Extraction of Natural Gum from Cold-Pressed Chia Seed, Flaxseed, and Rocket Seed Oil By-Product and Application in Low Fat Vegan Mayonnaise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gum Extraction
2.2. Gum Characterization
2.3. Preparation of Gum Solutions
2.4. Rheological Properties
2.5. Flow Behavior Rheological Properties
2.6. Thixotropic Properties
2.6.1. Constant Shear Rate
2.6.2. 3-ITT Rheological Properties
2.6.3. Dynamical Rheological Properties
2.7. Vegan Mayonnaise Preparation and Analysis
2.7.1. Rheological Analysis
2.7.2. Zeta Potential and Particle Size
2.7.3. Oxidative Stability (OXITEST)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Gum
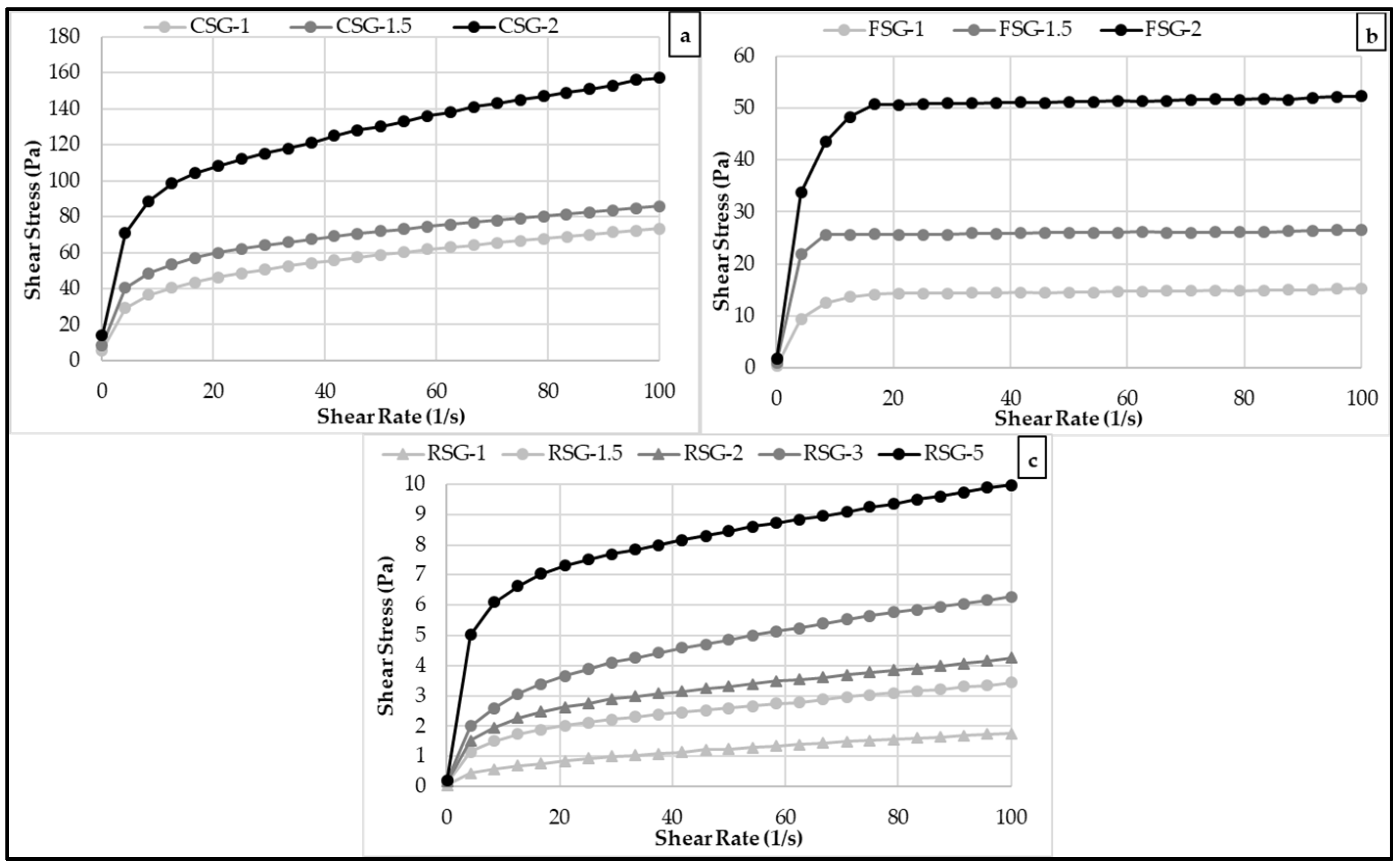
3.2. Flow Behavior Rheological Properties of the CSG, FSG, and RSG Solutions
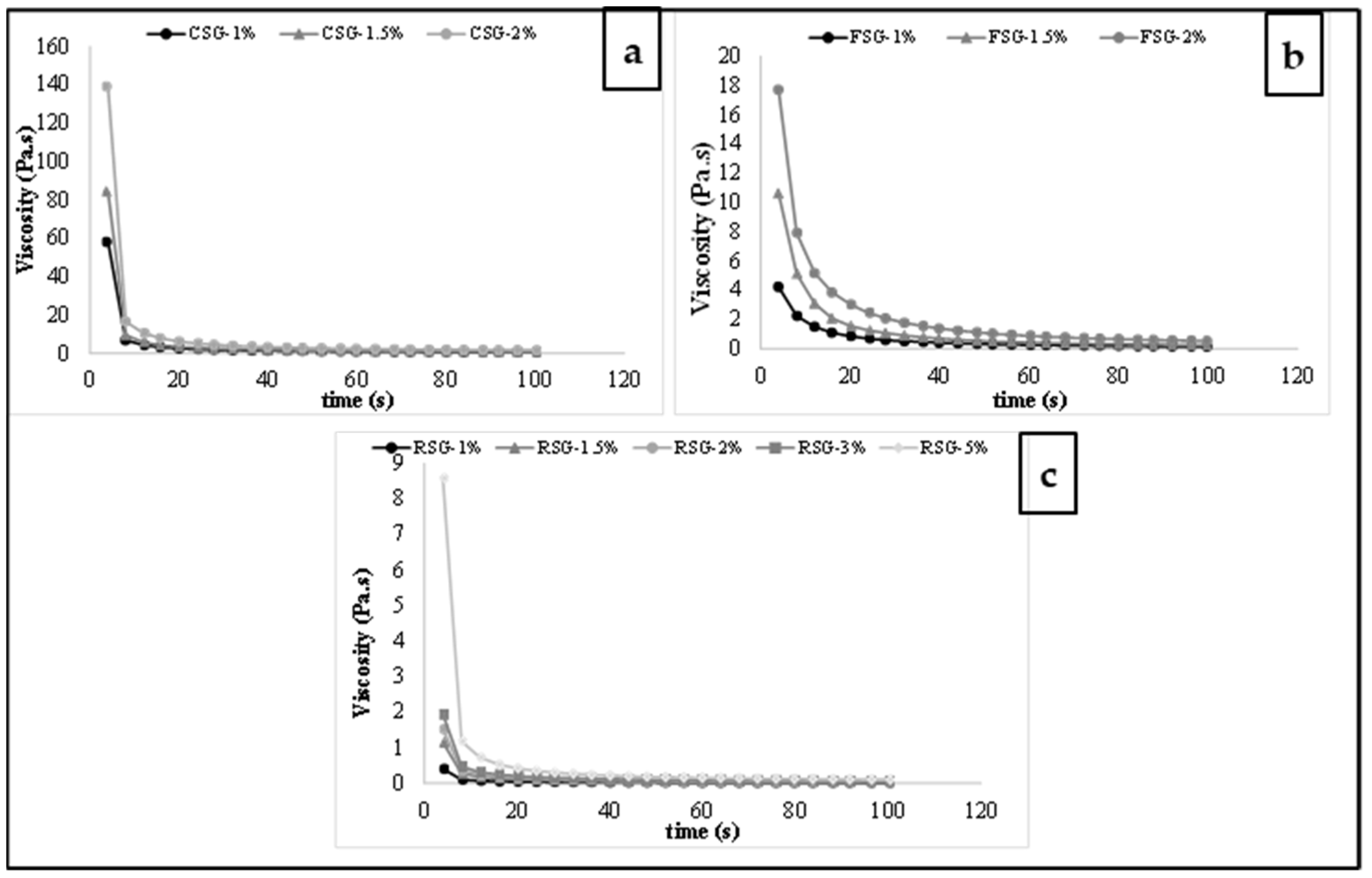
3.3. Thixotropic Properties
3.3.1. Constant Shear Rate
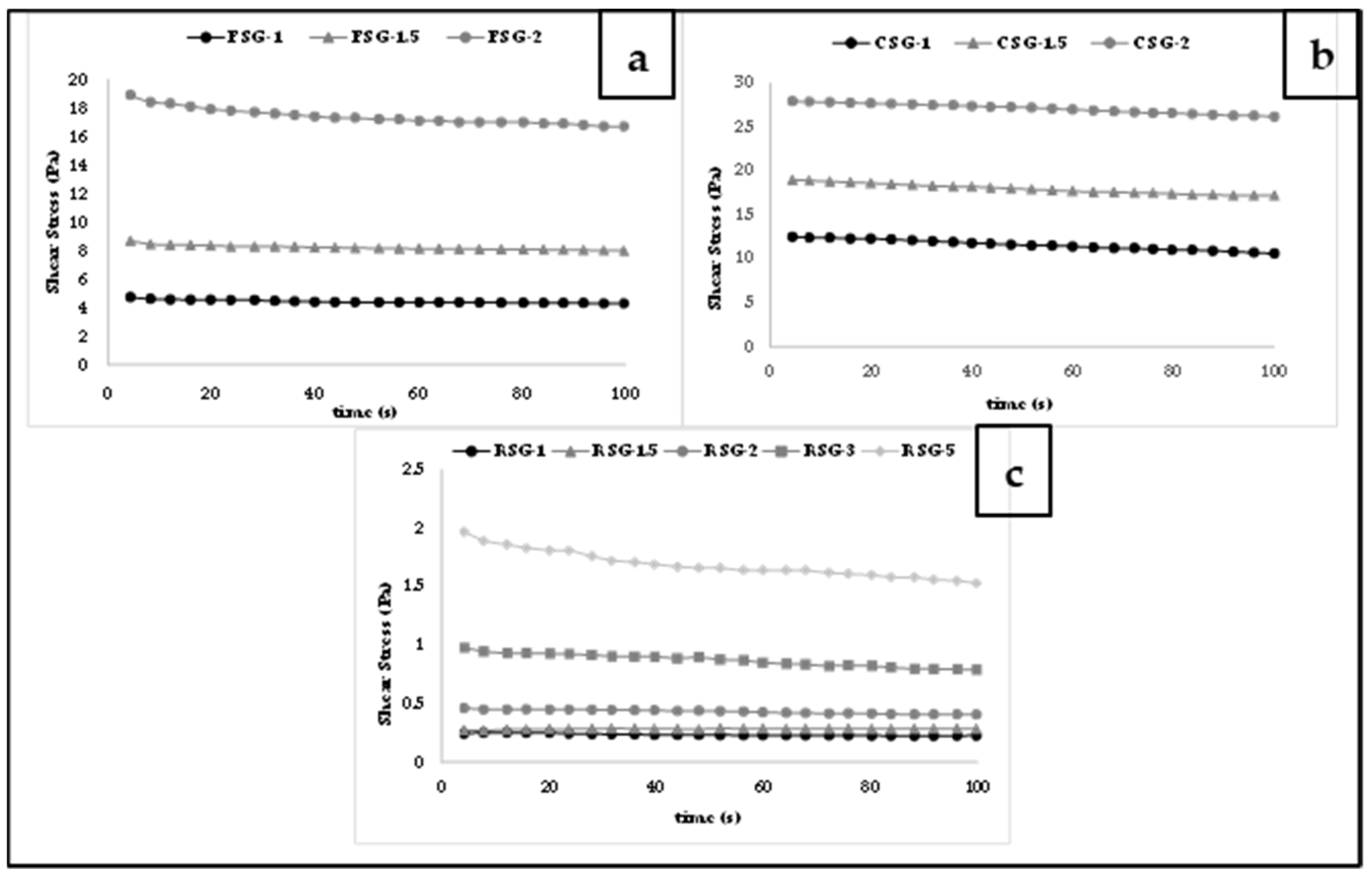
3.3.2. Three Interval Thixotropic Time Test (3-ITT)
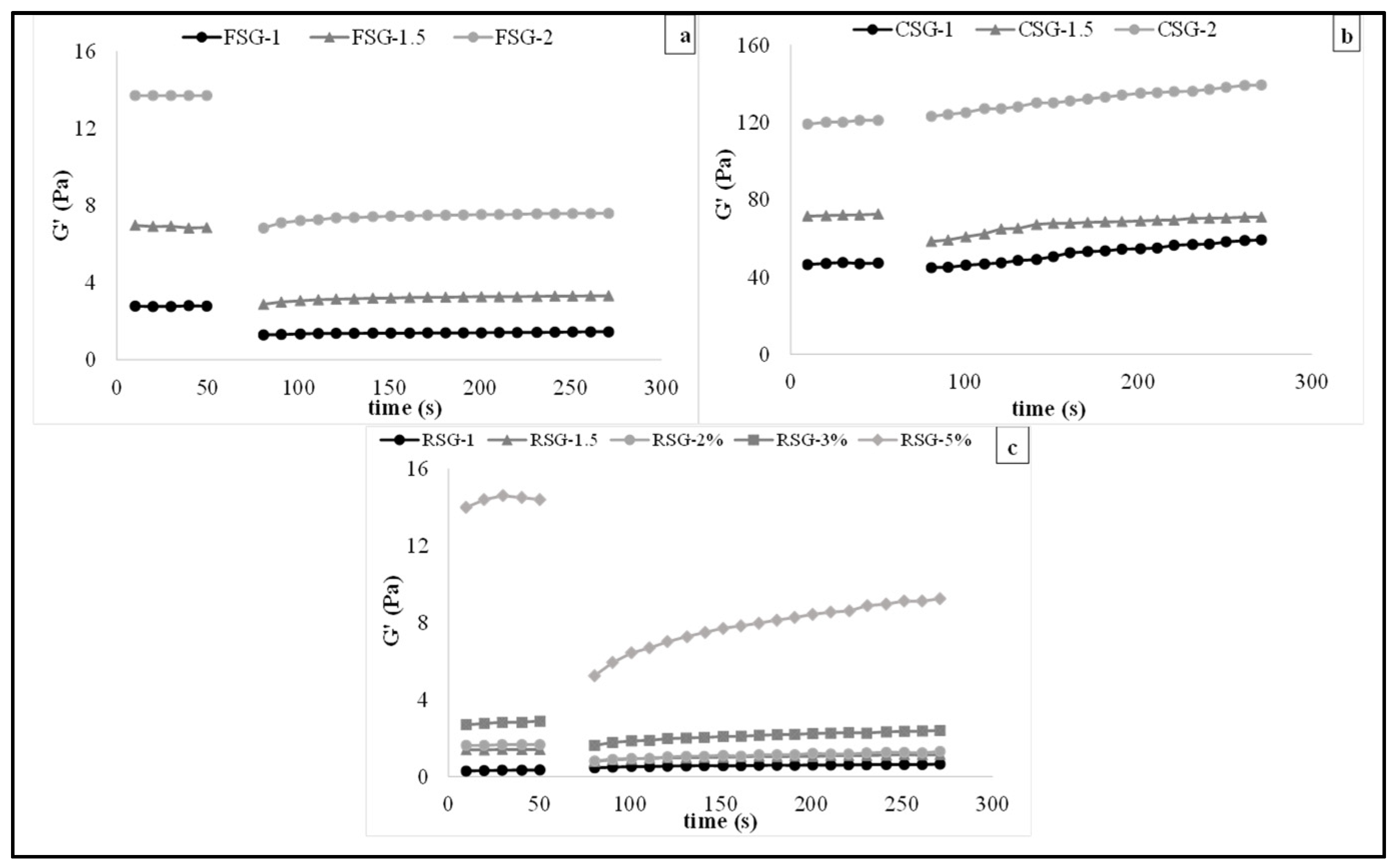
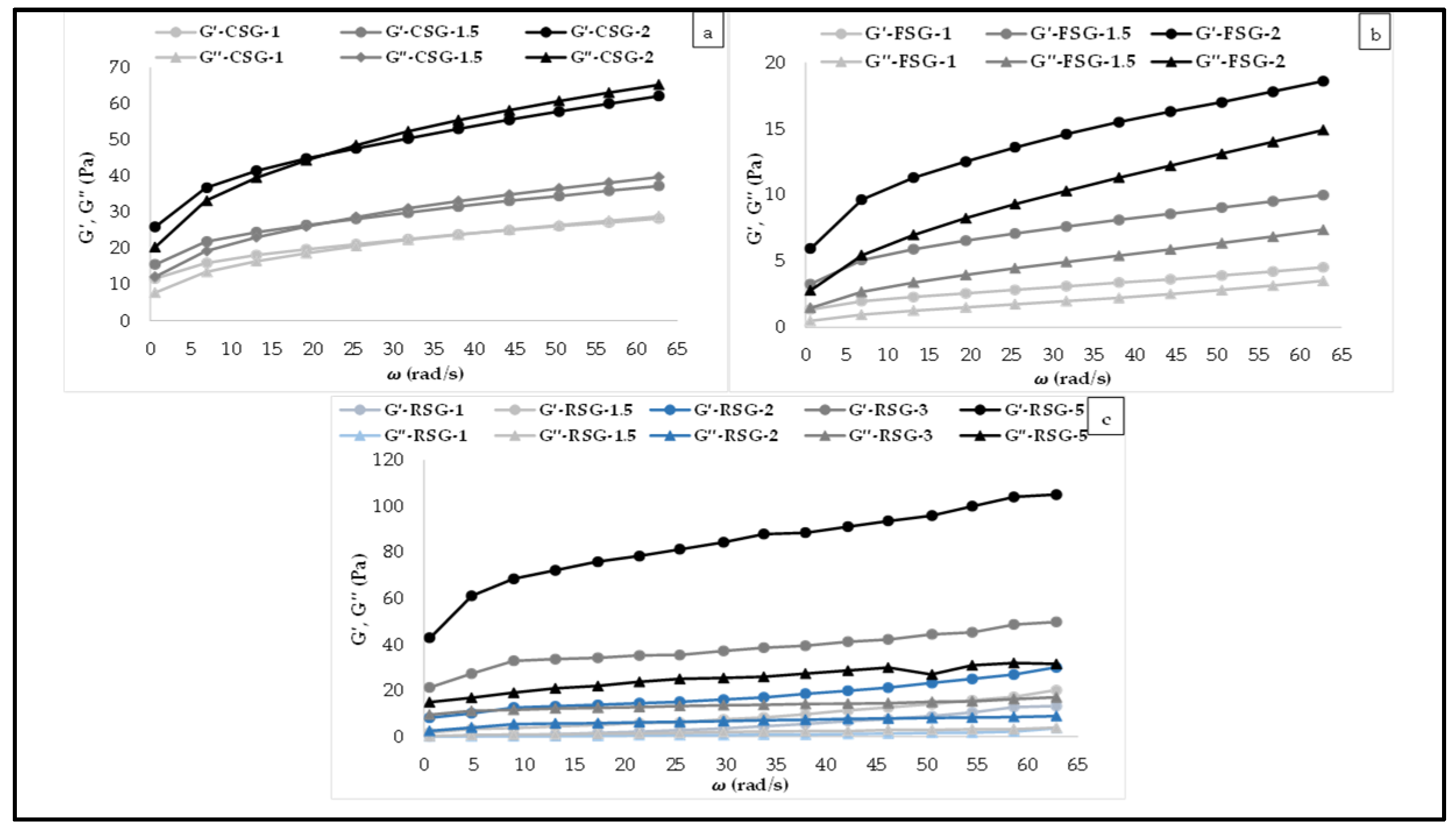
3.4. Viscoelastic Behavior of the CSG, FSG, and RSG Solutions
3.5. Rheological Properties, Zeta Potential, and Particle Size and Oxidative Stability of Low-Fat Vegan Mayonnaise Samples with Different Types of Gum
3.5.1. Rheological Properties
3.5.2. Zeta Potential and Particle Size
3.5.3. Oxidative Stability (at 90 °C)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty Acid Composition and Oxidative Stability of Cold-pressed Edible Seed Oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Siger, A.; Kowalczewski, P.Ł.; Rudzińska, M. Changes in chemical composition and oxidative stability of cold-pressed oils obtained from by-product roasted berry seeds. LWT 2019, 111, 541–547. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Tekin, Z.H.; Karasu, S. Cold-pressed flaxseed oil by-product as a new source of fat replacers in low-fat salad dressing formulation: Steady, dynamic and 3-ITT rheological properties. J. Food Process. Preserv. 2020, 44, e14650. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Wang, L.-J.; Li, D.; Adhikari, B. Effect of flaxseed gum on the rheological properties of peanut protein isolate dispersions and gels. LWT 2016, 74, 528–533. [Google Scholar] [CrossRef]
- Chen, H.-H.; Xu, S.-Y.; Wang, Z. Interaction between flaxseed gum and meat protein. J. Food Eng. 2007, 80, 1051–1059. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.-Y.; Ahmad, A. Linum usitatissimum L. seeds: Flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Preparation and characterization of chia seed protein isolate–chia seed gum complex coacervates. Food Hydrocoll. 2016, 52, 554–563. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Rheological and microstructural properties of the chia seed polysaccharide. Int. J. Biol. Macromol. 2015, 81, 991–999. [Google Scholar] [CrossRef]
