2–25 (μmol L−1) | 0.03 (μmol L−1) | [135] |
| 2,4,6-trinitrophenol | C-BTC MOF | GCE | Cu(NO3)2 | H3BTC | DMF and ethanol | 12 | 120 | water | 0.2–10 μM | 0.1 μM | [136] |
| Metronidazole | ZIF-67 MOF | GCE | Co(NO3)2·6H2O | 2-methyldinazole | water | 5 | 90 | water | 0.5–1000 μM | 0.05 μM | [137] |
| Hydrazine | ZIF-67 | CPE and AgN | Cobalt nitrate hexahydrate | 2-methylimidazole | methanol | 24 | room temperature (RT) | 4–326 μM | 1.45μM | [138] | |
| Parathion | ZIF-8MOF | CPE | Zn(NO3)2·6H2O | 2-methylimidazole | methanol | 24 | RT | vegetables | 5.0–700 μg/L | 2.0 μg/L | [139] |
| Malathion | Cu-BTC MOF | CUO | Zn(NO3)2·6H2O | H3BTC | Acetic acid and TEA | 24 | 85 | Chinese cabbage | 10−10–1.0 × 10−5 mol L−1 | 8.6 × 10−11 mol L−1 | [140] |
| estradiol | Cu-BDC MOF | CPE | Cu(OAc)2·H20 | H2BDC | DMF and water | 2 | RT | water | 5 to 650 nM | 3.8 nM | [141] |
| Malachite green | Ag/Cu MOF | GCE | Cu(NO3)2·3H2O and | BTC | Water and ethanol | 14 | 120 | fish | 10–140 nM | 2.2 nM | [142] |
| Hydroquinone (HQ) and catechol (CT) | FJU-40-H/NH2 MOFs | NPC | Zn(NO3)2·6H2O | BDC and Trz | Water, ethanol and DMF | 24 | 85 | water | HQ = 1–70 µmol L−1 | HQ = 0.18 µmol L−1 | [143] |
| CT = 1–100 µmol L−1 | CT = 0.31 µmol L−1 | ||||||||||
| Cd (II) and Pb (II) | Bi/MIL-101 (Cr) MOF | CrCl3·6H2O | TPA | water | 20 | 200 | water | Cd (II) and Pb (II) = 0.1 ~90 μg L−1 | Cd2+:0.06 μg L−1 | [144] | |
| Pb2+: 0.07 μg L−1 | |||||||||||
| Nitrite | NH2-MIL-101(Cr) MOF | SPCE | Cr (NO3)3·9H2O | 2-aminoterephthalicacid | NaOH | 16 | 160 | sausage and pickle | 5.00 × 10−6–1.5 × 10−4 nM | 1.3 nM | [145] |
| tetrabromobisphenol | PCN-222(Fe) MOF | acetylene black | ZrCl4 and Fe-TCPP | benzoic acid | DMF | 48 | 120 | water | 0.001–1.0 μmol L−1 | 0.57 nmol L−1 | [146] |
| Bisphenols (BPs: BPE, BPF, BPA, BPB, and BPZ) | Cu-MOF | GCE | copper nitrate trihydrate | Triethylenediamine and benzene dicarboxylic acid | DMF | 36 | 120 | wastewater | BPE: 5.0 × 10−8 to 3.0 × 10−6 nM | BPE:15 nM | [147] |
| BPF: 5.0 × 10−8 to 3.0 × 10−6 nM | BPF:16 nM | ||||||||||
| BPA: 5.0 × 10−8 to 3.0 × 10−6 nM | BPA:13 nM | ||||||||||
| BPB: 1.25 × 10−7 to 8.0 × 10−6 nM | BPB:56 nM | ||||||||||
| BPZ: 2.5 × 10−7 to 5.0 × 10−6 nM | BPZ:33 nM | ||||||||||
| Nitrite | Cu-MOF (MOF-14) | CPE | Cu(OH)2 | H3BTB | DMF, DMSO, DW and HNO3 | 48 | 100 | lake water | 50 nM–717.2 μM | 30 nM | [148] |
| chloramphenicol | IRMOF-8 | GCE | zinc nitrate hexahydrate | 2,6-naphthalenedicarboxylic acid | DMF | 20 | 120 | honey | 1 × 10−8–1 × 10−6 mol L−1 and 1 × 10−6–4 × 10−6 mol L−1 | 2.9 × 10−9 mol L−1 | [149] |
| nitrite | Cu-MOF | GCE | CuCl2 | PVP | Water, NaOH and ascorbic acid | 5 min | RT | water | 0.1–4000 and 4000–10,000 μM | 82 nM | [150] |
