Enzymatic Preparation of Gentiooligosaccharides by a Thermophilic and Thermostable β-Glucosidase at a High Substrate Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
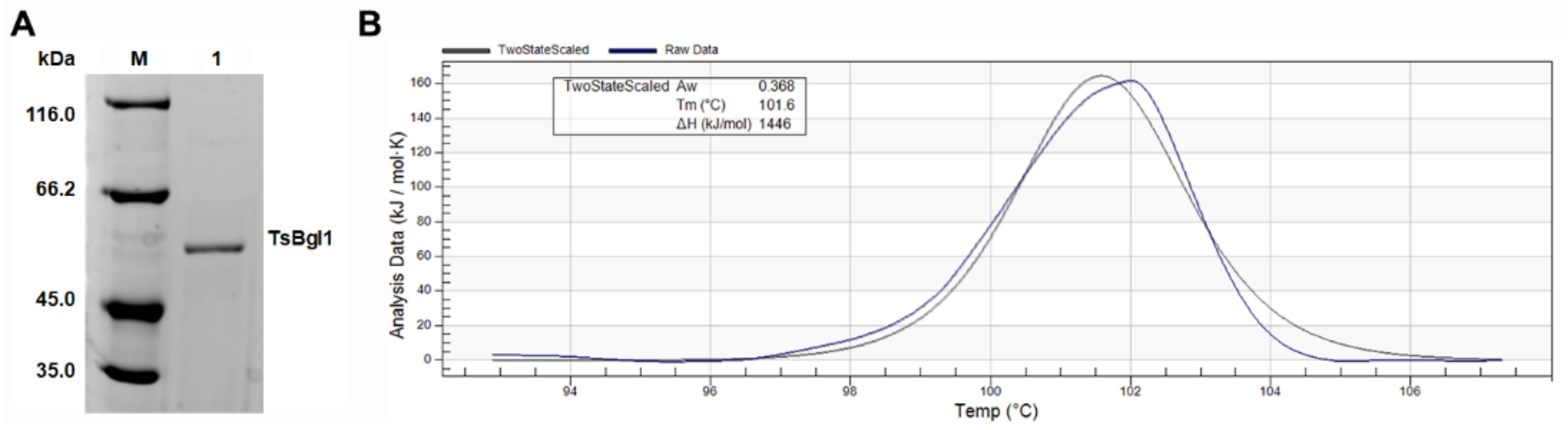
2.2. Expression and Purification of Recombinant TsBgl1
2.3. Enzyme Activity Assay
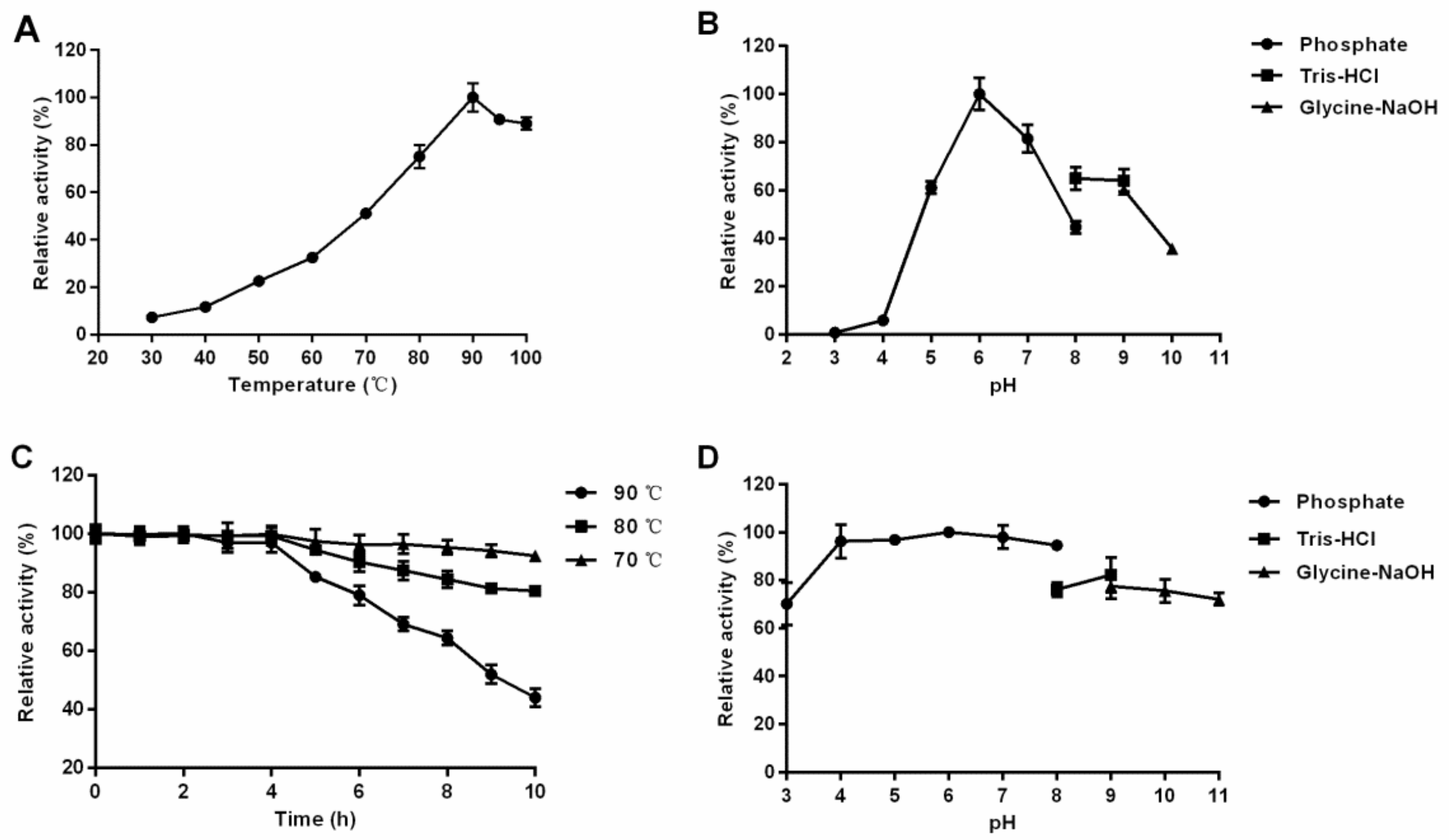
2.4. Enzymatic Properties of Recombinant TsBgl1
2.5. Optimization of Transglycosylation Reaction Conditions
2.6. Products Analysis
3. Results and Discussion
3.1. Expression, Purification and Characterization of β-Glucosidase TsBgl1
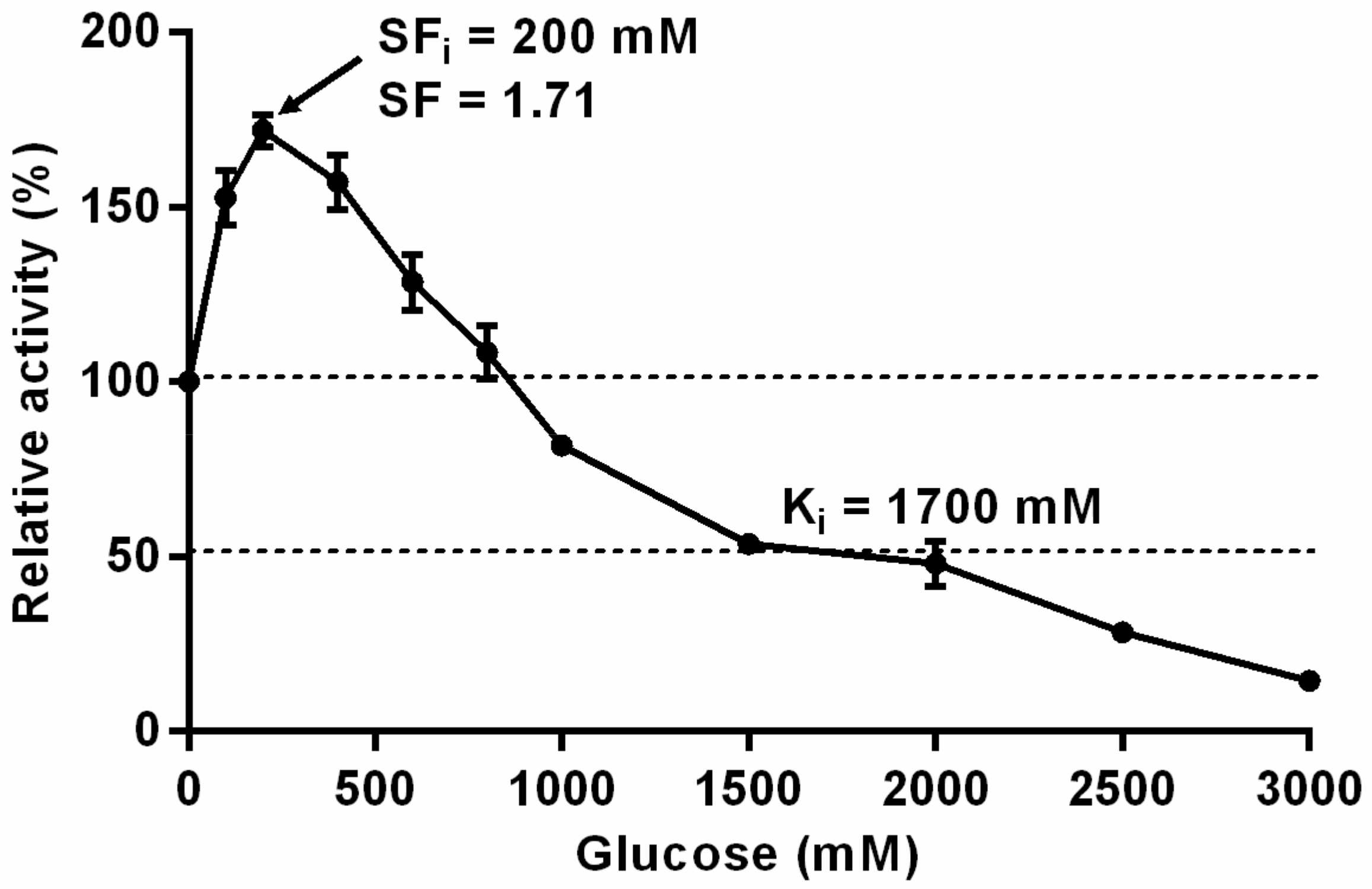
3.2. Substrate Specificities and Kinetic Parameters
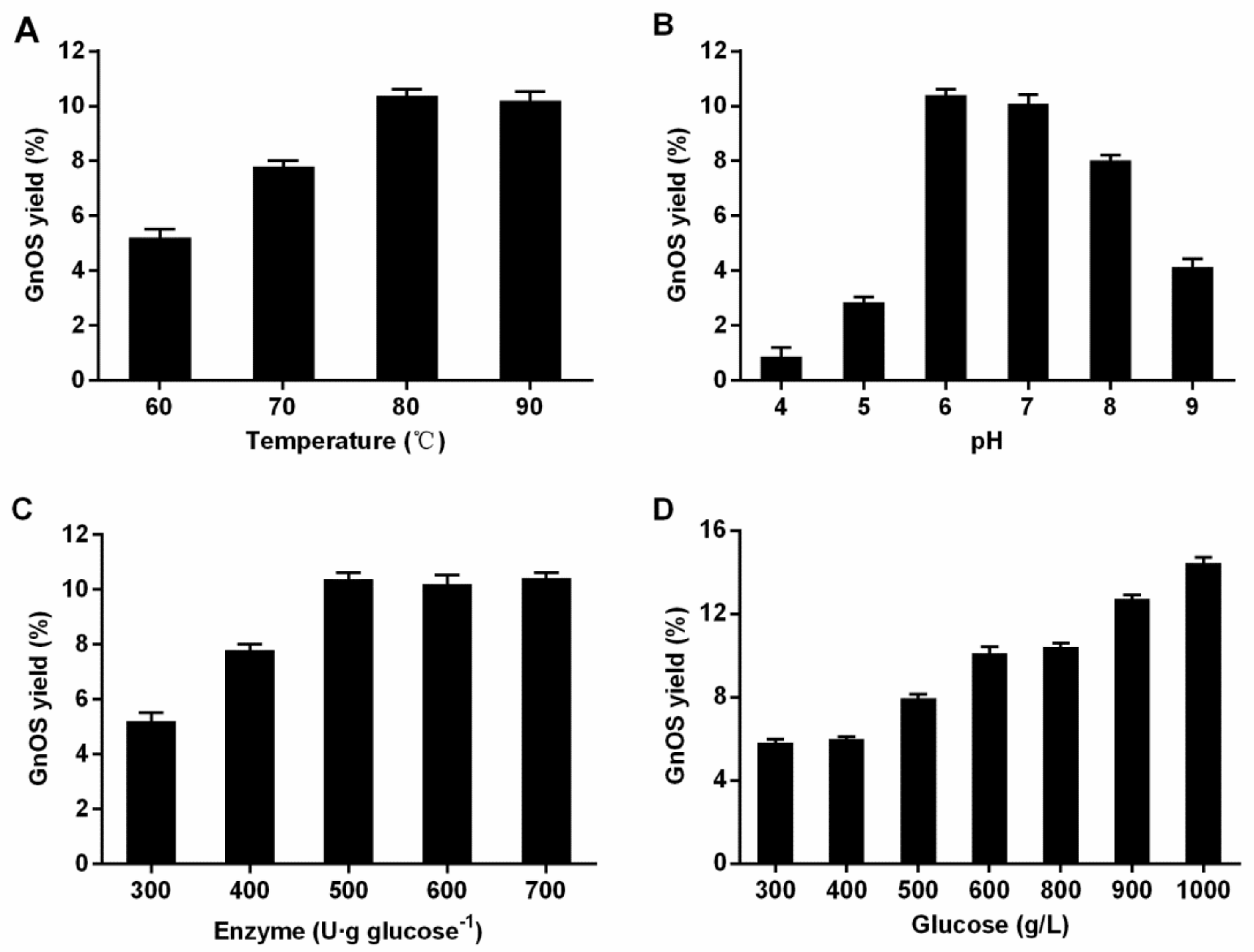
3.3. Optimization of Reaction Conditions for the Synthesis of GnOS by TsBgl1
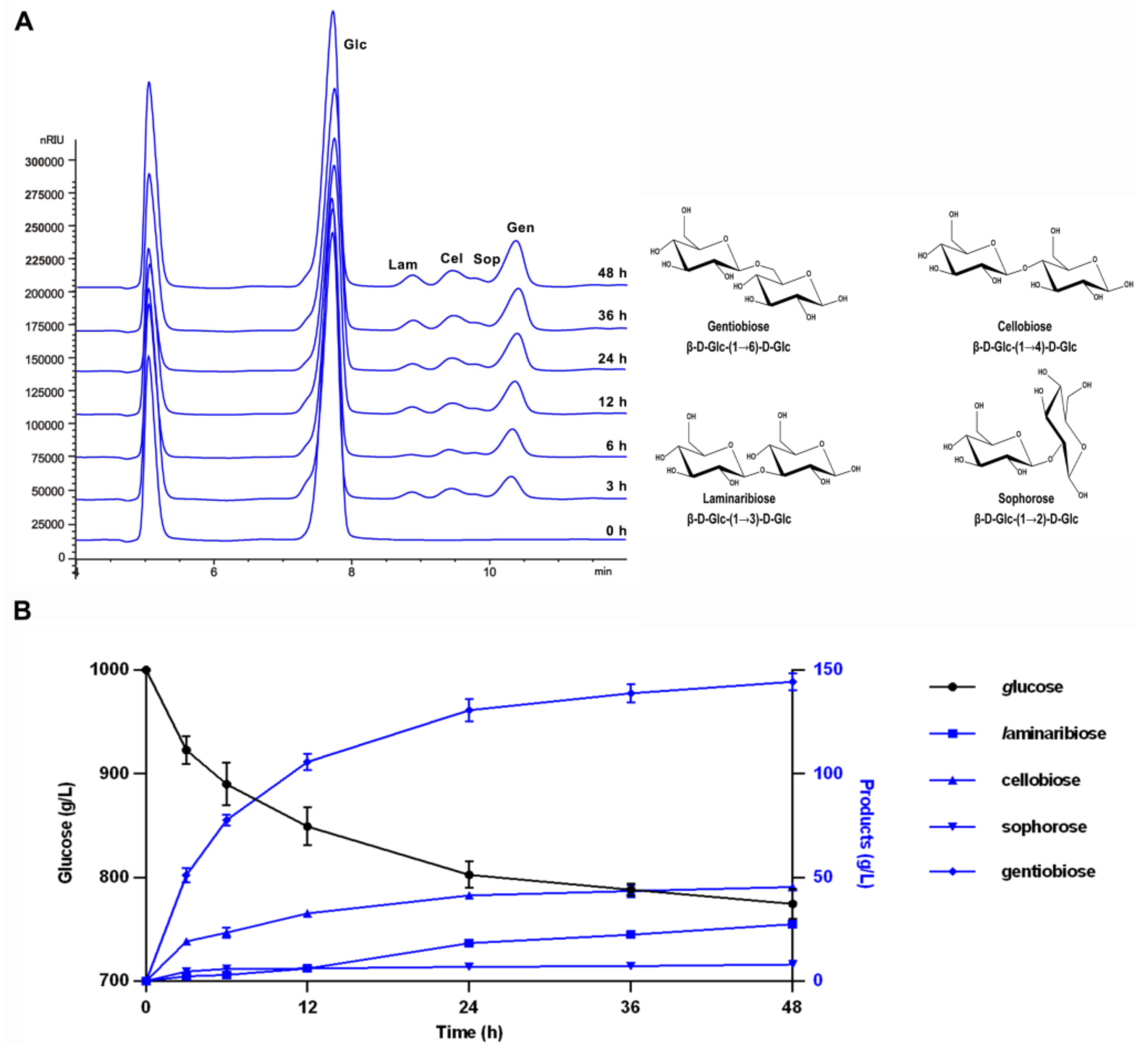
3.4. Reaction Time Course and Product Composition Analysis of TsBgl1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GnOS | Gentiooligosaccharides |
| GH | Glycoside hydrolase |
| pNP-β-Glc | 4-nitrophenyl-β-D-glucopyranoside |
| GOD-POD | Glucose oxidase-peroxidase |
| DSC | Differential scanning calorimeter |
| SF | Stimulating factor |
| Ki | Inhibition constant |
References
- Fujimoto, Y.; Hattori, T.; Uno, S.; Murata, T.; Usui, T. Enzymatic synthesis of gentiooligosaccharides by transglycosylation with β-glycosidases from Penicillium multicolor. Carbohyd. Res. 2009, 344, 