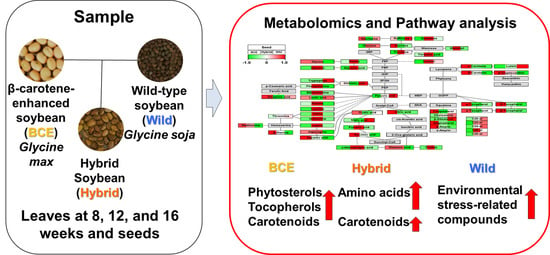
Metabolomic Variability of Different Soybean Genotypes: β-Carotene-Enhanced (Glycine max), Wild (Glycine soja), and Hybrid (Glycine max × Glycine soja) Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Extraction and Analysis of Hydrophilic Compounds
2.3. Carotenoid Extraction and Analysis
2.4. Extraction and Analysis of Policosanols, Tocopherols, and Sterols
2.5. Statistical Analysis
3. Results and Discussion
3.1. PCA of Seeds
3.2. Pearson’s Correlation Analysis and HCA of Seeds
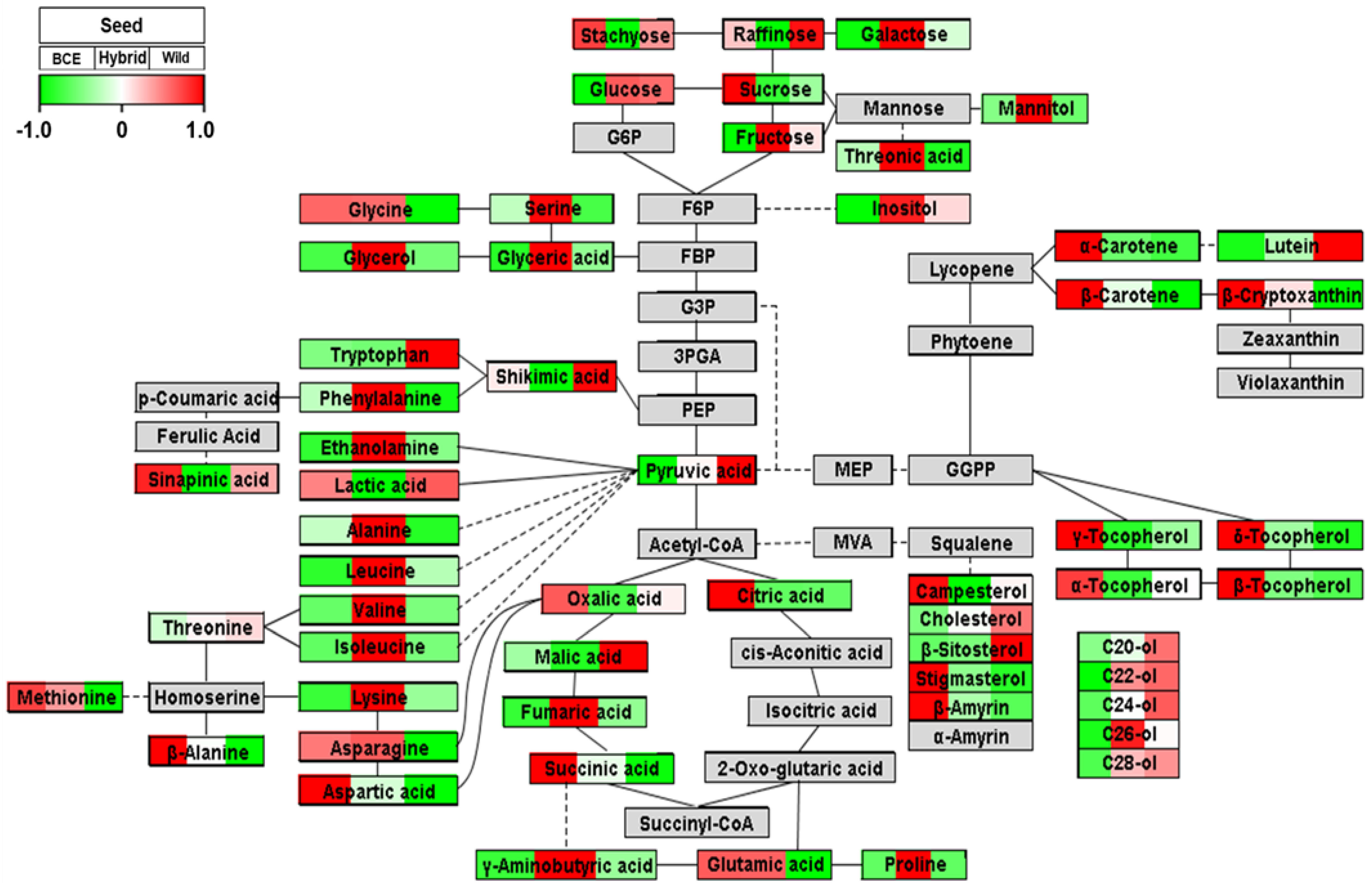
3.3. Pathway Analysis of Seeds
3.4. PCA of Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean—Worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Masuda, T.; Goldsmith, P.D. World soybean production: Area harvested, yield, and long-term projections. Int. Food Agribus. Manag. Rev. 2009, 12, 1–20. [Google Scholar]
- Kim, I.-S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Kerwin, S.M. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Juillerat, M.A.; Lee, C.-H. Cholesterol lowering mechanism of soybean protein hydrolysate. J. Agric. Food Chem. 2007, 55, 10599–10604. [Google Scholar] [CrossRef]
- Tikkanen, M.J.; Adlercreutz, H. Dietary soy-derived isoflavone phytoestrogens: Could they have a role in coronary heart disease prevention? Biochem. Pharmacol. 2000, 60, 1–5. [Google Scholar] [CrossRef]
- Hermansen, K.; Søndergaard, M.; Høie, L.; Carstensen, M.; Brock, B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care 2001, 24, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention: Clinical science review. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guan, R.; Liu, Z.; Ma, Y.; Wang, L.; Li, L.; Lin, F.; Luan, W.; Chen, P.; Yan, Z.; et al. Genetic structure and diversity of cultivated soybean (Glycine max (L.) Merr.) landraces in China. Theor. Appl. Genet. 2008, 117, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Yu, B.-J.; Guo, J.-S. Ameliorative effects of inoculation with Bradyrhizobium japonicum on Glycine max and Glycine soja seedlings under salt stress. Plant Growth Regul. 2016, 80, 137–147. [Google Scholar] [CrossRef]
- Hyeon, H.; Xu, J.L.; Kim, J.K.; Choi, Y. Comparative metabolic profiling of cultivated and wild black soybeans reveals distinct metabolic alterations associated with their domestication. Food Res. Int. 2020, 134, 109290. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, P.; de los Reyes, B.G. Differential responses of the cultivated and wild species of soybean to dehydration stress. Crop. Sci. 2006, 46, 2041–2046. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, W.; Zhang, C.; Yang, L.; Chang, R.Z.; Gaut, B.S.; Qiu, L.J. Genetic diversity in domesticated soybean (Glycine max) and its wild progenitor (Glycine soja) for simple sequence repeat and single-nucleotide polymorphism loci. N. Phytol. 2010, 188, 242–253. [Google Scholar] [CrossRef]