| hydroquinone (HQ) and catechol (CT). | Cu-MOF-199 | GCE and SWCNTs | Cu(NO3)2·3H2O | H3BTC | DMF and ethanol | 12 | 120 | water | HQ: 0.1 to 1453 μmol L−1 | HQ: 0.08 μmol L−1 | [151] |
| CT: 0.1–1150 μmol L−1 | CT: 0.1 μmol L−1 | ||||||||||
| uric acid (UA) catechol (CT) hydroquinone (HQ) | ZIF-8 | GCE | (NO3)2·6H2O | 2-methylimidazole | Water and 2-methylimidazole | RT | 30 min | water and seawater | UA and CT:0.001–0.3 HQ: 0.001–0.2 mM | UA: 1.4 × 10−8 M | [152] |
| CT: 2.78 × 10−7 M | |||||||||||
| HQ:2.15 × 10−7 M | |||||||||||
| HQ and CT | Cu-MOF | GCE | Cu(NO3)2·6H2O | H3BTC | DW and ethanol | 150 | 24 | tap water | HQ: 1.0 × 10−6–1.0 × 10−3 M | HQ: 5.9 × 10−7 M | [153] |
| CT: 1.0 × 10−6–1.0 × 10−3 M | CT: 3.3 × 10−7 M | ||||||||||
| HQ, CT and RS | UiO-66 MOF | GCE | ZrCl4 | BDG | DMF and acetic acid | 120 | 24 | lake water | HQ: 0.056 μM | [154] | |
| CT: 0.072 μM | |||||||||||
| RS: 3.51 μM | |||||||||||
| bisphenol A (BPA) | Ge-MOF | GCE | Ce(NO3)3·6H2O | 1,3,5-H3BTC | Water and ethanol | RT | 10 min | fresh milk | 0.005–50 μmo L−1 | 0.092 μmol L−1 | [155] |
| metolcarb | MIL-101 | MIP and QCM | Cr(NO3)·39H2O | terephthalic acid (TPA) | HF and DDW | 220 | 8 | pear juice | 0.1–0.9 mg L−1 | 0.0689 mg L−1 | [156] |
| Methamidophos (omethoate) | MIL-101(Cr) | GO | (Cr(NO3)3·9H2O), | (C6H4–1,4-(CO2H)2), | hydrofluoric acid and DDW | 200 | 8 | cucumber and kidney bean | 1.0 × 10−7–1.0 × 10−12 and 1.0 × 10−7–1.0 × 10−13 mol/L | 2.67 × 10−13 mol/L and 2.05 × 10−14 mol/L | [157] |
| MOF Synthesis | Application in Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target Food Hazards | MOF | Metal/Metal Cluster Source | Ligand Source | Solvent | Time (h) | Temperature (°C) | Sample | Linear Range | DL | Reference |
| UO22+ | EU-MOF | Eu(NO3)3·6H2O | H3TATAB | DMF and H2O | 72 | 120 | 12.5–87.5 μM | 0.9 μM | [168] | |
| Berberine hydrochloride (BRH) and tetracycline (TC) | Eu-MOF 1 | Eu(NO3)3·6H2O | Terephthalic acid and Hartz | DMF/H2O | 27 | 150 | urine | BRH = 0.5–320 Μm | BRH = 78 nM | [169] |
| TC = 0.05 to 160 | TC = 17 nM | |||||||||
| Fe2+ | SUMOF-7II | LaCl3·7H2O | 2,4,6-tri-p-carboxyphenylpyridine(H3L2) | DMF, Cyclohexane and water | 16 | 85 | 16.6 μM | 16.6–167 μM | [170] | |
| Clenbuterol | UiO-66 MOF | ZnCl4 | 1,4-benzenedicarboxylic acid | DMF and HCl | 16 | 220 | pig and sheep urine | 4–40 ng/mL | 0.17 μM | [171] |
| Acetone and Fe3+ | ([Cd1.5(DBPT)(DiPyDz)(H2O)]· 3.5H2O)n (1) MOF | Cd(NO3)2·4H2O | H3DBPT and 4-DiPyDz | DMA/water | 72 | 130 | 0.0025–0.025 mM | Acetone = 0.0013% (v/v%) Fe3+ = 78 ppb | [172] | |
| Sulphonamide Antibiotics | FSC-1 MOF | Zn(NO3)2·6H2O | H3L and NaHCO3 | water | 72 | 130 | wastewater | [173] | ||
| Cr(VI) | 1, H4mtb MOF | Eu(NO3)3·6H2O | N,N-dimethylacetamide | DMA and DW | 48 | 90 | water | 1 ppb to 300 ppm | DW = 0.56, LW = 2.88, and SW = 1.75 ppb | [174] |