- Suri, S.; Passi, S.J.; Goyat, J. Chia seed (Salvia hispanica L.)—A new age functional food. In Proceedings of the 4th International Conference on Recent Innovations in Science Engineering and Management, New Delh, India, 20 March 2016; pp. 286–299. [Google Scholar]
- Akcicek, A.; Karasu, S. Utilization of cold pressed chia seed oil waste in a low-fat salad dressing as natural fat replacer. J. Food Process Eng. 2018, 41, e12694. [Google Scholar] [CrossRef]
- Punia, S.; Dhull, S.B. Chia seed (Salvia hispanica L.) mucilage (a heteropolysaccharide): Functional, thermal, rheological behaviour and its utilization. Int. J. Biol. Macromol. 2019, 140, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Mhemdi, H.; Sanlaville, Q.; Vorobiev, E. Recovery of oil, erucic acid, and phenolic compounds from rapeseed and rocket seeds. Chem. Eng. Technol. 2016, 39, 1431–1437. [Google Scholar] [CrossRef]
- Nail, T.; Ali, M.; Salim, E. Phytochemical studies on Sudanese rocket (Eruca sativa) seeds and oil constituents. Am. J. Phytomed. Clin. Ther. 2017, 5, 1–5. [Google Scholar]
- Sherahi, M.H.; Fathi, M.; Zhandari, F.; Hashemi, S.M.B.; Rashidi, A. Structural characterization and physicochemical properties of Descurainia sophia seed gum. Food Hydrocoll. 2017, 66, 82–89. [Google Scholar] [CrossRef]
- Depree, J.A.; Savage, G.P. Physical and flavour stability of mayonnaise. Trends Food Sci. Technol. 2001, 12, 157–163. [Google Scholar] [CrossRef]
- Harrison, L.J.; Cunningham, F.E. Factors influencing the quality of mayonnaise: A review. J. Food Qual. 1985, 8, 1–20. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part I—Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Yebeyen, D.; Lemenih, M.; Feleke, S. Characteristics and quality of gum arabic from naturally grown Acacia senegal (Linne) Willd. trees in the Central Rift Valley of Ethiopia. Food Hydrocoll. 2009, 23, 175–180. [Google Scholar] [CrossRef]
- Shabani, H.; Askari, G.; Jahanbin, K.; Khodaeian, F. Evaluation of physicochemical characteristics and antioxidant property of Prunus avium gum exudates. Int. J. Biol. Macromol. 2016, 93, 436–441. [Google Scholar] [CrossRef]
- Sibaja-Hernández, R.; Román-Guerrero, A.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Physicochemical, shear flow behaviour and emulsifying properties of Acacia cochliacantha and Acacia farnesiana gums. Ind. Crops Prod. 2015, 67, 161–168. [Google Scholar] [CrossRef]
- Rincón, F.; Muñoz, J.; Ramírez, P.; Galán, H.; Alfaro, M.C. Physicochemical and rheological characterization of Prosopis juliflora seed gum aqueous dispersions. Food Hydrocoll. 2014, 35, 348–357. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.; Ding, H. Some physicochemical properties of sage (Salvia macrosiphon) seed gum. Food Hydrocoll. 2014, 35, 453–462. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Jia, F.; Jin, H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Jdayil, B. Modelling the time-dependent rheological behavior of semisolid foodstuffs. J. Food Eng. 2003, 57, 97–102. [Google Scholar] [CrossRef]