972–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, R.; Danson, M.; Eisenthal, R.; Lee, C.; Peterson, M. The effect of temperature on enzyme activity: New insights and their implications. Extrem. Life Under Extrem. Cond. 2008, 12, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hodoniczky, J.; Morris, C.A.; Rae, A.L. Oral and intestinal digestion of oligosaccharides as potential sweeteners: A systematic evaluation. Food Chem. 2012, 132, 1951–1958. [Google Scholar] [CrossRef]
- Moser, M.; Agemans, A.; Caers, W. Production and Bioactivity of Oligosaccharides from Chicory Roots; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Sanz, M.L.; Côté, G.L.; Gibson, G.R.; Rastall, R.A. Selective fermentation of gentiobiose-derived oligosaccharides by human gut bacteria and influence of molecular weight. Fems Microbiol. Ecol. 2006, 56, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, D.; Goyal, A. Gentio-oligosaccharides from Leuconostoc mesenteroides NRRL B-1426 dextransucrase as prebiotics and as supplement for functional foods with anti-cancer properties. Food Funct. 2014, 6, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kong, F. A facile and effective synthesis of f nt for functional foods with anti-cancer properties. rides by human gut bacteria and influinked glucose di-, tri-, tetra-, hexa-, and octasaccharides using sugar trichloroacetimidates as the donors and unprotected or partially protected glycosides as the acceptors. Carbohyd. Res. 2001, 332, 1–21. [Google Scholar] [CrossRef]
