NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity
Abstract
:1. Introduction
2. Materials and Methods
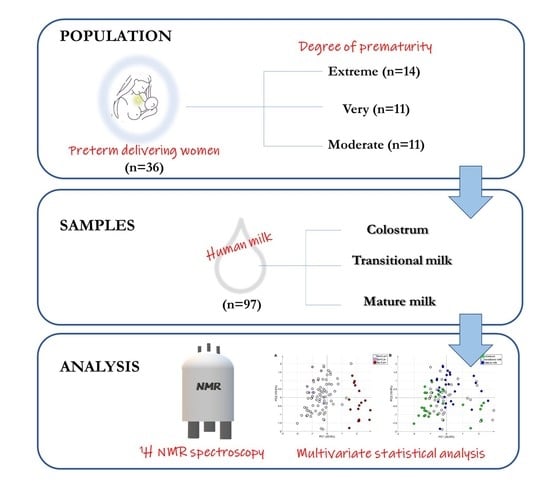
2.1. Study Population
2.2. Sample Collection
2.3. Sample Preparation
2.4. 1H NMR Spectroscopy and Spectral Processing
2.5. Statistical Data Analysis
3. Results
3.1. Study Population
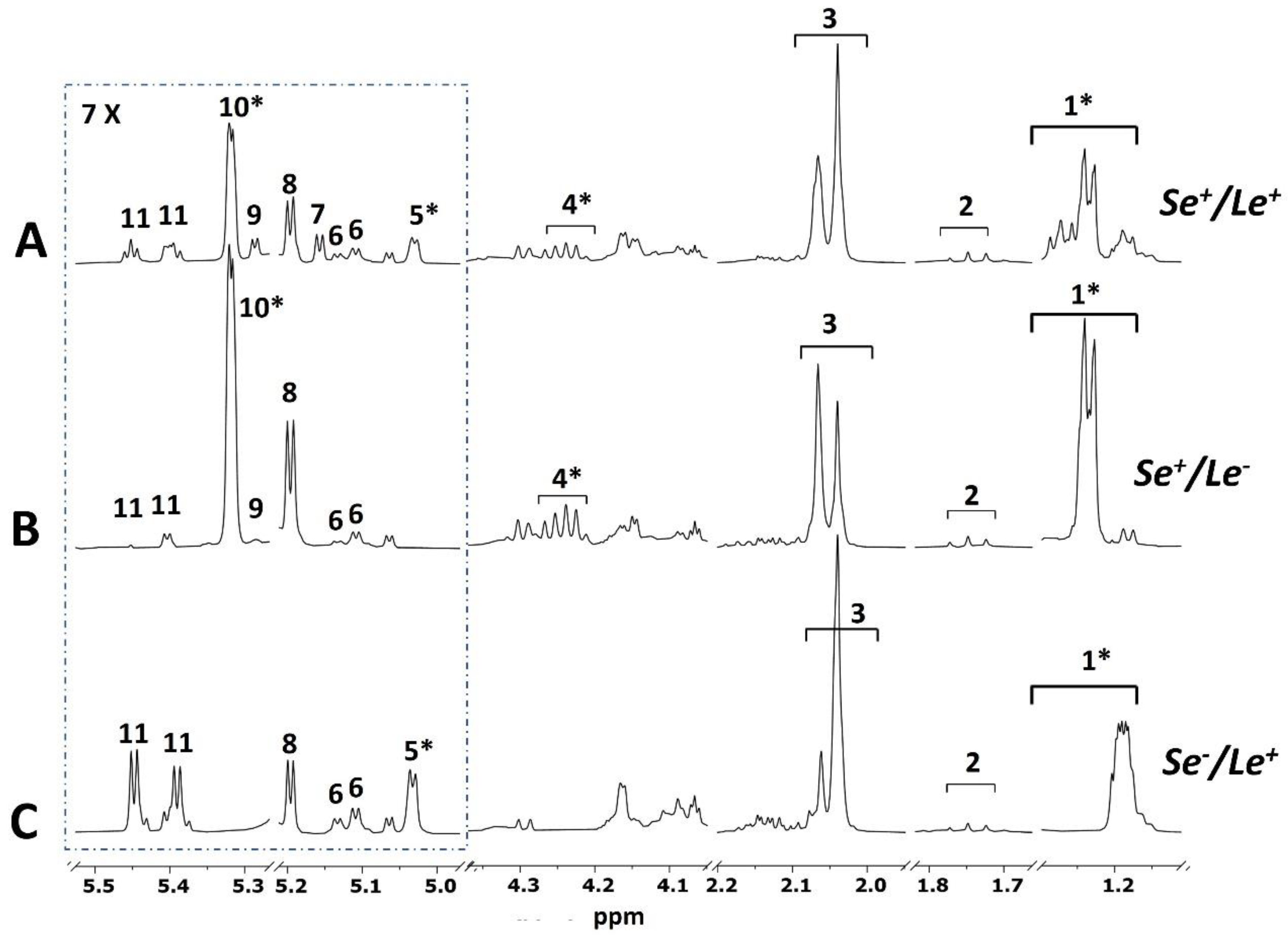
3.2. Mother Phenotype
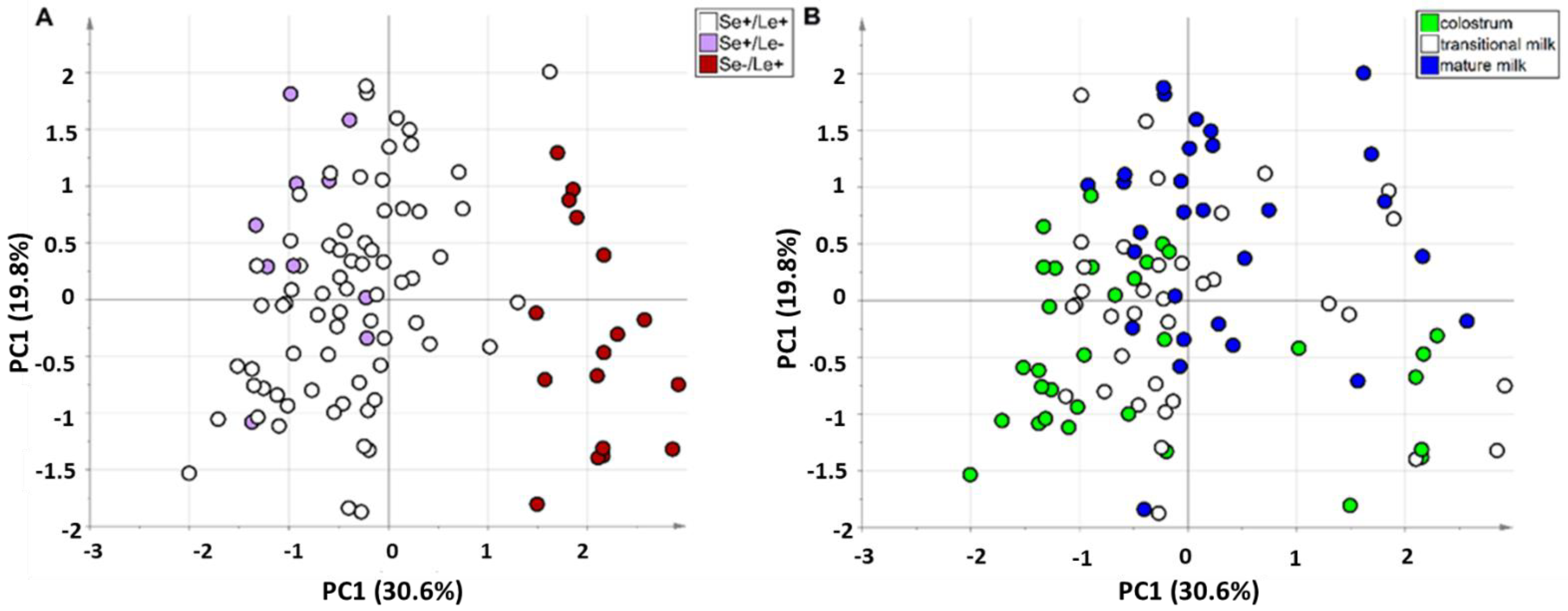
3.3. Exploratory Data Analysis
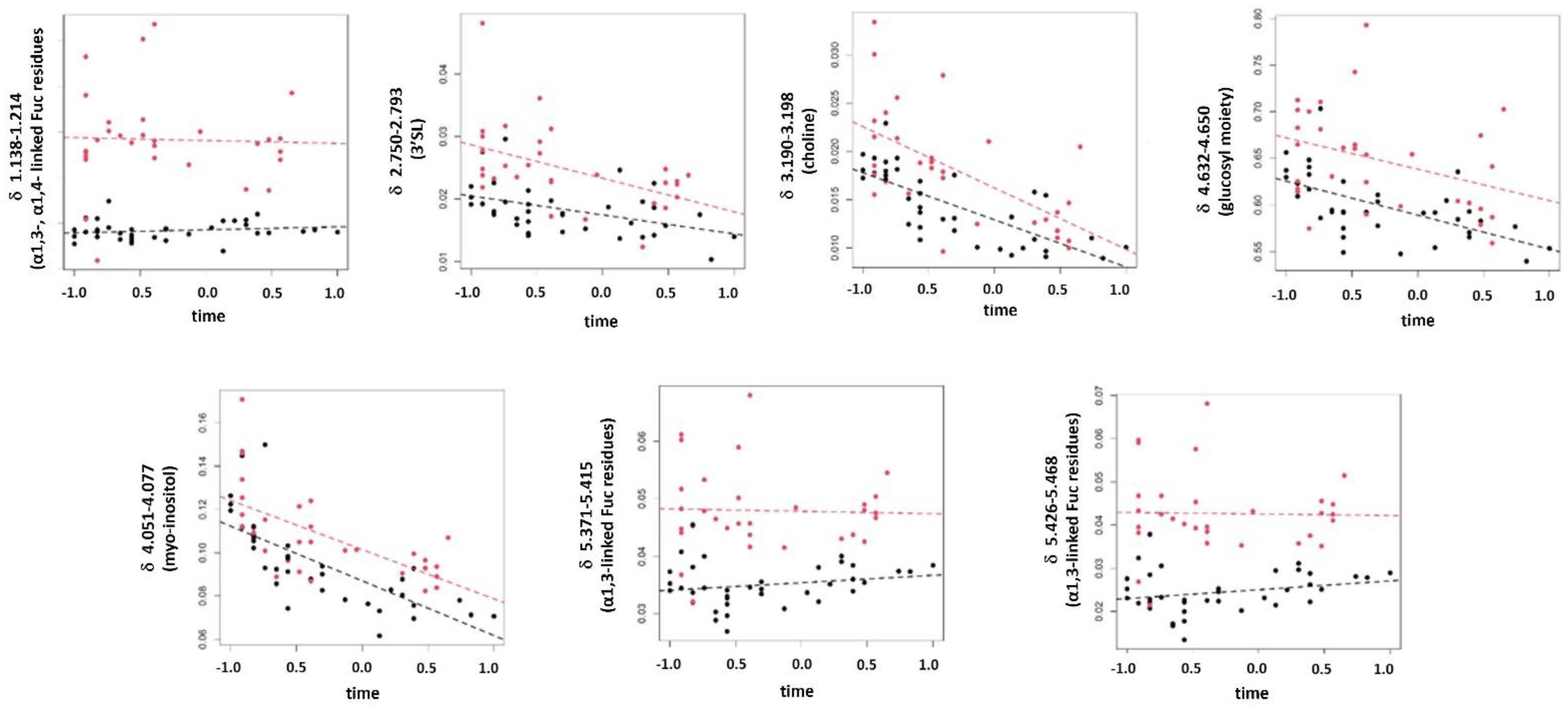
3.4. Studying the Changes in HM Metabolome Due to Degree of Prematurity and Lactation Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Stuebe, A. The Risks of Not Breastfeeding for Mothers and Infants. Rev. Obstet. Gynecol. 2009, 2, 222–231. [Google Scholar] [PubMed]
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef]
- Platt, M.J. Outcomes in preterm infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Committee on Nutrition. Nutritional needs of lowbirth-weight infants. Pediatrics 1985, 76, 976–986. [Google Scholar]
- Longini, M.; Tataranno, M.L.; Proietti, F.; Tortoriello, M.; Belvisi, E.; Vivi, A.; Tassini, M.; Perrone, S.; Buonocore, G. A metabolomic study of preterm and term human and formula milk by proton MRS analysis: Preliminary results. J. Matern. Neonatal Med. 2014, 27, 27–33. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Noto, A.; Caboni, P.; Reali, A.; Barberini, L.; Lussu, M.; Murgia, F.; Santoru, M.L.; Atzori, L.; Fanos, V. A metabolomic study of preterm human and formula milk by high resolution NMR and GC/MS analysis: Preliminary results. J. Matern. Neonatal Med. 2012, 25, 62–67. [Google Scholar] [CrossRef]
- Perrone, S.; Longini, M.; Zollino, I.; Bazzini, F.; Tassini, M.; Vivi, A.; Bracciali, C.; Calderisi, M.; Buonocore, G. Breast milk: To each his own. From metabolomic study, evidence of personalized nutrition in preterm infants. Nutrition 2019, 62, 158–161. [Google Scholar] [CrossRef]
- Briana, D.D.; Fotakis, C.; Kontogeorgou, A.; Gavrili, S.; Georgatzi, S.; Zoumpoulakis, P.; Malamitsi-Puchner, A. Early Human-Milk Metabolome in Cases of Intrauterine Growth–Restricted and Macrosomic Infants. J. Parenter. Enter. Nutr. 2020, 44, 1510–1518. [Google Scholar] [CrossRef]
