The Effects of Antioxidants and Packaging Methods on Inhibiting Lipid Oxidation in Deep Fried Crickets (Gryllus bimaculatus) during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Cricket Samples
2.3. Preparation of Deep Fried Crickets
2.4. Lipid Extraction
2.5. Analysis of Composition of Fatty Acids
2.6. Determination of the Contents of Free Fatty Acids (FFAs)
2.7. Determination of Peroxide Value (PV)
2.8. Determination of Thiobarbituric Acid Reactive Substances (TBARs)
2.9. Statistical Analysis
3. Results
3.1. Fatty Acid Compositions of Raw Gryllus bimaculatus and Palm Oil
3.2. Changes in Fatty Acid Composition of Deep Fried Samples
3.3. Contents of FFAs in Deep Fried Samples
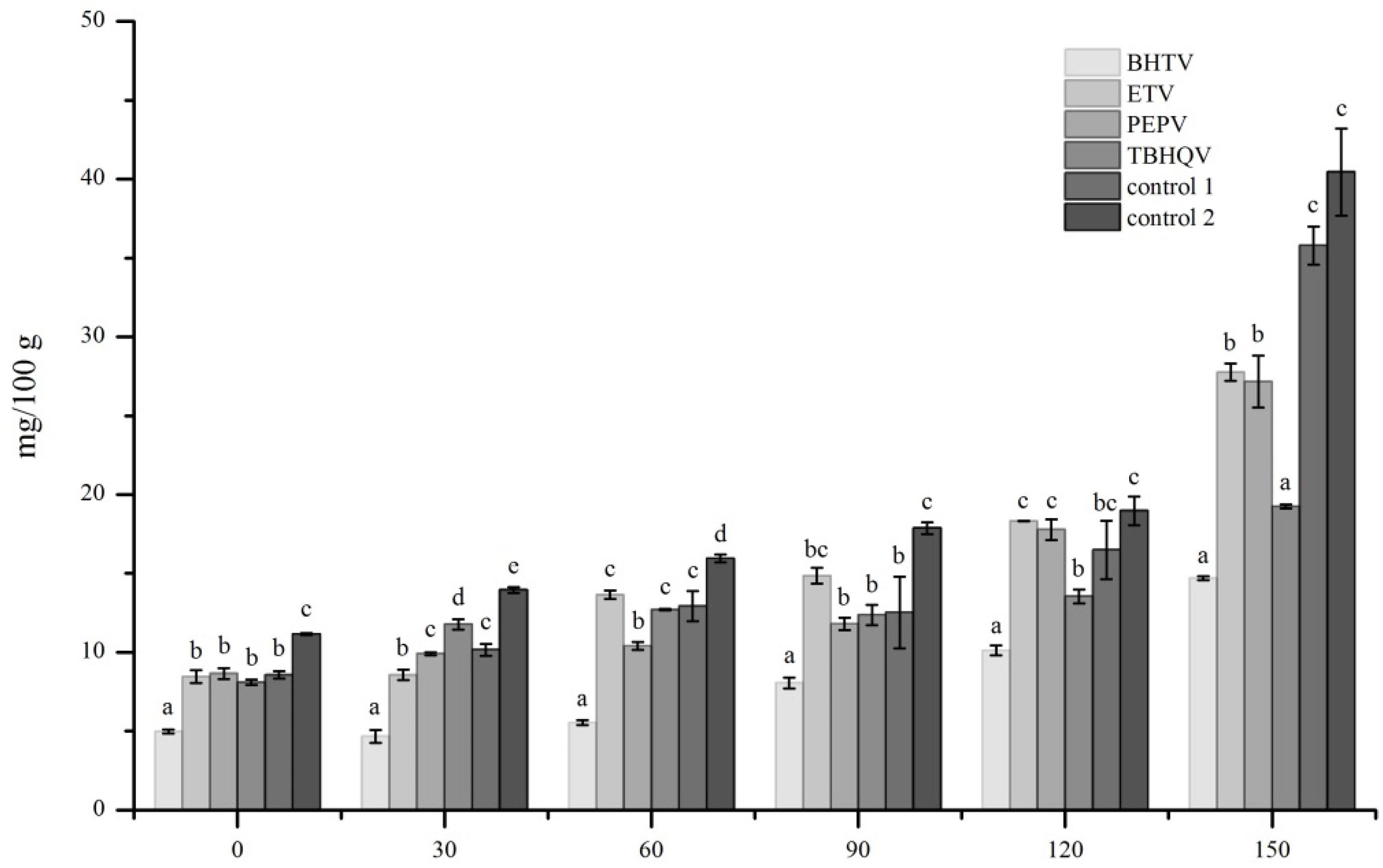
3.4. PV of Deep Fried Samples
3.5. TBARs of Deep Fried Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.Y.; Ding, W.F. Edible insects in China: Utilization and prospects. Insect Sci. 2017, 25, 184–198. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- Vantomme, P. Way forward to bring insects in the human food chain. J. Insects Food Feed. 2015, 1, 121–129. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Tanga, C.M.; Ayieko, M.A.; Hugel, S.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Niassy, S.; Sevgan, S.; Fiaboe, K.K.M.; et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2019, 112, 653–664. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Niassy, S.; Ayieko, A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible crickets (Orthoptera) around the world: Distribution, nutritional value, and other benefits-a review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef]
- Halloran, A.; Caparros-Megido, R.; Oloo, J.; Weigel, T.; Nsevolo, P.; Francis, T. Comparative aspects of cricket farming in Thailand, Cambodia, Lao People’s Democratic Republic, Democratic Republic of the Congo and Kenya. J. Insects Food Feed. 2018, 4, 101–114. [Google Scholar] [CrossRef]
- Wang, D.; Bai, Y.; Li, J.; Zhang, C. Nutritional value of the field cricket (gryllus testaceus walker). Entomol. Sin. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Ghosha, S.; Leeb, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Reverberi, M. Edible insects: Cricket farming and processing as an emerging market. J. Insects Food Feed. 2020, 6, 211–220. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Ghosh, S.; Jung, C. Farming of insects for food in South Korea: Tradition and innovation. Berl. Und Muenchener Tieraerztliche Wochenschr. 2019, 131, 236–244. [Google Scholar]
- Miech, P.; Berggren, A.; Lindberg, J.E.; Chhay, T.; Khieu, B.; Jansson, A. Growth and survival of reared Cambodian field crickets (Teleogryllu stestaceus) fed weeds, agricultural and food industry by-products. J. Insects Food Feed. 2016, 2, 285–292. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Kipkoech, C. Production and growth parameters of edible crickets: Experiences from a farm in a high altitude, cooler region of Kenya. J. Insects Food Feed. 2018, 4, 247–251. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Dossey, A.T. Age-dependent food utilization of Acheta domesticus (Orthoptera: Gryllidae) in small groups at two temperatures. J. Insects Food Feed. 2018, 4, 51–60. [Google Scholar] [CrossRef]