- Pitson, S.; Seviour, R.; McDougall, B. Effect of carbon source on extracellular (1 → 3)- and (1 → 6)-β-glucanase production by Acremonium persicinum. Can. J. Microbiol. 2011, 43, 432–439. [Google Scholar] [CrossRef]
- Adam, O.; Vercellone, A.; Paul, F.; Monsan, P.; Puzo, G. A nondegradative route for the removal of endotoxin from exopolysaccharides. Anal Biochem. 1995, 225, 321–327. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Bhatia, Y.; Mishra, S.; Bisaria, V.S. Microbial beta-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.R.K.; Mahong, B.; Baiya, S.; Jeon, J. β-Glucosidases: Multitasking, moonlighting or simply misunderstood? Plant Sci. 2015, 241, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Bairoch, A.M. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1993, 293, 781–788. [Google Scholar] [CrossRef] [PubMed]
- And, D.L.Z.; Withers, S.G. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Accounts Chem. Res. 1999, 33, 11. [Google Scholar]
- Davies, G.J.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar]
- Litzinger, S.; Fischer, S.; Polzer, P.; Diederichs, K.; Welte, W.; Mayer, C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010, 285, 35675–35684. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech. 2015, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Bucke, C. Oligosaccharide synthesis using glycosidases. J. Chem. Technol. Biot. 1996, 67, 217–220. [Google Scholar] [CrossRef]