- Praticò, G.; Capuani, G.; Tomassini, A.; Baldassarre, M.E.; Delfini, M.; Miccheli, A. Exploring human breast milk composition by NMR-based metabolomics. Nat. Prod. Res. 2014, 28, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; Morales, J.; Monleón, D.; du Toit, E.; Kumar, H.; Linderborg, K.; Zhang, Y.; Yang, B.; Isolauri, E.; Salminen, S.; et al. Human Breast Milk NMR Metabolomic Profile across Specific Geographical Locations and Its Association with the Milk Microbiota. Nutrients 2018, 10, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessì, A.; Briana, D.; Gavrili, S.; Corbu, S.; Cesare Marincola, F.; Georgantzi, S.; Pintus, R.; Briana, D.; Fanos, V.; Malamitsi-Puchner, A. Metabolomics of Breast Milk: The Importance of Phenotypes. Metabolites 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilowitz, J.T.; O’sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The Human Milk Metabolome Reveals Diverse Oligosaccharide Profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundekilde, U.; Downey, E.; O’Mahony, J.; O’Shea, C.-A.; Ryan, C.; Kelly, A.; Bertram, H. The Effect of Gestational and Lactational Age on the Human Milk Metabolome. Nutrients 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spevacek, A.R.; Smilowitz, J.T.; Chin, E.L.; Underwood, M.A.; German, J.B.; Slupsky, C.M. Infant Maturity at Birth Reveals Minor Differences in the Maternal Milk Metabolome in the First Month of Lactation. J. Nutr. 2015, 145, 1698–1708. [Google Scholar] [CrossRef] [Green Version]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef]
- Conta, G.; Del Chierico, F.; Reddel, S.; Marini, F.; Sciubba, F.; Capuani, G.; Tomassini, A.; Di Cocco, M.E.; Laforgia, N.; Baldassarre, M.E.; et al. Longitudinal Multi-Omics Study of a Mother-Infant Dyad from Breastfeeding to Weaning: An Individualized Approach to Understand the Interactions Among Diet, Fecal Metabolome and Microbiota Composition. Front. Mol. Biosci. 2021, 8, 688440. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Piccolo, B.D.; Andres, A. Maternal adiposity alters the human milk metabolome: Associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 2020, 112, 1228–1239. [Google Scholar] [CrossRef]