- Machado, C.D.R.; Thys, R.C.S. Cricket (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Homann, A.M.; Ayieko, M.A.; Konyole, S.O.; Roos, N. Acceptability of biscuits containing 10% cricket (Acheta domesticus) compared to milk biscuits among 5–10-year-old Kenyan schoolchildren. J. Insects Food Feed. 2017, 3, 95–103. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełminska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and nutritional/ textural properties of durum wheat pasta enriched with cricket powder. Foods 2017, 8, 46. [Google Scholar] [CrossRef]
- Kim, H.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.B. Effect of house cricket (Acheta domesticus) flour addition on physicochemical and textural properties of meat emulsion under various formulations. J. Food Sci. 2017, 81. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Flore, R.; Hanboonsong, Y. The development of the edible cricket industry in Thailand. J. Insects Food Feed. 2016, 2, 91–100. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, M.; Ding, W.F.; Chen, X.M. Overview of edible insect resources and common species utilisation in China. J. Insects Food Feed. 2020, 6, 13–25. [Google Scholar] [CrossRef]
- The National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard: Determination of Fatty Acid in Food GB 5009.168–2016; China Standards Press: Beijing, China, 2016; pp. 1–6. [Google Scholar]
- Wang, Y.; Jiang, Y.; Cao, J.; Chen, Y.; Sun, Y.; Zeng, X.; Pan, D.; Ou, C.; Gan, N. Study on lipolysis-oxidation and volatile flavour compounds of dry cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- The National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard: Determination of Peroxide Value in Food GB 5009.227–2016; China Standards Press: Beijing, China, 2016; pp. 1–4. [Google Scholar]
- The National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard: Determination of Malondialdehyde in Food GB 5009.181–2016; China Standards Press: Beijing, China, 2016; pp. 3–5. [Google Scholar]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Provijn, P.; Brouwers, J.F.; Meulenaer, B.; Oonincx, D. Lipidome of cricket species used as food. Food Chem. 2001, 349, 129077. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, L.; Wang, C.; Feng, Y.; Zhao, M. Nutritional composition analysis and evaluation of the two-spotted cricket Gryllus bimaculatus (Orthoptera:Gryllidae). Biot. Resour. 2021, 43, 303–308. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Lim, J.; Chang, Y.; Lee, Y.; Park, J.J.; Ahn, M. Physicochemical properties of edible cricket (Gryllus bimaculatus) in different districts. Korean J. Food Preserv. 2015, 22, 831–837. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Li, S.; Peng, Z. Effects of pepper (Zanthoxylum bungeanum Maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.; Ribeiro, C.P.; Victorio, A.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Garcia-Arias, M.T.; Pontes, E.A.; Garda-Linares, M.C.; Garcfa-Femandez, M.C.; Sanchez-Muniz, F.J. Cooking-freezing-reheating (CFR) of sardine (Sardina pilchardus) fillets. Effect of different cooking and reheating procedures on the proximate and fatty acid compositions. Food Chem. 2003, 83, 349–356. [Google Scholar] [CrossRef]