- Gao, L.; Gao, F.; Zhang, D.; Zhang, C.; Wu, G.; Chen, S. Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresource Technol. 2013, 147, 658–661. [Google Scholar]
- Jorgensen, F.; Hansen, O.; Stougaard, P. High-efficiency synthesis of oligosaccharides with a truncated β-galactosidase from Bifidobacterium bifidum. Appl. Microbiol. Biotech. 2001, 57, 647–652. [Google Scholar]
- Hassan, N.; Geiger, B.; Gandini, R.; Patel, B.K.C.; Kittl, R.; Haltrich, D.; Nguyen, T.; Divne, C.; Tan, T.C. Engineering a thermostable Halothermothrix orenii β-glucosidase for improved galacto-oligosaccharide synthesis. Appl. Microbiol. Biotech. 2016, 100, 3533–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, C.; Guerrero, C.; Conejeros, R.; Illanes, A. Synthesis of galacto-oligosaccharides by β-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzyme Microb. Tech. 2012, 50, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, P.; Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Wilson, L.; Illanes, A.; Plou, F.J. Detailed analysis of galactooligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. J. Agr. Food Chem. 2013, 61, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.; Rai, A.K.; Singh, S.P. A novel β-glucosidase from a hot-spring metagenome shows elevated thermal stability and tolerance to glucose and ethanol. Enzyme Microb. Tech. 2021, 145, 109764. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.M.O.; Vici, A.C.; Pinheiro, M.P.; Heinen, P.R.; de Oliveira, A.H.C.; Ward, R.J.; Prade, R.A.; Buckeridge, M.S.; Polizeli, M.d.L.T.d.M. A highly glucose tolerant ß-glucosidase from Malbranchea pulchella (MpBg3) enables cellulose saccharification. Sci. Rep.-UK 2020, 10, 6998. [Google Scholar] [CrossRef] [PubMed]
- Manna, B.; Ghosh, A. Molecular insight into glucose-induced conformational change to investigate uncompetitive inhibition of GH1 β-glucosidase. ACS Sustain. Chem. Eng. 2021, 9, 1613–1624. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, B.; Chattopadhyay, K.; Ghosh, A.; Datta, S. Role of conformational change and glucose binding sites in the enhanced glucose tolerance of Agrobacterium tumefaciens 5A GH1 β-glucosidase mutants. J. Phys. Chem. B 2021, 125, 9402–9416. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Molina, J.F.; Teixeira, R.S.S.; Valdivieso Gelves, L.G.; Bon, E.P.S.; Ferreira-Leitão, V.S. Synthesis of disaccharides using β-glucosidases from Aspergillus niger, A. awamori and Prunus dulcis. Biotechnol. Lett. 2017, 39, 1717–1723. [Google Scholar] [CrossRef]
- Wang, F.; Wu, J.; Chen, S. Preparation of gentiooligosaccharides using Trichoderma viride β-glucosidase. Food Chem. 2018, 248, 340–345. [Google Scholar] [CrossRef]