- Wang, A.; Koleva, P.; du Toit, E.; Geddes, D.T.; Munblit, D.; Prescott, S.L.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; et al. The Milk Metabolome of Non-secretor and Lewis Negative Mothers. Front. Nutr. 2021, 7, 380. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Gomez-Romero, M.; Lopez-Gonzalvez, M.A.; Villaseñor, A.; Wijeyesekera, A.; Barbas, C.; Modi, N.; Holmes, E.; Garcia-Perez, I. Multiplatform characterization of dynamic changes in breast milk during lactation. Electrophoresis 2015, 36, 2269–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten-doménech, I.; Ramos-garcia, V.; Piñeiro-ramos, J.D.; Gormaz, M.; Parra-llorca, A.; Vento, M.; Kuligowski, J.; Quintás, G. Current practice in untargeted human milk metabolomics. Metabolites 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, K.O.; Sundekilde, U.K. The Metabolomic Analysis of Human Milk Offers Unique Insights into Potential Child Health Benefits. Curr. Nutr. Rep. 2021, 10, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Human Milk Banking Association of North America. Best Practice for Expressing, Storing and Handling Human Milk in Hospitals, Homes, and Child Care Settings, 3rd ed.; Human Milk Banking Association of North America, Inc.: Fort Worth, TX, USA, 2011. [Google Scholar]
- Arslanoglu, S.; Bertino, E.; Tonetto, P.; De Nisi, G.; Ambruzzi, A.M.; Biasini, A.; Profeti, C.; Spreghini, M.R.; Moro, G.E. Guidelines for the establishment and operation of a donor human milk bank. J. Matern. Neonatal Med. 2010, 23, 1–20. [Google Scholar] [CrossRef]
- Wu, J.; Domell, M.; Zivkovic, A.M.; Larsson, G.; Ohman, A.; Nording, M.L. NMR-based metabolite profiling of human milk: A pilot study of methods for investigating compositional changes during lactation. Biochem. Biophys. Res. Commun. 2016, 469, 626–632. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Madsen, H.; Thyregod, P. Introduction to General and Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420091557. [Google Scholar]
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef]
- Benjamini, Y. Discovering the False Discovery Rate. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 72, 405–416. [Google Scholar] [CrossRef]
- Stocchero, M. Iterative deflation algorithm, eigenvalue equations, and PLS2. J. Chemometr. 2019, 33, e3144. [Google Scholar] [CrossRef]