- Timón, M.L.; Ventanas, J.; Carrapiso, A.I.; Jurado, A.; GarcõÂa, C. Subcutaneous and intermuscular fat characterisation of dry-cured Iberian hams. Meat Sci. 2001, 58, 85–91. [Google Scholar] [CrossRef]
- Huang, L.; Xiong, Y.; Kong, B.; Huang, X.; Li, J. Influence of storage temperature and duration on lipid and protein oxidation and flavour changes in frozen pork dumpling filler. Meat Sci. 2013, 95, 295–301. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, C.; Guo, A.; Han, D. Effect of drying temperature on fatty acid composition in air-dried beef during chilled storage. Food Sci. 2019, 40, 14–21. [Google Scholar] [CrossRef]
- Shin, D.; Yang, A.; Min, B.; Narciso-Gaytán, C.; Sánchez-Plata, M.; Ruiz-Feria, C. Evaluation of antioxidant effects of vitamins C and E alone and in combination with sorghum bran in a cooked and stored chicken sausage. Korean J. Food Sci. Anim. Resour. 2011, 31, 693–700. [Google Scholar] [CrossRef][Green Version]
- Cuong, T.V.; Chin, K.B. Effects of annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant and antimicrobial activities of pork patties during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Kinyuru, J.N. Oil characteristics and influence of heat processing on fatty acid profile of wild harvested termite (Macrotermes subhylanus) and long-horned grasshopper (Ruspolia differens). Int. J. Trop. Insect Sci. 2021, 41, 1427–1433. [Google Scholar] [CrossRef]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Effect of drying processes in the chemical, physico-chemical, techno-functional and antioxidant properties of flours obtained from house cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451–1458. [Google Scholar] [CrossRef]
- Dobermann, D.; Field, L.M.; Michaelson, L.V. Impact of heat processing on the nutritional content of Gryllus bimaculatus (black cricket). Nutr. Bull. 2019, 44, 116–122. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E.; Chang, Y.; Ahn, M.; Lee, Y.; Park, J.J.; Lim, J. Determination of the shelf life of cricket powder and effects of storage on its quality characteristics. Korean J. Food Preserv. 2016, 23, 211–217. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Aringo, R.O.; Mukisa, I.M.; Nakimbugwe, D. Effect of processing, packaging and storage-temperature based hurdles on the shelf stability of sautéed ready-to-eat Ruspolia nitidula. J. Insects Food Feed. 2016, 2, 245–253. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed over-flavour in different species of meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- Nissen, L.R.; Byrne, D.V.; Bertelsen, G.; Skibsted, L.H. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004, 68, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; JaramilloFlores, M.E.; Guerrero Legarreta, I. Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and colour of model raw pork batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef]