- Van Hecke, E.; Nguyen, P.U.; Clausse, D.; Lanoisellé, J.L. Flow behaviour of carrot puree: Modelling the influence of time, temperature and potato flakes addition. Int. J. Food Sci. Technol. 2012, 47, 177–185. [Google Scholar] [CrossRef]
- Toker, O.S.; Karasu, S.; Yilmaz, M.T.; Karaman, S. Three interval thixotropy test (3ITT) in food applications: A novel technique to determine structural regeneration of mayonnaise under different shear conditions. Food Res. Int. 2015, 70, 125–133. [Google Scholar] [CrossRef]
- Yoo, B.; Rao, M.A.; Steffe, J.F. Yield stress of food dispersions with the vane method at controlled shear rate and shear stress. J. Texture Stud. 1995, 26, 1–10. [Google Scholar] [CrossRef]
- Aksoy, F.S.; Tekin-Cakmak, Z.H.; Karasu, S.; Aksoy, A.S. Oxidative stability of the salad dressing enriched by microencapsulated phenolic extracts from cold-pressed grape and pomegranate seed oil by-products evaluated using OXITEST. Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Bhushette, P.R.; Annapure, U.S. Physicochemical, functional and rheological investigation of Soymida febrifuga exudate gum. Int. J. Biol. Macromol. 2018, 111, 1116–1123. [Google Scholar] [CrossRef]
- Hellebois, T.; Tsevdou, M.; Soukoulis, C. Chapter Five—Functionalizing and bio-preserving processed food products via probiotic and synbiotic edible films and coatings. In Advances in Food and Nutrition Research; da Cruz, A.G., Prudencio, E.S., Esmerino, E.A., da Silva, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 161–221. [Google Scholar]
- Kontogiorgos, V. Galactomannans (Guar, Locust Bean, Fenugreek, Tara). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Oomah, B.D.; Kenaschuk, E.; Cui, S.; Mazza, G. Variation in the composition of water-soluble polysaccharides in flaxseed. J. Agric. Food Chem. 1995, 43, 1484–1488. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Mehmood, A.; Rashid, M.T.; Ali, N.; Naveed, M. Flaxseed gum: Extraction, bioactive composition, structural characterization, and its potential antioxidant activity. J. Food Biochem. 2019, 43, e13014. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Effect of extraction temperature on composition, structure and functional properties of flaxseed gum. Food Chem. 2017, 215, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Souza, B.W.S.; Simões, J.; Teixeira, J.A.; Domingues, M.R.M.; Coimbra, M.A.; Vicente, A.A. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr. Polym. 2011, 83, 179–185. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Matia-Merino, L.; Chiang, J.H.; Quek, R.; Soh, S.J.B.; Lentle, R.G. The physico-chemical properties of chia seed polysaccharide and its microgel dispersion rheology. Carbohydr. Polym. 2016, 149, 297–307. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Suresh, K.I.; Rama Rao, B.; Vijaya Saradhi, U.V.R.; Prabhakar Rao, T. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Fonseca, P.R.M.S.; Dekker, R.F.H.; Barbosa, A.M.; Silveira, J.L.M.; Vasconcelos, A.F.D.; Monteiro, N.K.; Aranda-Selverio, G.; Da Silva, M.D.L.C. Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05. Molecules 2011, 16, 7488–7501. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-H.; Liu, X.; Shen, M.-Y.; Nie, S.-P.; Zhang, H.; Li, C.; Gong, D.-M.; Xie, M.-Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Hadad, S.; Goli, S.A.H. Fabrication and characterization of electrospun nanofibers using flaxseed (Linum usitatissimum) mucilage. Int. J. Biol. Macromol. 2018, 114, 408–414. [Google Scholar] [CrossRef]
- Darwish, A.; El-Sohaimy, S. Functional Properties of Chia Seed Mucilage Supplemented In Low Fat Yoghurt. Alex. Med. J. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Bortnowska, G.; Balejko, J.; Schube, V.; Tokarczyk, G.; Krzemińska, N.; Mojka, K. Stability and physicochemical properties of model salad dressings prepared with pregelatinized potato starch. Carbohydr. Polym. 2014, 111, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Coello, R.G.; Rodríguez-Huezo, M.E.; Carrillo-Navas, H.; Hernández-Jaimes, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. In vitro digestibility, physicochemical, thermal and rheological properties of banana starches. Carbohydr. Polym. 2014, 101, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Dakia, P.A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244, 160–170. [Google Scholar] [CrossRef]
- Sanchez, C.; Renard, D.; Robert, P.; Schmitt, C.; Lefebvre, J. Structure and rheological properties of acacia gum dispersions. Food Hydrocoll. 2002, 16, 257–267. [Google Scholar] [CrossRef]
- Marcotte, M.; Taherian Hoshahili, A.R.; Ramaswamy, H.S. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Yamazaki, E.; Kurita, O.; Matsumura, Y. High viscosity of hydrocolloid from leaves of Corchorus olitorius L. Food Hydrocoll. 2009, 23, 655–660. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Taheri, H.; Quinchia, L.A. Steady shear flow properties of wild sage (Salvia macrosiphon) seed gum as a function of concentration and temperature. Food Hydrocoll. 2011, 25, 451–458. [Google Scholar] [CrossRef]
- Chaharlang, M.; Samavati, V. Steady shear flow properties of Cordia myxa leaf gum as a function of concentration and temperature. Int. J. Biol. Macromol. 2015, 79, 56–62. [Google Scholar] [CrossRef]
- Yaseen, E.I.; Herald, T.J.; Aramouni, F.M.; Alavi, S. Rheological properties of selected gum solutions. Food Res. Int. 2005, 38, 111–119. [Google Scholar] [CrossRef]
- Adeli, M.; Samavati, V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int. J. Biol. Macromol. 2015, 72, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Taherian, A.R.; Bostan, A. Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2013, 50, 446–456. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food Hydrocoll. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Torres, M.D.; Hallmark, B.; Wilson, D.I. Effect of concentration on shear and extensional rheology of guar gum solutions. Food Hydrocoll. 2014, 40, 85–95. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, W.; Jia, L.; He, Q. The rheological properties of tara gum (Caesalpinia spinosa). Food Chem. 2015, 168, 366–371. [Google Scholar] [CrossRef]