| Dipicolinic acid (DPA) | Tb-MOF | Tb(NO3)3·5H2O | H3BTC | DW and ethanol | 1 | room temperature | 1 nM to 100 μM | 0.04 nM | [175] | |
| DPA | Bio-MOF-1 | zinc acetate dihydrate | 4,4′-biphenyl dicarboxylic acid and adenine | DMF, water and nitric acid | 48 | 130 | human serum | 34 nM | [176] | |
| 3-nitropropionic acid (3-NPA) | Cd(L)·solvent]n (1) | Cd(NO3)2·6H2O | H2L | DMF | 72 | 85 | sugarcane | 0.135 M | [177] | |
| oridazole antibiotics | CTGU-7 MOF | Eu(NO3)3·6H2O and Na3TATAB | DMF | DMF and water | 140 | 72 | 1 μM to 50 μM | 0.8 μM | [178] | |
| quercetin | ZIF-8 | Zn(NO3)2·6H2O | 2-Hmin | Methanol | RT | 1 | Ginkgo biloba extract capsules | 0–50.0 μM | 2.9 nM | [179] |
| Cr(VI) | Eu-MOFs) | Eu(NO3)3·6H2O | H3BTC | DMF and water | 100 | 24 | water | 2 μM to 100 μM | 0.21 μM | [180] |
| Cu2+ | Eu-DPA MOFs | Eu(NO3)3·6H2O | DPA | Ethanol | 180 | 73 | water | 50−1 × 104 nM | 26.3 nM | [181] |
| parathion-methyl | znPO-MOFs | Zn(NO3)2·6H2O | H4TCPB | DMF | 100 | 48 | water | 1.0 μg L−1–10 mg L−1 | 0.12 μg L−1 | [182] |
| MOF Synthesis | Application in Detection | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensing Method | Target Food Hazards | MOF | Biomolecules | Metal/Metal Cluster Source | Ligand Source | Solvent | Time (h) | Temperature (°C) | Sample | Linear Range | DL | Reference |
| Electrochemical | triazophos (TRS) and thiacloprid (THD) | UiO-66-NH2 | antibody | ZnCl4 | 2-aminoterephthalic acid | DMF and acetic acid (AC) | 8 | 120 | rice | TRS = 0.2–750 ng. mL−1 | TRS = 0.07 ng. mL−1 | [197] |
| THD = 0.2–750 ng. mL−1 | THD = 0.1 ng. mL−1 | |||||||||||
| Electrochemical | Malathion | Cu/Ce-BTC MOF | enzyme | Cu(OAc)2·3H2O and Ce(NO3)3·6H2O (n(Cu):n(Ce) | H3BDC | DMF and water | 4 | 100 | water | 10 fM–100 nM | 3.3 fM | [198] |
| Fluorescent | Ochratoxin A | HKUST-1 | Aptamer | Cu(NO3)2·3H2O | H3BTC | Water and ethanol | 12 | 120 | corn | 5.0–160 ng/mL | 2.57 ng/mL | [199] |
| Colorimetric | kanamycin | Fe-MIL-88NH2 | Aptamer | FeCl3·6H2O | H2N-BDC | DMF and AC | 4 | 120 | milk | 0.0005–30 ng mL–1 | 0.2 pg | [200] |
| chloramphenicol | Cu-TCPP | Aptamer | Cu (NO3)2·3H2O and CF3COOH | PVP and TCPP | DMF and ethanol | 3 | 80 | milk and fish | 0.001–10 ng mL−1 | 0.3 pg mL−1 | [201] | |
| photoluminescence | S. aureus | NH2-MIL-53(Fe) | Bacteriophages | FeCl3·6H2O | NH2-BDC | DW | 72 | 150 | pastry cream | 40–4 × 108 CFU/mL | 31 CFU/mL | [202] |
| fluorescent | S. arlettae | IRMOF-3′ | Bacteriophage | Zn(NO3)2·6H2O | 2-amino terephthalic acid | DMF | RT | 2 | river water | 102–108 cfu mL−1 S | 100 cfu /mL | [196] |
| Electrochemical | oxytetracycline | Ce-MOF@COF | Aptamer | Ce(NO3)3·6H2O | Cyanure acid and melamine H3BTC | Water and ethanol | 90 | 2 | milk, water, and urine | 2 × 10−4–1.0 nM | 35.0 fM | [203] |