- Fujimoto, A.; Masuda, T. Antioxidation mechanism of rosmarinic acid, identification of an unstable quinone derivative by the addition of odourless thiol. Food Chem. 2012, 132, 901–906. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Saldanha, T.; Soares, R.A.M.; Torres, E.A.F.S. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012, 135, 1383–1390. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Gan, J.; He, Z.; Zhang, H.; Feng, Y. Optimization of polyphenols extraction from Phyllanthus emblica L. pomace and its antioxidant activity analysis. Sci. Technol. Food Ind. 2019, 40, 171–177. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, J.; Estévez, M. The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Sci. 2016, 116, 253–259. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, X.; Hu, Y.; Zhou, J.; Liu, Y. Inhibitory activity of tea polyphenols against lipolysis and lipid oxidation in white fish. Jiangsu J. Agric. Sci. 2016, 32, 216–221. [Google Scholar] [CrossRef]

| Content of Fatty Acids (% of Total Fatty Acids) | ||
|---|---|---|
| Gryllus bimaculatus | Palm Oil | |
| Myristic acid (C14:0) | 0.58 ± 0.01 | 0.97 ± 0.02 |
| Palmitic acid (C16:0) | 24.31 ± 0.14 | 40.67 ± 0.2 |
| Palmitoleic acid (C16:1) | 0.44 ± 0.00 | - |
| Stearic acid (C18:0) | 9.32 ± 0.07 | 4.24 ± 0.03 |
| Oleic acid (C18:1) | 22.75 ± 0.09 | 42.8 ± 0.04 |
| Linoleic acid (C18:2) | 41.75 ± 0.23 | 11.23 ± 0.03 |
| Linolenic acid (C18:3) | 0.85 ± 0.01 | - |
| ΣUFA 2 | 65.79 ± 0.19 | 54.03 ± 0.07 |
| ΣSFA 3 | 34.21 ± 0.2 | 45.89 ± 0.21 |
| UFA/SFA | 1.92 ± 0.02 | 1.18 ± 0.01 |
| Fatty Acids | Treatments 2 | Storage Time/Days | Sign. 3 | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |||
| Myristic acid (C14:0) | BHTV | 0.88 ± 0.00 | 1.01 ± 0.02 | 0.73 ± 0.01 B | 0.85 ± 0.00 | 1.34 ± 0.53 | 0.93 ± 0.02 AB | n.s. |
| ETV | 0.89 ± 0.00 | 1.02 ± 0.28 | 0.76 ± 0.00 B | 0.86 ± 0.02 | 1.04 ± 0.05 | 0.94 ± 0.00 AB | n.s. | |
| PEPV | 0.87 ± 0.00 b | 0.93 ± 0.03 c | 0.80 ± 0.01 Ba | 0.86 ± 0.01 b | 0.96 ± 0.02 c | 0.94 ± 0.01 ABc | *** | |
| TBHQV | 0.89 ± 0.03 b | 0.90 ± 0.02 bc | 0.81 ± 0.01 Ba | 0.89 ± 0.01 b | 0.93 ± 0.01 c | 0.93 ± 0.01 ABc | *** | |
| control 1 | 0.87 ± 0.00 b | 0.94 ± 0.06 b | 0.68 ± 0.07 Aa | 0.85 ± 0.01 b | 0.95 ± 0.06 b | 0.95 ± 0.01 Ab | *** | |
| control 2 | 0.87 ± 0.00 b | 090 ± 0.02 c | 0.81 ± 0.01 Ba | 0.87 ± 0.01 b | 0.92 ± 0.02 c | 0.91 ± 0.01 Bc | *** | |
| Sign. | n.s. | n.s. | ** | n.s. | n.s. | * | ||
| Palmitic acid (C16:0) | BHTV | 38.4 ± 0.04 b | 37.69 ± 0.06 a | 39.90 ± 0.10 Cd | 39.26 ± 0.17 ABc | 40.55 ± 0.37 e | 40.66 ± 0.21 BCe | *** |
| ETV | 38.37 ± 0.02 b | 37.84 ± 0.08 a | 39.32 ± 0.11 BCd | 39.07 ± 0.20 ABc | 40.16 ± 0.24 e | 40.68 ± 0.02 BCf | *** | |
| PEPV | 37.84 ± 0.09 a | 37.70 ± 0.26 a | 39.09 ± 0.15 ABCc | 38.57 ± 0.21 Ab | 39.73 ± 0.10 d | 40.75 ± 0.06 BCe | *** | |