- Koocheki, A.; Mortazavi, S.A.; Shahidi, F.; Razavi, S.M.A.; Taherian, A.R. Rheological properties of mucilage extracted from Alyssum homolocarpum seed as a new source of thickening agent. J. Food Eng. 2009, 91, 490–496. [Google Scholar] [CrossRef]
- Fijan, R.; Šostar-Turk, S.; Lapasin, R. Rheological study of interactions between non-ionic surfactants and polysaccharide thickeners used in textile printing. Carbohydr. Polym. 2007, 68, 708–717. [Google Scholar] [CrossRef]
- Weltmann, R.N. Breakdown of Thixotropic Structure as Function of Time. J. Appl. Phys. 1943, 14, 343–350. [Google Scholar] [CrossRef]
- Dzuy Nguyen, Q.; Jensen, C.T.B.; Kristensen, P.G. Experimental and modelling studies of the flow properties of maize and waxy maize starch pastes. Chem. Eng. J. 1998, 70, 165–171. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Canet, W. Time-independent and time-dependent rheological characterization of vegetable-based infant purees. J. Food Eng. 2013, 114, 449–464. [Google Scholar] [CrossRef] [Green Version]
- Koocheki, A.; Razavi, S.M. Effect of Concentration and Temperature on Flow Properties of Alyssum homolocarpum Seed Gum Solutions: Assessment of Time Dependency and Thixotropy. Food Biophys. 2009, 4, 353–364. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Purification of cress seed (Lepidium sativum) gum: A comprehensive rheological study. Food Hydrocoll. 2016, 61, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Chaisawang, M.; Suphantharika, M. Pasting and rheological properties of native and anionic tapioca starches as modified by guar gum and xanthan gum. Food Hydrocoll. 2006, 20, 641–649. [Google Scholar] [CrossRef]
- Kutlu, G.; Akcicek, A.; Bozkurt, F.; Karasu, S.; Tekin-Cakmak, Z.H. Rocket seed (Eruca sativa Mill) gum: Physicochemical and comprehensive rheological characterization. Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Toker, O.S.; Karaman, S.; Yuksel, F.; Dogan, M.; Kayacier, A.; Yilmaz, M.T. Temperature dependency of steady, dynamic, and creep-recovery rheological properties of ice cream mix. Food Bioprocess Technol. 2013, 6, 2974–2985. [Google Scholar] [CrossRef]
- Yılmaz, M.T.; Karaman, S.; Cankurt, H.; Kayacier, A.; Sagdic, O. Steady and dynamic oscillatory shear rheological properties of ketchup–processed cheese mixtures: Effect of temperature and concentration. J. Food Eng. 2011, 103, 197–210. [Google Scholar] [CrossRef]
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. β-Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Rudra, S.G.; Hanan, E.; Sagar, V.; Bhardwaj, R.; Basu, S.; Sharma, V. Manufacturing of mayonnaise with pea pod powder as a functional ingredient. J. Food Meas. Charact. 2020, 14, 2402–2413. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jin, W.; Zhou, B.; Li, B. Application of micronized konjac gel for fat analogue in mayonnaise. Food Hydrocoll. 2014, 35, 375–382. [Google Scholar] [CrossRef]