| Electrochemical | ampicillin (AMP) | Co-MOF | Aptamer | Co(NO3)2·6H2O | 2-methylimidazole | water | RT | 2 | water and milk | 0.001–2000 pg mL−1 | 0.217 fg mL−1 | [204] |
| fluorescence | Bisphenol A | Fe-MIL-88B–NH2 | Aptamer | FeCl3·6H2O | H2N-BDC | Water and AC | 4 | 120 | 2.0 × 10−9 to 5.0 × 10−14 mol L−1 | 4.1 × 10−14 mol L−1 | [12] | |
| Immunosensing | Atrazine | Cu-MOF | Antibody | Cu (OAc)2·H2O and (TEOS) | (H3BTC) | Water ethanol and NaOH | 2 | RT | water | 0.01 nM–1 μM | 0.01 nM | [190] |
| electrochemical | Antibiotics (CAP and OTC) | UiO-66-NH2 and UiO-66 | Aptamer | ZrCl4 | H2N-H2BDC and H2BDC | DMF and AC | 8 | 120 | milk | 0.0001–50 nM | CAP:33 fM OTC:48 fM | [205] |
| Antibiotics (KANA CAP) | UiO-66-NH2 | Aptamer | ZrCl4 | H2N-H2BDC and H2BDC | DMF and AC | 8 | 120 | milk | KANA:0.16 pM CAP: 0.19 pM | [205] | ||
| electrochemical | kanamycin and neomycin | MIL-53(Fe) | Aptamer | FeCl3·6H2O | H2BDC | DMF | 65 | 120 | Milk and honey | 1.0 × 10−10–1.0 × 10−6 M | 1.7 × 10−11 M | [206] |
| patulin (PAT) | UiO-66-NH2 | Aptamer | ZrCl4 | BDC-NH2 | DMF | 8 | 120 | Apple Juice | 5 × 10−8−5 × 10−1 μg mL−1 | 1.46 × 10−8 μg mL−1 | [207] | |
| Fluorescent | OTA | HKUST-1 | Aptamer | Cu(NO3)2·3H2O | H3BTC | Water and ethanol | 12 | 120 | corn | 5.0–160.0 ng/mL | 2.57 ng/mL | [199] |
| OTA | MOF-74 | Aptamer | Cu(NO3)2·3H2O cadmium acetate dihydrate | DHTA | DMF | 125 | 20 | Red wine | 0.05–100 ng mL−1 | 10 pg mL−1 | [11] | |
| Colorimetric | chloramphenicol | Fe-MIL-88 | Aptamer | FeCl3·6H2O | terephthalic acid | DMF and AC | 4 | 120 | milk | 0.1 pM–1000 pM | 0.03 pM | [208] |
| Electrochemical | acetamiprid | Au-Cu-MOF | Aptamer | CuCl2 | Trimesic acid (TMA) | Water and NaOH | RT | 12 | tea | 0.1 pM to 10.0 nM | 2.9 fM | [209] |
| Electrochemical | Tobramycin (TOB) | Ce/Cu-MOF | Aptamer | Ce(NO3)3·6H2O and Cu(NO3)2·3H2O | H3BTC | Ethanol and water | RT | 24 | Milk and human serum | 0.01 pg mL−1–10 ng mL−1 | 2.0 fg mL−1 | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitabatuma, A.; Wang, P.; Su, X.; Ma, M. Metal-Organic Frameworks-Based Sensors for Food Safety. Foods 2022, 11, 382. https://doi.org/10.3390/foods11030382
Hitabatuma A, Wang P, Su X, Ma M. Metal-Organic Frameworks-Based Sensors for Food Safety. Foods. 2022; 11(3):382. https://doi.org/10.3390/foods11030382
Chicago/Turabian StyleHitabatuma, Aloys, Peilong Wang, Xiaoou Su, and Mengmeng Ma. 2022. "Metal-Organic Frameworks-Based Sensors for Food Safety" Foods 11, no. 3: 382. https://doi.org/10.3390/foods11030382
APA StyleHitabatuma, A., Wang, P., Su, X., & Ma, M. (2022). Metal-Organic Frameworks-Based Sensors for Food Safety. Foods, 11(3), 382. https://doi.org/10.3390/foods11030382