- Boudabbous, M.; Ben Hmad, I.; Saibi, W.; Mssawra, M.; Belghith, H.; Gargouri, A. Trans-glycosylation capacity of a highly glycosylated multi-specific β-glucosidase from Fusarium solani. Bioproc. Biosyst. Eng. 2017, 40, 559–571. [Google Scholar] [CrossRef]
- Seidle, H.F.; Huber, R.E. Transglucosidic reactions of the Aspergillus niger family 3 beta-glucosidase: Qualitative and quantitative analyses and evidence that the transglucosidic rate is independent of pH. Arch. Biochem. Biophys. 2005, 436, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Christakopoulos, P.; Bhat, M.K.; Kekos, D.; Macris, B.J. Enzymatic synthesis of trisaccharides and alkyl beta-D-glucosides by the transglycosylation reaction of beta-glucosidase from Fusarium oxysporum. Int. J. Biol. Macromol. 1994, 16, 331–334. [Google Scholar] [CrossRef]
| Substrate | Specific Activity (U/mg) | Km (mM) a | kcat (s−1) b | kcat/Km (mM−1·s−1) |
|---|---|---|---|---|
| pNP-β-Glc | 181.3 | 0.24 | 123.7 | 509.1 |
| Cellobiose (Glc-β-1,4-Glc) | 85.5 | 2.13 | 246.2 | 115.6 |
| Sophorose (Glc-β-1,2-Glc) | 91.2 | 3.95 | 218.2 | 55.3 |
| Gentiobiose (Glc-β-1,6-Glc) | 87.8 | 3.68 | 240.0 | 65.2 |
| Laminaribiose (Glc-β-1,3-Glc) | 140.8 | 2.17 | 295.0 | 135.7 |
| Source Species | Substrate | Yield (g·L−1) a | Reference |
|---|---|---|---|
| Thermotoga sp. KOL6 | 1000 g·L−1 glucose | 144.3 | This work |
| Prunus dulcis | 900 g·L−1 glucose | 128 | Da Silva et al. [29] |