- Dickie, A.M.; Kokini, J.L. An Improved Model for Food Thickness from non-Newtonian Fluid Mechanics in the Mouth. J. Food Sci. 1983, 48, 57–61. [Google Scholar] [CrossRef]
- Juszczak, L.; Fortuna, T.; Kośla, A. Sensory and rheological properties of Polish commercial mayonnaise. Food/Nahrung 2003, 47, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, D.; Sierakowski, M.-R.; Waszczynskyj, N.; Haminiuk, C.W.; de Paula Scheer, A. Sensory evaluation and rheological behavior of commercial mayonnaise. Int. J. Food Eng. 2007, 3, 1–15. [Google Scholar] [CrossRef]
- Singla, N.; Verma, P.; Ghoshal, G.; Basu, S. Steady state and time dependent rheological behaviour of mayonnaise (egg and eggless). Int. Food Res. J. 2013, 20, 2009–2016. [Google Scholar]
- Moschakis, T.; Murray, B.; Dickinson, E. Microstructural evolution of viscoelastic emulsions stabilised by sodium caseinate and xanthan gum. J. Colloid Interface Sci. 2005, 284, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Khalloufi, S.; Corredig, M.; Goff, H.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]


| CSG | FSG | RSG | |
|---|---|---|---|
| Carbohydrate (%w/w) | 73.59 ± 0.29 b | 78.56 ± 0.06 a | 70.48 ± 0.20 c |
| Protein (%w/w) | 9.45 ± 0.07 b | 11.38 ± 0.10 a | 11.00 ± 0.01 a |
| Fat (%w/w) | 1.01 ± 0.02 b | 2.16 ± 0.10 a | 1.94 ± 0.04 a |
| Moisture (%w/w) | 9.60 ± 0.18 s | 9.08 ± 0.06 b | 9.95 ± 0.08 a |
| Ash (%w/w) | 6.26 ± 0.14 b | 7.89 ± 0.11 a | 6.63 ± 0.05 b |
| Monosaccharides (%) | |||
| Glucose | 20.46 ± 0.16 a | 20.27 ± 0.15 a | 10.26 ± 0.06 b |
| Galactose | 6.70 ± 0.07 c | 19.91 ± 0.03 b | 22.08 ± 0.08 a |
| Mannose | 4.09 ± 0.01 b | 0.41 ± 0.01 c | 39.12 ± 0.12 a |
| Xylose | 36.15 ± 0.15 b | 38.75 ± 0.05 a | - |
| Gum (%) | K (Pa·sn) | n | R2 | |
|---|---|---|---|---|
| FSG-1 | 1.0 | 8.256 ± 0.043 C | 0.143 ± 0.000 | 0.942 |
| FSG-1.5 | 1.5 | 15.699 ± 0.120 B | 0.143 ± 0.001 | 0.972 |
| FSG-2 | 2.0 | 28.731 ± 0.341 A | 0.145 ± 0.001 | 0.956 |
| CSG-1 | 1.0 | 18.578 ± 0.087 C | 0.296 ± 0.011 | 0.998 |
| CSG-1.5 | 1.5 | 27.889 ± 0.092 B | 0.243 ± 0.003 | 0.995 |
| CSG-2 | 2.0 | 49.028 ± 0.0359 A | 0.252 ± 0.001 | 0.994 |
| RSG-1 | 1.0 | 0.209 ± 0.001 E | 0.460 ± 0.004 | 0.999 |
| RSG-1.5 | 1.5 | 0.694 ± 0.015 D | 0.342 ± 0.003 | 0.997 |
| RSG-2 | 2.0 | 0.985 ± 0.002 C | 0.313 ± 0.001 | 0.996 |
| RSG-3 | 3.0 | 1.217 ± 0.003 B | 0.305 ± 0.000 | 0.998 |
| RSG-5 | 5.0 | 3.553 ± 0.021 A | 0.224 ± 0.001 | 0.987 |
| Weltman Model | Second-Order Structural Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gum (%) | A (Pa) | -B (Pa) | R2 | k ∗ 1000 | η0 (Pa·s) | ηe (Pa·s) | η0/ηe | R2 | |
| FSG-1 | 1.0 | 4.910 C | 0.133 C | 0.984 | 3.666 C | 23.346 C | 5.276 C | 4.425 C | 0.996 |
| FSG-1.5 | 1.5 | 8.911 B | 0.193 B | 0.986 | 7.641 B | 59.080 B | 6.415 B | 9.209 B | 0.993 |
| FSG-2 | 2.0 | 19.923 A | 0.683 A | 0.996 | 13.522 A | 138.530 A | 9.166 A | 15.113 A | 0.997 |
| CSG-1 | 1.0 | 13.988 C | 0.550 C | 0.921 | 5.566 C | 74.176 C | 11.842 C | 6.264 C | 0.997 |