- Robison, J.; Arora, N.; Yamasaki, Y.; Saito, M.; Boone, J.; Blacklock, B.; Randall, S. Glycine max and Glycine soja are capable of cold acclimation. J. Agron. Crop. Sci. 2017, 203, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, X.; Yuan, X.; Shi, J.; Zhang, C.; Yan, N.; Jing, C. Comparison of phenolic and flavonoid compound profiles and antioxidant and α-glucosidase inhibition properties of cultivated soybean (Glycine max) and wild Soybean (Glycine soja). Plants 2021, 10, 813. [Google Scholar] [CrossRef]
- Chaudhary, J.; Deshmukh, R.; Mir, Z.A.; Bhat, J.A. Metabolomics: An Emerging Technology for Soybean Improvement. In Biotechnology Products in Everyday Life; Khoobchandani, M., Saxena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–186. [Google Scholar]
- Kim, M.-J.; Kim, J.K.; Kim, H.J.; Pak, J.H.; Lee, J.-H.; Kim, D.-H.; Choi, H.K.; Jung, H.W.; Lee, J.-D.; Chung, Y.-S.; et al. Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS ONE 2012, 7, e48287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Park, S.-Y.; Oh, S.-W.; Lim, M.-H.; Shin, K.-S.; Cho, H.-S.; Lee, S.-K.; Woo, H.-J. Nutritional composition analysis for beta-carotene-enhanced transgenic soybeans (Glycine max L.). Appl. Biol. Chem. 2017, 60, 299–309. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant. 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Lu, B.-R. Conserving biodiversity of soybean gene pool in the biotechnology era. Plant Species Biol. 2004, 19, 115–125. [Google Scholar] [CrossRef]
- Scurrah, M.; Celis-Gamboa, C.; Chumbiauca, S.; Salas, A.; Visser, R.G.F. Hybridization between wild and cultivated potato species in the Peruvian Andes and biosafety implications for deployment of GM potatoes. Euphytica 2008, 164, 881–892. [Google Scholar] [CrossRef]
- Finger, R.; El Benni, N.; Kaphengst, T.; Evans, C.; Herbert, S.; Lehmann, B.; Morse, S.; Stupak, N. A meta analysis on farm-level costs and benefits of GM crops. Sustainability 2011, 3, 743–762. [Google Scholar] [CrossRef] [Green Version]