| Penicillium multicolor | 500 g·L−1 gentiotriose | 157 | Fujimoto et al. [1] |
| Fusarium solani | 30 g·L−1 cellobiose | 16.8 | Boudabbous et al. [31] |
| Aspergillus niger | 17.1 g·L−1 cellobiose | 6.35 | Seidle et al. [32] |
| Fusarium oxysporum | 200 g·L−1 cellobiose | 28.2 | Christakopoulos et al. [33] |
| Trichoderma viride | 800 g·L−1 glucose | 130 | Wang et al. [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Sheng, L.; Mu, W.; Shi, Y.; Wu, J. Enzymatic Preparation of Gentiooligosaccharides by a Thermophilic and Thermostable β-Glucosidase at a High Substrate Concentration. Foods 2022, 11, 357. https://doi.org/10.3390/foods11030357
Xia W, Sheng L, Mu W, Shi Y, Wu J. Enzymatic Preparation of Gentiooligosaccharides by a Thermophilic and Thermostable β-Glucosidase at a High Substrate Concentration. Foods. 2022; 11(3):357. https://doi.org/10.3390/foods11030357
Chicago/Turabian StyleXia, Wei, Lingling Sheng, Wanmeng Mu, Yuping Shi, and Jing Wu. 2022. "Enzymatic Preparation of Gentiooligosaccharides by a Thermophilic and Thermostable β-Glucosidase at a High Substrate Concentration" Foods 11, no. 3: 357. https://doi.org/10.3390/foods11030357
APA StyleXia, W., Sheng, L., Mu, W., Shi, Y., & Wu, J. (2022). Enzymatic Preparation of Gentiooligosaccharides by a Thermophilic and Thermostable β-Glucosidase at a High Substrate Concentration. Foods, 11(3), 357. https://doi.org/10.3390/foods11030357