| CSG-1.5 | 1.5 | 20.407 B | 0.661 B | 0.952 | 7.480 B | 256.477 B | 15.567 B | 16.475 B | 0.996 |
| CSG-2 | 2.0 | 29.349 A | 0.701 A | 0.999 | 14.329 A | 423.919 A | 20.661 A | 20.517 A | 0.994 |
| RSG-1 | 1.0 | 0.281 E | 0.012 E | 0.966 | 1.309 E | 1.219 D | 0.947 E | 1.287 E | 0.998 |
| RSG-1.5 | 1.5 | 0.309 D | 0.017 D | 0.992 | 3.157 D | 6.626 C | 3.976 B | 1.668 D | 0.999 |
| RSG-2 | 2.0 | 0.731 C | 0.041 C | 0.993 | 6.787 C | 8.837 B | 4.466 A | 1.978 C | 0.990 |
| RSG-3 | 3.0 | 2.195 B | 0.137 B | 0.987 | 8.602 B | 8.953 B | 3.092 C | 2.896 B | 0.992 |
| RSG-5 | 5.0 | 3.281 A | 0.212 A | 0.996 | 10.656 A | 17.499 A | 2.899 D | 6.036 A | 0.993 |
| G0 | Ge | Ge/G0 | k × 1000 | R2 | % D | % R | |
|---|---|---|---|---|---|---|---|
| FSG-1 | 1.285 ± 0.011 C | 1.542 ± 0.107 C | 1.200 C | 7.41 C | 0.970 | 53.79 | 55.47 |
| FSG-1.5 | 2.694 ± 0.049 B | 3.390 ± 0.012 B | 1.259 B | 35.56 B | 0.998 | 61.35 | 48.64 |
| FSG-2 | 6.368 ± 0.124 A | 8.200 ± 0.300 A | 1.288 A | 56.71 A | 0.997 | 53.52 | 59.85 |
| CSG-1 | 38.788 ± 1.561 C | 59.525 ± 0.565 C | 1.535 C | 33.27 C | 0.909 | 16.41 | 128.29 |
| CSG-1.5 | 44.234 ± 0.095 B | 75.015 ± 0.352 B | 1.696 B | 38.99 B | 0.990 | 38.05 | 61.95 |
| CSG-2 | 76.001 ± 0.219 A | 133.000 ± 0.435 A | 1.750 A | 41.80 A | 0.948 | 36.13 | 111.76 |
| RSG-1 | 0.461 ± 0.003 E | 0.518 ± 0.019 E | 1.124 E | 11.00 E | 0.983 | 17.67 | 256.47 |
| RSG-1.5 | 0.744 ± 0.014 D | 0.842 ± 0.005 D | 1.132 D | 14.91 D | 0.980 | 46.47 | 89.33 |
| RSG-2 | 0.800 ± 0.020 C | 0.914 ± 0.259 C | 1.143 C | 7.08 C | 0.983 | 50.62 | 99.60 |
| RSG-3 | 1.268 ± 0.209 B | 1.557 ± 0.083 B | 1.228 B | 8.56 B | 0.996 | 47.89 | 105.48 |
| RSG-5 | 4.617 ± 0.308 A | 5.869 ± 0.438 A | 1.271 A | 9.47 A | 0.998 | 65.59 | 81.91 |
| K′ (Pa·s) | n′ | R2 | K″ (Pa·s) | n″ | R2 | |
|---|---|---|---|---|---|---|
| FSG-1 | 0.949 ± 0.002 C | 0.358 ± 0.002 | 0.9994 | 0.217 ± 0.004 C | 0.656 ± 0.069 | 0.9981 |
| FSG-1.5 | 2.933 ± 0.025 B | 0.285 ± 0.015 | 0.9975 | 1.049 ± 0.006 B | 0.459 ± 0.010 | 0.9880 |
| FSG-2 | 5.666 ± 0.031 A | 0.279 ± 0.005 | 0.9997 | 2.216 ± 0.033 A | 0.453 ± 0.006 | 0.9985 |
| CSG-1 | 10.679 ± 0.031 C | 0.326 ± 0.002 | 0.9978 | 7.328 ± 0.012 C | 0.326 ± 0.002 | 0.9917 |
| CSG-1.5 | 14.655 ± 0.093 B | 0.304 ± 0.001 | 0.9990 | 11.013 ± 0.307 B | 0.304 ± 0.011 | 0.9964 |
| CSG-2 | 24.849 ± 0.101 A | 0.212 ± 0.000 | 0.9992 | 19.543 ± 0.076 A | 0.287 ± 0.000 | 0.9807 |
| RSG-1 | 0.011 ± 0.001 E | 0.118 ± 0.103 | 0.9993 | 0.007 ± 0.000 E | 0.117 ± 0.079 | 0.9989 |
| RSG-1.5 | 0.074 ± 0.002 D | 0.809 ± 0.098 | 0.989 | 0.060 ± 0.003 D | 0.801 ± 0.106 | 0.988 |
| RSG-2 | 0.151 ± 0.002 C | 0.679 ± 0.045 | 0.9987 | 0.150 ± 0.034 C | 0.721 ± 0.009 | 0.9983 |
| RSG-3 | 0.232 ± 0.001 B | 0.508 ± 0.002 | 0.9899 | 0.219 ± 0.019 B | 0.720 ± 0.032 | 0.9983 |
| RSG-5 | 2.178 ± 0.041 A | 0.202 ± 0.000 | 0.9982 | 0.634 ± 0.092 A | 0.213 ± 0.001 | 0.9910 |
| Gum (%) | K (Pa·sn) | R2 | |||
|---|---|---|---|---|---|