- Brookes, G.; Barfoot, P. Economic impact of GM crops: The global income and production effects 1996–2012. GM Crop. Food 2014, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Lam, H.-M.; Pi, E.; Zhan, Q.; Tsai, S.; Wang, C.; Kwan, Y.; Ngai, S. Comparative metabolomics in Glycine max and Glycine soja under salt stress to reveal the phenotypes of their offspring. J. Agric. Food Chem. 2013, 61, 8711–8721. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, D.-Y.; Kang, Y.-G.; Kim, M.; Kim, D.; Kim, E.-H.; Hong, Y.-S. Metabotyping of different soybean genotypes and distinct metabolism in their seeds and leaves. Food Chem. 2020, 330, 127198. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jang, Y.; Park, S.; Suh, S.; Lee, B. Characterization of hybrid soybean seeds between β-carotene enhanced transgenic soybean and wild soybean. Weed Turfgrass Sci. 2020, 9, 129–139. [Google Scholar]
- Oh, S.-D.; Jang, Y.-J.; Lee, G.M.; Lee, K.; Suh, S.J.; Ryu, T.-H.; Park, S.-Y. Comparison of the nutritional compositions of hybrid soybean between β–carotene enhanced transgenic soybean and wild soybean. J. Korean Soc. Int. Agric. 2020, 32, 339–347. [Google Scholar] [CrossRef]
- Kim, M.S.; Baek, S.-H.; Park, S.U.; Im, K.-H.; Kim, J.K. Targeted metabolite profiling to evaluate unintended metabolic changes of genetic modification in resveratrol-enriched rice (Oryza sativa L.). Appl. Biol. Chem. 2017, 60, 205–214. [Google Scholar] [CrossRef]
- Creek, D.J.; Dunn, W.B.; Fiehn, O.; Griffin, J.L.; Hall, R.D.; Lei, Z.; Mistrik, R.; Neumann, S.; Schymanski, E.L.; Sumner, L.W.; et al. Metabolite identification: Are you sure? And how do your peers gauge your confidence? Metabolomics 2014, 10, 350–353. [Google Scholar] [CrossRef]
- Kim, T.J.; Choi, J.; Kim, K.W.; Ahn, S.K.; Ha, S.-H.; Choi, Y.; Park, N.I.; Kim, J.K. Metabolite profiling of peppers of various colors reveals relationships between tocopherol, carotenoid, and phytosterol content. J. Food Sci. 2017, 82, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Ho, H.M.; Chen, R.Y.; Leung, L.K.; Chan, F.L.; Huang, Y.; Chen, Z.-Y. Difference in flavonoid and isoflavone profile between soybean and soy leaf. Biomed. Pharmacother. 2002, 56, 289–295. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Cherif, A. The Peanut Used as a Source of Phytochemicals. In Bioactivities and Allergies; Expósito, I.L., Blazquez, A.B., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 77–177. [Google Scholar]
- Austin, J.R.; Frost, E.; Vidi, P.-A.; Kessler, F.; Staehelin, L.A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef] [Green Version]