- Thiel, M.; Féraud, B.; Govaerts, B. ASCA+ and APCA+: Extensions of ASCA and APCA in the analysis of unbalanced multifactorial designs. J. Chemometr. 2017, 31, e2895. [Google Scholar] [CrossRef]
- Stocchero, M. Relevant and irrelevant predictors in PLS2. J. Chemometr. 2020, 34, e3237. [Google Scholar] [CrossRef]
- Van Leeuwen, S.S.; Schoemaker, R.J.W.; Gerwig, G.J.; Van Leusen-Van Kan, E.J.M.; Dijkhuizen, L.; Kamerling, J.P. Rapid milk group classification by 1H NMR analysis of Le and H epitopes in human milk oligosaccharide donor samples. Glycobiology 2014, 24, 728–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Academy of Paediatrics. Breastfeeding and use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Hay, W.W. Strategies for feeding the preterm infant. Neonatology 2008, 94, 245–254. [Google Scholar]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of human milk for preterm infants: Update and recommendations of the european milk bank association (emba) working group on human milk fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- de Halleux, V.; Rigo, J. Variability in human milk composition: Benefit of individualized fortification in very-low-birth-weight infants. Am. J. Clin. Nutr. 2013, 98, 529S–535S. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Poindexter, B.; Uauy, R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev. Nutr. Diet. 2014, 110, 297–299. [Google Scholar]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Wen, L.; Wu, Y.; Yang, Y.; Han, T.; Wang, W.; Fu, H.; Zheng, Y.; Shan, T.; Chen, J.; Xu, P.; et al. Gestational Diabetes Mellitus Changes the Metabolomes of Human Colostrum, Transition Milk and Mature Milk. Med. Sci. Monit. 2019, 25, 6128–6152. [Google Scholar] [CrossRef]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino Acid Profiles in Term and Preterm Human Milk through Lactation: A Systematic Review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef] [Green Version]
- Columbus, D.A.; Fiorotto, M.L.; Davis, T.A. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015, 47, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm Milk Oligosaccharides During the First Month of Lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L. Human milk oligosaccharides in the milk of mothers delivering term versus preterm infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Mu¨ller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Sosa, S.; Martín, M.J.; García-Pardo, L.A.; Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: Variations with the progression of lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Jose Costeira, M.