| TBHQV | 37.94 ± 0.06 ab | 37.80 ± 0.06 a | 38.49 ± 0.26 Ab | 39.39 ± 0.40 ABc | 39.67 ± 0.47 c | 40.38 ± 0.38 Bd | *** | |
| control 1 | 38.3 ± 0.47 b | 37.41 ± 0.07 a | 39.51 ± 0.72 BCc | 39.56 ± 0.19 Bc | 40.45 ± 0.03 d | 41.01 ± 0.06 Cd | *** | |
| control 2 | 38.00 ± 0.01 a | 37.77 ± 0.29 a | 39.00 ± 0.22 ABb | 39.04 ± 0.41 ABb | 39.77 ± 0.45 c | 39.87 ± 0.08 Ac | *** | |
| Sign. | n.s. | n.s. | ** | * | n.s. | *** | ||
| Stearic acid (C18:0) | BHTV | 5.37 ± 0.00 Dd | 5.17 ± 0.01 Ac | 4.43 ± 0.06 BCa | 5.07 ± 0.02 CDb | 5.15 ± 0.01 Bc | 5.25 ± 0.02 Cd | *** |
| ETV | 5.21 ± 0.01 Bc | 5.45 ± 0.02 De | 4.29 ± 0.07 Ba | 4.98 ± 0.01 BCb | 5.05 ± 0.02 Ab | 5.01 ± 0.02 ABb | *** | |
| PEPV | 5.43 ± 0.01 Ee | 5.28 ± 0.05 Bd | 4.76 ± 0.01 Da | 5.17 ± 0.04 Dec | 5.19 ± 0.02 Bc | 5.08 ± 0.04 Bb | *** | |
| TBHQV | 5.19 ± 0.01 Ad | 5.16 ± 0.02 Ad | 4.62 ± 0.05 CDa | 4.91 ± 0.03 Bb | 5.06 ± 0.06 Ac | 5.00 ± 0.07 ABc | *** | |
| control 1 | 5.29 ± 0.02 Cc | 5.16 ± 0.02 A | 3.92 ± 0.29 Aa | 4.70 ± 0.16 Ab | 5.22 ± 0.06 Bc | 4.98 ± 0.02 Ac | *** | |
| control 2 | 5.44 ± 0.01 Ed | 5.36 ± 0.04 Cc | 5.18 ± 0.03 Ea | 5.27 ± 0.04 Eb | 5.20 ± 0.02 Ba | 5.28 ± 0.01 Cb | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
| Oleic acid (C18:1) | BHTV | 40.47 ± 0.02 Ac | 41.40 ± 0.13 BCd | 40.45 ± 0.03 Bc | 40.09 ± 0.19 Ab | 37.45 ± 0.09 Aa | 37.39 ± 0.16 Aa | *** |
| ETV | 41.07 ± 0.03 Bd | 40.46 ± 0.41 Ac | 41.66 ± 0.06 Ce | 40.55 ± 0.22 Bc | 38.25 ± 0.08 Bb | 37.84 ± 0.03 Ba | *** | |
| PEPV | 40.36 ± 0.06 Abc | 40.97 ± 0.37 Abd | 40.47 ± 0.10 Bc | 40.00 ± 0.22 Ab | 37.88 ± 0.23 Aa | 37.82 ± 0.13 Ba | *** | |
| TBHQV | 41.7 ± 0.07 Cd | 41.71 ± 0.05 Cd | 42.12 ± 0.15 Cd | 40.71 ± 0.31 Bc | 38.97 ± 0.32 Cb | 38.31 ± 0.24 Ca | *** | |
| control 1 | 40.62 ± 0.37 Ab | 41.38 ± 0.02 BCb | 40.95 ± 0.82 Bb | 40.87 ± 0.23 Bb | 37.80 ± 0.01 Aa | 37.70 ± 0.08 Ba | *** | |
| control 2 | 40.29 ± 0.01 Ad | 40.88 ± 0.36 Abe | 39.45 ± 0.13 Ac | 39.62 ± 0.33 Ac | 38.32 ± 0.27 Bb | 37.43 ± 0.07 Aa | *** | |
| Sign. | *** | ** | *** | *** | *** | *** | ||
| Linoleic acid (C18:2) | BHTV | 14.88 ± 0.04 Cd | 14.30 ± 0.04 Aa | 14.48 ± 0.04 Bb | 14.73 ± 0.07 Cc | 15.51 ± 0.13 Abe | 15.79 ± 0.07 Bf | *** |
| ETV | 14.45 ± 0.04 Bb | 15.22 ± 0.12 Dc | 13.97 ± 0.05 Aa | 14.54 ± 0.05 Bb | 15.50 ± 0.18 ABd | 15.53 ± 0.07 Ad | *** | |
| PEPV | 15.50 ± 0.03 Ec | 15.12 ± 0.04 CDb | 14.87 ± 0.06 Ca | 15.40 ± 0.02 Ec | 16.25 ± 0.16 Cd | 15.42 ± 0.10 Ac | *** | |
| TBHQV | 14.28 ± 0.01 Ac | 14.30 ± 0.05 Ac | 13.95 ± 0.06 Aa | 14.11 ± 0.14 Aab | 15.38 ± 0.14 Ad | 15.38 ± 0.09 Ad | *** | |
| control 1 | 14.92 ± 0.09 Cb | 14.70 ± 0.03 Bb | 14.96 ± 0.27 Cb | 14.01 ± 0.05 Aa | 15.56 ± 0.05 ABc | 15.36 ± 0.06 Ac | *** | |
| control 2 | 15.39 ± 0.01 Dbc | 15.04 ± 0.09 Ca | 15.55 ± 0.11 Dc | 15.20 ± 0.13 Dab | 15.79 ± 0.19 Bd | 16.50 ± 0.08 Ce | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
| ΣUFA 4 | BHTV | 55.35 ± 0.04 Ac | 55.95 ± 0.06 d | 54.94 ± 0.05 b | 54.82 ± 0.17 b | 52.97 ± 0.20 Aa | 53.17 ± 0.23 Aa | *** |
| ETV | 55.53 ± 0.01 ABd | 55.68 ± 0.36 d | 55.63 ± 0.08 d | 55.09 ± 0.22 c | 53.75 ± 0.18 BCb | 53.36 ± 0.03 Aa | *** | |