- Yun, D.-Y.; Kang, Y.-G.; Yun, B.; Kim, E.-H.; Kim, M.; Park, J.S.; Lee, J.H.; Hong, Y.-S. Distinctive metabolism of flavonoid between cultivated and semiwild soybean unveiled through metabolomics approach. J. Agric. Food Chem. 2016, 64, 5773–5783. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Graham, I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Ponce, M.A.; Cao, X.; Yang, Z.; Ohlrogge, J.B. Lipid turnover during senescence. Plant Sci. 2013, 205, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Lee, S.Y.; Chu, S.M.; Lim, S.H.; Suh, S.-C.; Lee, Y.-T.; Cho, H.S.; Ha, S.-H. Variation and correlation analysis of flavonoids and carotenoids in Korean pigmented rice (Oryza sativa L.) cultivars. J. Agric. Food Chem. 2010, 58, 12804–12809. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, T.; Jing, Y.; Fan, L.; Wan, Y.; Lin, J. γ-Aminobutyric acid (GABA) homeostasis regulates pollen germination and polarized growth in Picea wilsonii. Planta 2013, 238, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.-M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.-L.; Li, M.-W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.U.; Ha, S.-H.; Lim, S.H.; Kim, J.K. Improved quantification of γ-aminobutyric acid in rice using stable isotope dilution gas chromatography-mass spectrometry. Food Chem. 2018, 266, 375–380. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-W.; Park, S.-Y.; Oh, S.-D.; Jang, Y.; Suh, S.-J.; Park, S.-K.; Ha, S.-H.; Park, S.-U.; Kim, J.-K. Metabolomic Variability of Different Soybean Genotypes: β-Carotene-Enhanced (Glycine max), Wild (Glycine soja), and Hybrid (Glycine max × Glycine soja) Soybeans. Foods 2021, 10, 2421. https://doi.org/10.3390/foods10102421
Jung J-W, Park S-Y, Oh S-D, Jang Y, Suh S-J, Park S-K, Ha S-H, Park S-U, Kim J-K. Metabolomic Variability of Different Soybean Genotypes: β-Carotene-Enhanced (Glycine max), Wild (Glycine soja), and Hybrid (Glycine max × Glycine soja) Soybeans. Foods. 2021; 10(10):2421. https://doi.org/10.3390/foods10102421
Chicago/Turabian StyleJung, Jung-Won, Soo-Yun Park, Sung-Dug Oh, Yejin Jang, Sang-Jae Suh, Soon-Ki Park, Sun-Hwa Ha, Sang-Un Park, and Jae-Kwang Kim. 2021. "Metabolomic Variability of Different Soybean Genotypes: β-Carotene-Enhanced (Glycine max), Wild (Glycine soja), and Hybrid (Glycine max × Glycine soja) Soybeans" Foods 10, no. 10: 2421. https://doi.org/10.3390/foods10102421
APA StyleJung, J.-W., Park, S.-Y., Oh, S.-D., Jang, Y., Suh, S.-J., Park, S.-K., Ha, S.-H., Park, S.-U., & Kim, J.-K. (2021). Metabolomic Variability of Different Soybean Genotypes: β-Carotene-Enhanced (Glycine max), Wild (Glycine soja), and Hybrid (Glycine max × Glycine soja) Soybeans. Foods, 10(10), 2421. https://doi.org/10.3390/foods10102421