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Ann. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef]
- Ilcol, Y.O.; Ozbek, R.; Hamurtekin, E.; Ulus, I.H. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J. Nutr. Biochem. 2005, 16, 489–499. [Google Scholar] [CrossRef]
- Hallman, M. Inositol during perinatal transition. NeoReviews 2015, 16, e84–e93. [Google Scholar] [CrossRef]
- Cavalli, C.; Teng, C.; Battaglia, F.C.; Bevilacqua, G. Free sugar and sugar alcohol concentrations in human breast milk. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, P.; Hallman, M. Myoinositol in small preterm infants: Relationship between intake and serum concentration. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.E.; Zechalko, A.; Murphy, J.; Brooke, O.G. Comparison of the B vitamin composition of milk from mothers of preterm and term babies. Arch. Dis. Child. 1983, 58, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Bijur, A.M.; Kumbhat, M.M. Vitamin-B composition of breast milk from mothers of preterm and term babies. Indian Pediatr. 1987, 24, 33–37. [Google Scholar] [PubMed]
- Nolan, L.S.; Lewis, A.N.; Gong, Q.; Sollome, J.J.; DeWitt, O.N.; Williams, R.D.; Good, M. Untargeted Metabolomic Analysis of Human Milk from Mothers of Preterm Infants. Nutrients 2021, 13, 3604. [Google Scholar] [CrossRef]
- Redeuil, K.; Lévêques, A.; Oberson, J.-M.; Bénet, S.; Tissot, E.; Longet, K.; de Castro, A.; Romagny, C.; Beauport, L.; Fischer Fumeaux, C.J.; et al. Vitamins and carotenoids in human milk delivering preterm and term infants: Implications for preterm nutrient requirements and human milk fortification strategies. Clin. Nutr. 2021, 40, 222–228. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Beard, E.; Uldry, J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 2011, 14, 1315–1324. [Google Scholar] [CrossRef] [Green Version]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef]