| PEPV | 55.86 ± 0.09 Bd | 56.09 ± 0.34 d | 55.34 ± 0.15 c | 55.40 ± 0.22 c | 54.13 ± 0.09 Cb | 53.24 ± 0.08 Aa | *** | |
| TBHQV | 55.98 ± 0.08 Bc | 56.01 ± 0.04 c | 56.08 ± 0.21 c | 54.82 ± 0.44 b | 54.34 ± 0.45 Cb | 53.68 ± 0.33 Ba | *** | |
| control 1 | 55.54 ± 0.45 ABbc | 56.31 ± 0.02 c | 55.90 ± 1.09 bc | 54.89 ± 0.27 b | 53.37 ± 0.05 Aba | 53.06 ± 0.06 Aa | *** | |
| control 2 | 55.68 ± 0.01 ABc | 55.91 ± 0.28 c | 55.00 ± 0.24 b | 54.82 ± 0.46 b | 54.11 ± 0.46 Ca | 53.94 ± 0.08 Ba | *** | |
| Sign. | * | n.s. | n.s. | n.s. | ** | *** | ||
| ΣSFA 5 | BHTV | 44.65 ± 0.04 Bb | 44.05 ± 0.06 a | 45.06 ± 0.05 c | 45.18 ± 0.17 c | 47.03 ± 0.20 Bd | 46.83 ± 0.23 Bd | *** |
| ETV | 44.47 ± 0.01 Aba | 44.32 ± 0.36 a | 44.37 ± 0.08 a | 44.91 ± 0.22 b | 46.25 ± 0.18 ABc | 46.64 ± 0.03 Bd | *** | |
| PEPV | 44.14 ± 0.09 Aa | 43.91 ± 0.34 a | 44.66 ± 0.15 b | 44.60 ± 0.22 b | 45.87 ± 0.09 Ac | 46.76 ± 0.08 Bd | *** | |
| TBHQV | 44.02 ± 0.08 Aa | 43.99 ± 0.04 a | 43.92 ± 0.21 a | 45.18 ± 0.44 b | 45.66 ± 0.45 Ab | 46.32 ± 0.33 Ac | *** | |
| control 1 | 44.46 ± 0.45 Aab | 43.69 ± 0.02 a | 44.10 ± 1.09 ab | 45.11 ± 0.27 b | 46.63 ± 0.05 BCc | 46.94 ± 0.06 Bc | *** | |
| control 2 | 44.32 ± 0.01 Aba | 44.09 ± 0.28 a | 45.00 ± 0.24 b | 45.18 ± 0.46 b | 45.89 ± 0.46 Ac | 46.06 ± 0.08 Ac | *** | |
| Sign. | * | n.s. | n.s. | n.s. | ** | *** | ||
| UFA/SFA | BHTV | 1.24 ± 0.00 Ac | 1.27 ± 0.00 d | 1.22 ± 0.00 b | 1.21 ± 0.01 b | 1.13 ± 0.01 Aa | 1.14 ± 0.01 Aa | *** |
| ETV | 1.25 ± 0.00 ABd | 1.26 ± 0.02 d | 1.25 ± 0.00 d | 1.23 ± 0.01 c | 1.16 ± 0.01 BCb | 1.14 ± 0.00 Aa | *** | |
| PEPV | 1.27 ± 0.00 Bd | 1.28 ± 0.02 d | 1.24 ± 0.01 c | 1.24 ± 0.01 c | 1.18 ± 0.00 Cb | 1.14 ± 0.00 Aa | *** | |
| TBHQV | 1.27 ± 0.00 Bc | 1.27 ± 0.00 c | 1.28 ± 0.01 c | 1.21 ± 0.02 b | 1.19 ± 0.02 Cb | 1.16 ± 0.02 Ba | *** | |
| control 1 | 1.25 ± 0.02 ABbc | 1.29 ± 0.00 c | 1.27 ± 0.06 bc | 1.22 ± 0.01 b | 1.14 ± 0.00 Aba | 1.13 ± 0.00 Aa | *** | |
| control 2 | 1.26 ± 0.00 ABc | 1.27 ± 0.01 c | 1.22 ± 0.01 b | 1.21 ± 0.02 b | 1.18 ± 0.02 Ca | 1.17 ± 0.00 Ba | *** | |
| Sign. | * | n.s. | n.s. | n.s. | *** | ** | ||
| Free Fatty Acid | Treatments 2 | Storage Time/Day | Sign. 3 | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |||
| Palmitic acid (C16:0) | BHTV | 39.91 ± 2.66 Ba | 46.98 ± 1.82 Cab | 52.76 ± 5.47 ABbc | 53.86 ± 1.15 Bbc | 59.32 ± 7.50 ABc | 55.43 ± 0.94 ABbc | ** |
| ETV | 29.64 ± 1.00 Aa | 42.22 ± 0.35 ABb | 56.56 ± 5.71 ABc | 58.07 ± 0.48 Bc | 57.92 ± 3.48 ABc | 63.90 ± 5.96 Bc | *** | |
| PEPV | 30.75 ± 3.31 Aa | 44.10 ± 5.51 ABb | 46.76 ± 1.00 Ab | 61.92 ± 5.76 Bc | 52.82 ± 6.57 Abc | 62.91 ± 3.46 Bc | *** | |
| TBHQV | 33.15 ± 0.72 Aa | 36.09 ± 1.46 Aa | 46.38 ± 6.37 Ab | 44.73 ± 5.96 Ab | 53.83 ± 4.13 Ab | 47.91 ± 4.28 Ab | ** | |
| control 1 | 50.23 ± 3.82 Ca | 47.05 ± 3.62 Ca | 63.49 ± 6.62 BCbc | 59.01 ± 0.1 Bb | 68.72 ± 4.66 BCc | 79.63 ± 3.88 Cd | *** | |
| control 2 | 54.45 ± 1.39 Ca | 64.25 ± 6.69 Dab | 72.57 ± 3.42 Cbc | 62.52 ± 5.37 Bab | 76.41 ± 5.18 Cc | 92.68 ± 6.58 Dd | *** | |