| CG-VM | 2.0 | 38.582 ± 0.183 A | 0.985 | ||
| FG-VM | 2.0 | 35.913 ± 0.238 B | 0.986 | ||
| RG-VM | 5.0 | 10.647 ± 0.232 C | 0.999 | ||
| AG-VM | 1.0 | 0.007 ± 0.002 E | 0.997 | ||
| GG-VM | 1.0 | 10.888 ± 0.313 C | 0.994 | ||
| XG-VM | 0.4 | 7.050 ± 0.069 D | 0.994 | ||
| K′ (Pa·sn) | n′ | K″ (Pa·sn) | n″ | R2 | |
| CG-VM | 43.317 ± 0.098 A | 0.299 ± 0.000 | 30.423 ± 0.032 A | 0.094 ± 0.005 | 0.9973 |
| FG-VM | 31.321 ± 0.291 C | 0.309 ± 0.001 | 11.688 ± 0.020 C | 0.370 ± 0.016 | 0.9960 |
| RG-VM | 32.511 ± 0.310 C | 0.191 ± 0.009 | 5.534 ± 0.197 D | 0.301 ± 0.002 | 0.9835 |
| AG-VM | 0.012 ± 0.001 E | 0.992 ± 0.031 | 0.006 ± 0.000 F | 1.201 ± 0.021 | 0.9993 |
| GG-VM | 36.752 ± 0.180 B | 0.264 ± 0.002 | 12.489 ± 0.129 B | 0.361 ± 0.024 | 0.9790 |
| XG-VM | 12.721 ± 0.201 D | 0.274 ± 0.008 | 4.364 ± 0.082 E | 0.238 ± 0.007 | 0.9941 |
| G0 | Ge | Ge/G0 | k × 1000 | R2 | |
| CG-VM | 64.613 | 104.270 | 1.614 A | 60.789 B | 0.9844 |
| FG-VM | 53.781 | 70.591 | 1.313 D | 37.57 C | 0.9990 |
| RG-VM | 38.071 | 47.951 | 1.260 E | 24.987 E | 0.9874 |
| AG-VM | 0.258 | 0.180 | 0.698 F | 4.32 F | 0.9938 |
| GG-VM | 65.192 | 97.138 | 1.490 B | 68.876 A | 0.9974 |
| XG-VM | 14.478 | 20.176 | 1.394 C | 29.90 D | 0.9981 |
| ζ-Potential (mV) | PdI | d32 (µm) | |
|---|---|---|---|
| CG-VM | −42.0 ± 1.58 A | 0.507 ± 0.121 D | 5418.00 ± 268.88 C |
| FG-VM | −40.4 ± 1.79 A | 0.701 ± 0.050 A | 8581.33 ± 284.39 A |
| RG-VM | −39.5 ± 1.21 A | 0.302 ± 0.000 E | 3689.67 ± 282.13 D |
| AG-VM | −31.9 ± 0.62 B | 0.249 ± 0.048 F | 1240.00 ± 102.77 E |
| GG-VM | −42.8 ± 1.04 A | 0.539 ± 0.092 C | 5851.33 ± 114.01 C |
| XG-VM | −41.2 ± 0.89 A | 0.613 ± 0.074 B | 6720.32 ± 160.99 B |
| Samples | IP (h) |
|---|---|
| CG-VM | 6:15 ± 0.02 B |
| FG-VM | 5:35 ± 0.08 D |
| RG-VM | 13:44 ± 0.12 A |
| AG-VM | 6:00 ± 0.01 C |
| GG-VM | 5:27 ± 0.04 D |
| XG-VM | 4:38 ± 0.03 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijazi, T.; Karasu, S.; Tekin-Çakmak, Z.H.; Bozkurt, F. Extraction of Natural Gum from Cold-Pressed Chia Seed, Flaxseed, and Rocket Seed Oil By-Product and Application in Low Fat Vegan Mayonnaise. Foods 2022, 11, 363. https://doi.org/10.3390/foods11030363
Hijazi T, Karasu S, Tekin-Çakmak ZH, Bozkurt F. Extraction of Natural Gum from Cold-Pressed Chia Seed, Flaxseed, and Rocket Seed Oil By-Product and Application in Low Fat Vegan Mayonnaise. Foods. 2022; 11(3):363. https://doi.org/10.3390/foods11030363
Chicago/Turabian StyleHijazi, Taha, Salih Karasu, Zeynep Hazal Tekin-Çakmak, and Fatih Bozkurt. 2022. "Extraction of Natural Gum from Cold-Pressed Chia Seed, Flaxseed, and Rocket Seed Oil By-Product and Application in Low Fat Vegan Mayonnaise" Foods 11, no. 3: 363. https://doi.org/10.3390/foods11030363
APA StyleHijazi, T., Karasu, S., Tekin-Çakmak, Z. H., & Bozkurt, F. (2022). Extraction of Natural Gum from Cold-Pressed Chia Seed, Flaxseed, and Rocket Seed Oil By-Product and Application in Low Fat Vegan Mayonnaise. Foods, 11(3), 363. https://doi.org/10.3390/foods11030363