- Berry, M.J.; Schlegel, M.; Kowalski, G.M.; Bruce, C.R.; Callahan, D.L.; Davies-Tuck, M.L.; Dickinson, H.; Goodson, A.; Slocombe, A.; Snow, R.J. UNICORN Babies: Understanding Circulating and Cerebral Creatine Levels of the Preterm Infant. An Observational Study Protocol. Front. Physiol. 2019, 10, 142. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.M.; Yun, B.; Kim, M.; Song, M.; Kim, Y.H.; Lee, S.H.; Lee, H.; Lee, S.M.; Lee, S.M. Postoperative serum metabolites of patients on a low carbohydrate ketogenic diet after pancreatectomy for pancreatobiliary cancer: A nontargeted metabolomics pilot study. Sci. Rep. 2019, 9, 16820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, S.; Rolfo, C.; Maksymiuk, A.W.; Tappia, P.S.; Sitar, D.S.; Russo, A.; Akhtar, P.S.; Khatun, N.; Rahnuma, P.; Rashiduzzaman, A.; et al. Liquid biopsy in lung cancer screening: The contribution of metabolomics. Results A Pilot Study. Cancers 2019, 11, 1069. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, M.; Paris, D.; Molino, A.; Cuomo, P.; Fulgione, A.; Sorrentino, N.; Palomba, L.; Maniscalco, M.; Motta, A. The Immune-Modulator Pidotimod Affects the Metabolic Profile of Exhaled Breath Condensate in Bronchiectatic Patients: A Metabolomics Pilot Study. Front. Pharmacol. 2019, 10, 1115. [Google Scholar] [CrossRef] [Green Version]

| Extremely Preterm (n = 14) | Very Preterm (n = 11) | Moderately Preterm (n = 11) | |
|---|---|---|---|
| Mothers | |||
| Maternal age, y (ANOVA, p = 0.48) | 34.6 ± 5.1 | 34.6 ± 4.3 | 35.2 ± 3.4 |
| Maternal BMI, kg/m2 (ANOVA, p = 0.41) | 23.4 ± 3.9 | 22.9± 3.6 | 24.6 ± 6.9 |
| Type of pregnancy (Singleton/Twins) (Chi-squared test, p = 0.69) | 12/2 | 9/2 | 8/3 |
| Mode of delivery (vaginal/casarean section) (Chi-squared test, p = 0.006) | 10/4 | 2/9 | 2/9 |
| Infants | |||
| Gender (Male/Female) (Chi-squared test, p = 0.70) | 6/10 | 6/7 | 5/9 |
| Birth weight, g (ANOVA, p = 0.002) | 977 ± 233 | 1382 ± 357 | 1369 ± 375 |
| Gestational age, wk [min–max] | 26 [23–28] | 30 [29–31] | 33 [32,33] |
| Milk Samples | |||
| Colostrum (3−6 lactation days) | 12 | 9 | 11 |
| Transitional milk (7−15 lactation days) | 14 | 11 | 11 |
| Mature milk (16–26 lactation days) | 12 | 8 | 9 |
| Lewis (Le) and Secretor (Se) phenotype of mothers2 (Chi-square test, p = 0.33) | |||
| Se+/Le+ | 11 | 9 | 6 |
| Se−/Le+ | 1 | 1 | 4 |
| Se+/Le− | 2 | 1 | 1 |