| Sign. 2 | *** | *** | *** | ** | ** | *** | ||
| Stearic acid (C18:0) | BHTV | 14.83 ± 0.67 Aa | 20.86 ± 2.08 BCb | 27.27 ± 4.54 Cc | 27.74 ± 0.69 ABc | 19.52 ± 1.37 ABb | 25.27 ± 1.96 Bc | ** |
| ETV | 14.23 ± 0.66 Aa | 17.61 ± 0.42 ABa | 13.35 ± 1.55 Aa | 23.78 ± 1.56 Ab | 17.61 ± 3.41 Aa | 37.82 ± 4.51 Cc | *** | |
| PEPV | 13.55 ± 4.20 Aa | 17.47 ± 1.50 ABa | 23.94 ± 0.47 BCb | 29.05 ± 3.39 Bb | 16.93 ± 1.75 Cb | 15.20 ± 1.19 Aa | *** | |
| TBHQV | 16.73 ± 0.65 ABb | 11.74 ± 1.70 Aa | 19.88 ± 3.29 Bb | 23.38 ± 1.48 Ab | 23.12 ± 2.14 BCb | 21.82 ± 4.43 ABb | ** | |
| control 1 | 20.07 ± 1.76 Ba | 21.76 ± 1.22 BCa | 19.21 ± 0.87 Ba | 35.82 ± 0.23 Cc | 25.87 ± 1.13 Cb | 19.12 ± 1.86 ABa | *** | |
| control 2 | 24.61 ± 0.57 Ca | 26.93 ± 6.48 Ca | 28.30 ± 1.95 Ca | 37.44 ± 3.54 Cb | 31.56 ± 1.55 Dab | 47.15 ± 3.60 Dc | *** | |
| Sign. | *** | ** | *** | *** | *** | *** | ||
| Oleic acid (C18:1) | BHTV | 16.36 ± 2.33 Aa | 23.64 ± 5.97 ABab | 18.61 ± 0.70 Aa | 27.53 ± 0.46 ABb | 27.74 ± 4.63 Ab | 24.58 ± 3.08 Aab | ** |
| ETV | 14.86 ± 0.74 Aa | 16.89 ± 0.11 Aa | 35.83 ± 1.29 BCb | 18.69 ± 4.12 Aa | 30.11 ± 6.65 Ab | 31.04 ± 4.98 ABb | *** | |
| PEPV | 14.71 ± 4.55 Aa | 22.76 ± 7.40 ABab | 27.75 ± 0.99 Bab | 25.09 ± 7.66 ABab | 25.48 ± 5.43 Aab | 33.75 ± 3.89 ABb | * | |
| TBHQV | 15.88 ± 0.51 Aa | 15.35 ± 4.56 Aa | 16.52 ± 2.24 Aa | 21.72 ± 3.68 ABa | 20.36 ± 2.48 Aa | 47.38 ± 13.54 Bb | *** | |
| control 1 | 20.54 ± 2.32 Aa | 23.38 ± 2.95 ABab | 28.26 ± 4.71 Bbc | 32.72 ± 0.18 Cc | 28.68 ± 2.06 Abc | 39.45 ± 4.68 ABd | *** | |
| control 2 | 24.61 ± 0.57 Ca | 26.93 ± 6.48 Ca | 28.30 ± 1.95 Ca | 37.44 ± 3.54 Cb | 31.56 ± 1.55 Dab | 47.15 ± 3.60 Dc | *** | |
| Sign. | *** | * | *** | ** | *** | * | ||
| Linoleic acid (C18:2) | BHTV | 11.39 ± 0.86 Ba | 22.25 ± 3.19 Bc | 16.55 ± 1.17 ABb | 25.1 ± 0.32 Bc | 29.79 ± 2.93 Bd | 24.81 ± 2.17 Bc | *** |
| ETV | 11.49 ± 0.64 Ba | 13.64 ± 0.15 ABa | 11.23 ± 2.99 Aa | 16.34 ± 3.97 Aa | 27.57 ± 4.08 Bb | 35.08 ± 1.88 Cc | *** | |
| PEPV | 8.05 ± 0.20 Aa | 13.61 ± 2.39 ABb | 15.48 ± 0.14 ABb | 16.42 ± 2.97 Ab | 24.31 ± 1.90 Bc | 28.23 ± 3.52 BCc | *** | |
| TBHQV | 11.19 ± 0.15 Bb | 6.12 ± 0.30 Aa | 12.83 ± 1.40 Abc | 16.44 ± 1.52 Abc | 14.10 ± 4.54 Abc | 17.77 ± 3.29 Ac | ** | |
| control 1 | 17.01 ± 1.03 Ca | 20.52 ± 3.20 Ba | 18.96 ± 1.78 Ba | 18.32 ± 0.07 Aa | 25.73 ± 7.61 Bab | 29.02 ± 4.12 BCb | * | |
| control 2 | 41.48 ± 0.90 Dab | 48.32 ± 8.70 Cab | 39.79 ± 3.80 Ca | 53.57 ± 3.62 Cb | 45.80 ± 1.38 Cab | 49.83 ± 5.18 Dab | * | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
| ΣUFA 4 | BHTV | 27.74 ± 2.82 Aa | 45.89 ± 9.11 Bb | 35.16 ± 1.85 ABa | 52.64 ± 0.19 Bb | 57.54 ± 7.56 Bb | 49.40 ± 5.17 Ab | *** |
| ETV | 26.36 ± 1.36 A | 30.53 ± 0.21 ABa | 47.06 ± 1.79 Cab | 35.03 ± 8.08 Aa | 57.68 ± 6.83 Bc | 66.12 ± 6.84 Ac | *** | |
| PEPV | 22.76 ± 4.71 Aa | 36.36 ± 8.04 ABb | 43.24 ± 0.91 BCb | 41.51 ± 10.55 ABb | 49.79 ± 7.32 ABbc | 61.98 ± 7.26 Ac | ** | |
| TBHQV | 27.07 ± 0.59 Aa | 21.46 ± 4.35 Aa | 29.35 ± 3.58 Aa | 38.15 ± 5.20 ABa | 34.46 ± 6.00 Aa | 65.16 ± 14.53 Ab | *** | |