| Integrated Region 1 (ppm) | Annotation 2 | Coefficient 3 | p[time] 4 | BH 5 | R2total 6 |
|---|---|---|---|---|---|
| 4.051–4.077 | myo-inositol | −2.5 × 10−2 | 8.5 × 10−11 | 1 | 6.6 × 10−1 |
| 4.632–4.650 | glucosyl moiety | −1.3 × 10−2 | 9.2 × 10−9 | 1 | 9.7 × 10−1 |
| 4.515–4.548 | galactose moiety in α1,2-linked Fuc | −4.3 × 10−2 | 1.5 × 10−8 | 1 | 8.1 × 10−1 |
| 4.203–4.274 | α1,2-linked Fuc residues 7 | −6.2 × 10−2 | 1.9 × 10−8 | 1 | 8.7 × 10−1 |
| 5.304–5.336 | α1,2-linked Fuc residues 8 | −5.2 × 10−2 | 2.6 × 10−8 | 1 | 8.9 × 10−1 |
| 4.278–4.322 | α1,2-linked Fuc residues | −1.7 × 10−2 | 4.8 × 10−8 | 1 | 9.7 × 10−1 |
| 1.215–1.294 | CH3 in Fuc(α1-2) 9 | −2.6 × 10−1 | 1.2 × 10−7 | 1 | 8.9 × 10−1 |
| 5.220–5.254 | lactose | 5.1 × 10−2 | 2.1 × 10−7 | 1 | 8.6 × 10−1 |
| 3.274–3.322 | lactose | 8.3 × 10−2 | 3.8 × 10−7 | 1 | 8.2 × 10−1 |
| 3.190–3.198 | choline | −6.6 × 10−3 | 4.4 × 10−7 | 1 | 7.1 × 10−1 |
| 3.033–3.055 | creatine and creatinine | −2.7 × 10−3 | 8.6 × 10−7 | 1 | 2.9 × 10−1 |
| 5.181–5.210 | glucosyl moieties | −1.6 × 10−2 | 1.8 × 10−6 | 1 | 8.8 × 10−1 |
| 1.467–1.498 | alanine | 6.2 × 10−3 | 3.1 × 10−6 | 1 | 5.1 × 10−1 |
| 0.926–0.941 | pantothenate | −4.8 × 10−3 | 8.1 × 10−6 | 1 | 3.9 × 10−1 |
| 3.001–3.015 | U | −8.9 × 10−4 | 2.0 × 10−5 | 1 | 6.1 × 10−1 |
| 3.455–3.522 | U | −3.9 × 10−2 | 2.1 × 10−5 | 1 | 7.1 × 10−1 |
| 2.397–2.485 | glutamine | 5.7 × 10−3 | 2.1 × 10−5 | 1 | 5.4 × 10−1 |
| 2.648–2.703 | citrate | −4.6 × 10−2 | 2.2 × 10−5 | 1 | 5.7 × 10−1 |
| 3.226–3.237 | GPC | 5.0 × 10−2 | 2.4 × 10−5 | 1 | 2.8 × 10−1 |
| 2.750–2.793 | 3′SL | −4.8 × 10−3 | 3.7 × 10−5 | 1 | 8.2 × 10−1 |
| 2.518–2.703 10 | citrate | −8.7 × 10−2 | 4.1 × 10−5 | 1 | 5.7 × 10−1 |
| 2.518–2.574 | citrate | −4.1 × 10−2 | 8.5 × 10−5 | 1 | 5.8 × 10−1 |
| 2.331–2.385 | glutamate | 1.9 × 10−2 | 1.2 × 10−4 | 1 | 5.5 × 10−1 |
| 4.133–4.155 | galactose moiety | −1.6 × 10−2 | 1.4 × 10−4 | 1 | 9.5 × 10−1 |
| 2.015–2.086 | N-Acetylglucosammine | −1.4 × 10−1 | 1.9 × 10−4 | 1 | 8.4 × 10−1 |
| 1.691–1.781 | 3′SL, 6′SL | −1.1 × 10−2 | 2.8 × 10−4 | 1 | 9.0 × 10−1 |
| 3.124–3.177 | U | 2.4 × 10−3 | 3.7 × 10−4 | 1 | 7.9 × 10−1 |
| 8.368–8.453 | U | −4.7 × 10−3 | 4.3 × 10−4 | 1 | 9.7 × 10−1 |
| 3.199–3.207 | U | −6.4 × 10−3 | 5.5 × 10−4 | 1 | 7.5 × 10−1 |