| control 1 | 37.56 ± 3.32 Ba | 43.90 ± 4.96 Bab | 47.22 ± 6.48 Cab | 51.04 ± 0.16 Bab | 54.41 ± 9.20 Bb | 68.46 ± 8.79 Ac | ** | |
| control 2 | 73.24 ± 1.41 Ca | 79.55 ± 11.58 Ca | 78.77 ± 8.47 Da | 85.88 ± 6.03 Ca | 110.47 ± 13.87 Cb | 87.69 ± 8.79 Ba | ** | |
| Sign. | *** | *** | *** | *** | *** | ** | ||
| ΣSFA 5 | BHTV | 54.73 ± 2.34 Ba | 67.84 ± 3.63 Bb | 80.04 ± 9.90 Ab | 81.6 ± 1.44 Bb | 78.84 ± 8.85 Ab | 80.70 ± 2.90 Ab | ** |
| ETV | 43.87 ± 1.56 Aa | 59.83 ± 0.75 ABb | 69.92 ± 7.17 A | 81.84 ± 1.69 Bd | 75.54 ± 0.39 Acd | 101.72 ± 10.45 Be | *** | |
| PEPV | 44.29 ± 5.23 Aa | 61.57 ± 6.55 ABb | 70.70 ± 1.44 Abc | 90.97 ± 4.94 BCd | 79.75 ± 8.32 Ac | 78.10 ± 4.44 Ac | *** | |
| TBHQV | 19.89 ± 0.85 ABa | 47.83 ± 2.87 Aa | 66.26 ± 9.64 Ab | 68.11 ± 7.15 Ab | 76.95 ± 5.86 Ab | 69.73 ± 6.79 Ab | *** | |
| control 1 | 70.30 ± 5.15 Ca | 68.82 ± 4.84 Ba | 82.70 ± 7.49 Ab | 94.83 ± 0.14 Cc | 94.59 ± 5.48 Bc | 98.76 ± 5.63 Bc | *** | |
| control 2 | 79.07 ± 1.71 Da | 91.19 ± 13.10 Cab | 100.87 ± 4.46 Bb | 99.96 ± 8.90 Cb | 107.98 ± 5.50 Cb | 139.84 ± 10.17 Cc | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
| Total FFAs | BHTV | 82.48 ± 5.16 Ba | 113.73 ± 12.01 Bb | 115.20 ± 11.20 ABb | 134.24 ± 1.52 BCb | 136.37 ± 16.18 ABb | 130.10 ± 7.84 Ab | *** |
| ETV | 70.23 ± 2.91 ABa | 90.36 ± 0.96 ABb | 116.98 ± 8.88 ABc | 116.87 ± 9.57 ABc | 133.22 ± 7.23 ABc | 167.84 ± 16.30 Ad | *** | |
| PEPV | 67.06 ± 9.82 Aa | 97.93 ± 14.30 ABb | 113.94 ± 2.35 ABbc | 132.48 ± 9.66 BCc | 129.54 ± 15.61 ABc | 140.08 ± 10.33 Ac | *** | |
| TBHQV | 76.95 ± 0.72 ABab | 69.29 ± 6.85 Aa | 95.61 ± 11.58 Abc | 106.26 ± 11.24 Ac | 111.41 ± 9.00 Ac | 134.89 ± 20.02 Ad | *** | |
| control 1 | 107.86 ± 7.97 Ca | 112.72 ± 9.78 Ba | 129.92 ± 13.98 Bab | 145.87 ± 0.26 Cbc | 149.00 ± 14.61 Bbc | 167.22 ± 13.46 Ac | *** | |
| control 2 | 152.30 ± 3.11 Da | 170.73 ± 24.56 Ca | 179.63 ± 12.49 Ca | 185.84 ± 14.85 Da | 218.45 ± 17.46 Cb | 227.53 ± 18.91 Bb | ** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Zhao, M.; He, Z.; Sun, L.; Li, X.; Feng, Y. The Effects of Antioxidants and Packaging Methods on Inhibiting Lipid Oxidation in Deep Fried Crickets (Gryllus bimaculatus) during Storage. Foods 2022, 11, 326. https://doi.org/10.3390/foods11030326
Gan J, Zhao M, He Z, Sun L, Li X, Feng Y. The Effects of Antioxidants and Packaging Methods on Inhibiting Lipid Oxidation in Deep Fried Crickets (Gryllus bimaculatus) during Storage. Foods. 2022; 11(3):326. https://doi.org/10.3390/foods11030326
Chicago/Turabian StyleGan, Jin, Min Zhao, Zhao He, Long Sun, Xian Li, and Ying Feng. 2022. "The Effects of Antioxidants and Packaging Methods on Inhibiting Lipid Oxidation in Deep Fried Crickets (Gryllus bimaculatus) during Storage" Foods 11, no. 3: 326. https://doi.org/10.3390/foods11030326
APA StyleGan, J., Zhao, M., He, Z., Sun, L., Li, X., & Feng, Y. (2022). The Effects of Antioxidants and Packaging Methods on Inhibiting Lipid Oxidation in Deep Fried Crickets (Gryllus bimaculatus) during Storage. Foods, 11(3), 326. https://doi.org/10.3390/foods11030326