| 0.890–0.941 11 | pantothenate | −1.1 × 10−2 | 6.4 × 10−4 | 1 | 3.9 × 10−1 |
| 0.945–0.979 | leucine | −3.1 × 10−3 | 4.2 × 10−3 | 1 | 3.5 × 10−1 |
| 0.890–0.906 | pantothenate | −5.8 × 10−3 | 9.6 × 10−3 | 1 | 3.9 × 10−1 |
| 4.156–4.173 | galactose moieties | −1.2 × 10−2 | 1.9 × 10−2 | 1 | 8.9 × 10−1 |
| 5.277–5.296 | α1,2-linked Fuc residues 12 | −5.7 × 10−3 | 2.0 × 10−2 | 1 | 6.9 × 10−1 |
| 1.315–1.344 | threonine | −1.5 × 10−2 | 7.8 × 10−2 | 0 | 4.1 × 10−1 |
| 3.215–3.225 | phosphocholine | 2.8 × 10−2 | 8.4 × 10−2 | 0 | 6.9 × 10−1 |
| 5.019–5.047 | α1,4-linked Fuc residues | 2.4 ×10−3 | 1.3 × 10−1 | 0 | 9.3 × 10−1 |
| 5.148–5.169 | α1,2-linked Fuc residues 13 | −1.4 × 10−3 | 1.9 × 10−1 | 0 | 9.6 × 10−1 |
| 0.980–1.002 | valine | −4.3 × 10−4 | 3.0 × 10−1 | 0 | 2.6 × 10−1 |
| 1.032–1.057 | valine | 1.7 × 10−4 | 5.5 × 10−1 | 0 | 4.3 × 10−1 |
| 1.138–1.214 | CH3 in α1,3-Fuc and α1,4-Fuc | −8.5 × 10−3 | 6.0 × 10−1 | 0 | 9.3 × 10−1 |
| 0.98–1.057 14 | valine | −2.7 × 10−4 | 7.0 × 10−1 | 0 | 3.3 × 10−1 |
| 5.371–5.415 | α1,3-linked Fuc residues 15 | −5.5 × 10−4 | 8.0 × 10−1 | 0 | 8.4 × 10−1 |
| 5.426–5.468 | α1,3-linked Fuc residues 15 | −5.2 × 10−5 | 9.8 × 10−1 | 0 | 8.4 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peila, C.; Sottemano, S.; Cesare Marincola, F.; Stocchero, M.; Pusceddu, N.G.; Dessì, A.; Baraldi, E.; Fanos, V.; Bertino, E. NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity. Foods 2022, 11, 345. https://doi.org/10.3390/foods11030345
Peila C, Sottemano S, Cesare Marincola F, Stocchero M, Pusceddu NG, Dessì A, Baraldi E, Fanos V, Bertino E. NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity. Foods. 2022; 11(3):345. https://doi.org/10.3390/foods11030345
Chicago/Turabian StylePeila, Chiara, Stefano Sottemano, Flaminia Cesare Marincola, Matteo Stocchero, Nicoletta Grazia Pusceddu, Angelica Dessì, Eugenio Baraldi, Vassilios Fanos, and Enrico Bertino. 2022. "NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity" Foods 11, no. 3: 345. https://doi.org/10.3390/foods11030345
APA StylePeila, C., Sottemano, S., Cesare Marincola, F., Stocchero, M., Pusceddu, N. G., Dessì, A., Baraldi, E., Fanos, V., & Bertino, E. (2022). NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity. Foods, 11(3), 345. https://doi.org/10.3390/foods11030345