Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Ultrasound Assisted Extraction Process
2.4. HPLC-DAD Analysis
2.5. Experimental Design and Statistical Analyses
3. Result and Discussion
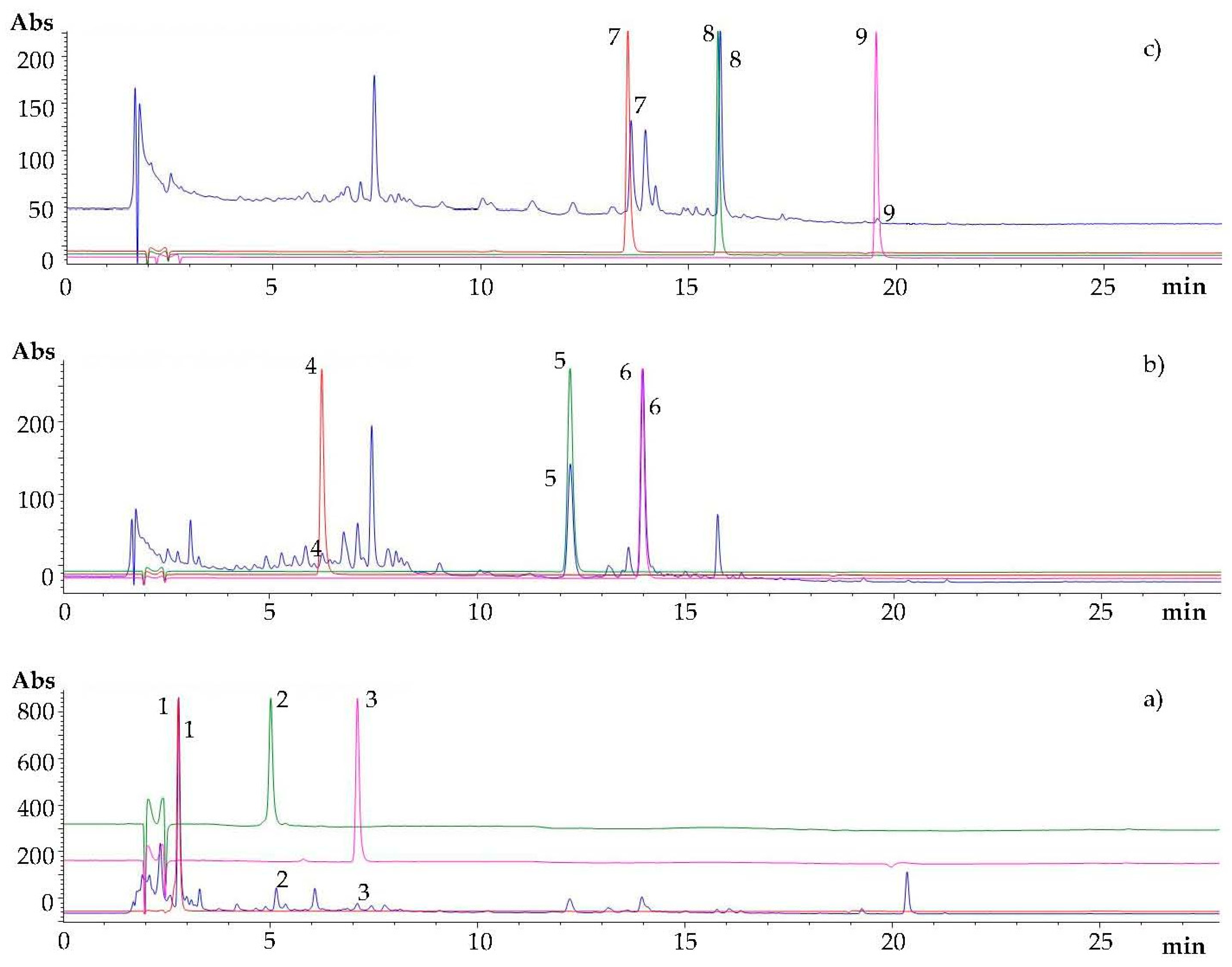
3.1. Qualitative Analysis of Polyphenols in Ripe Carob Pods Extracts
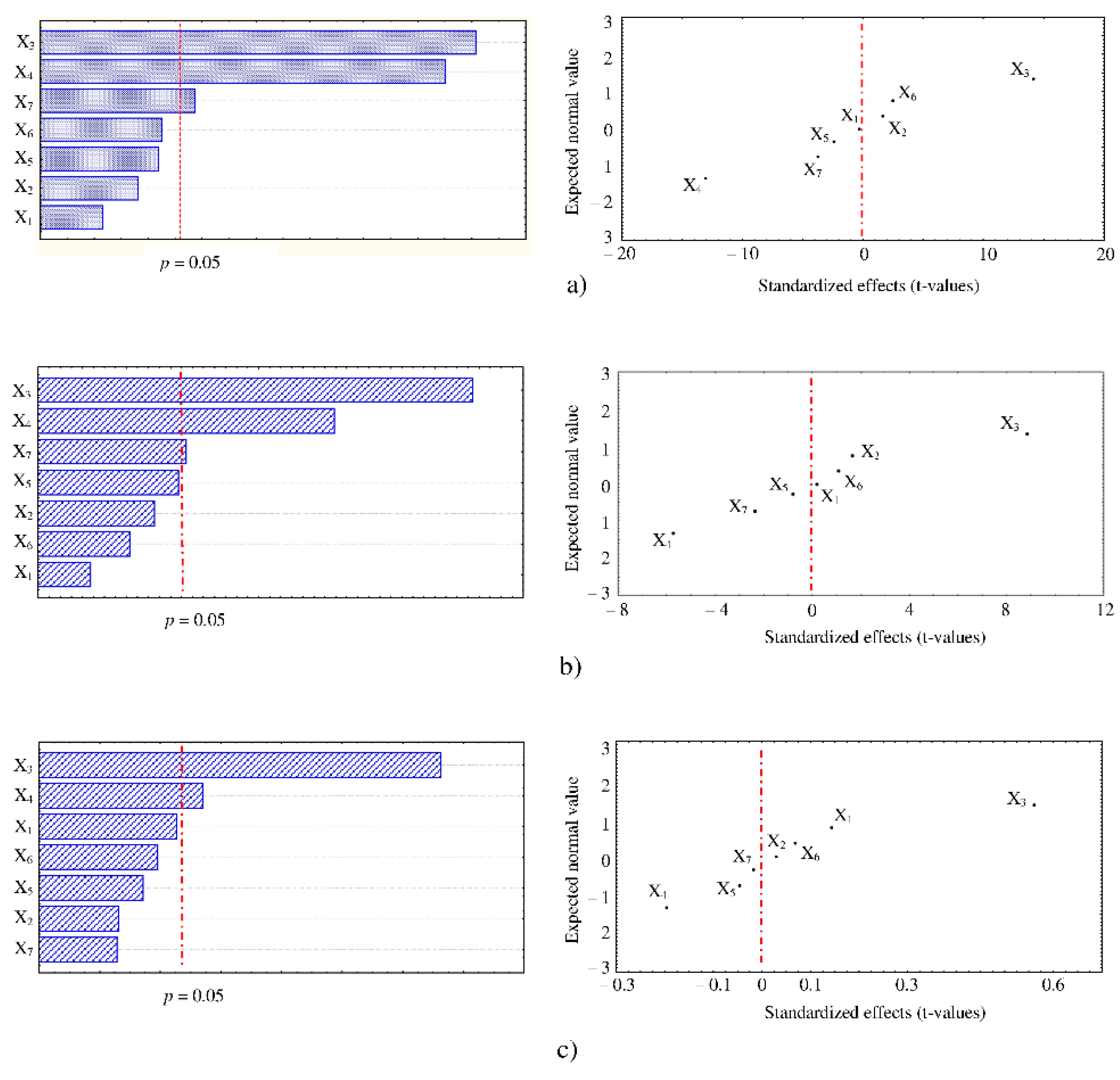
3.2. Screening of Factors Influencing UAE Efficiency
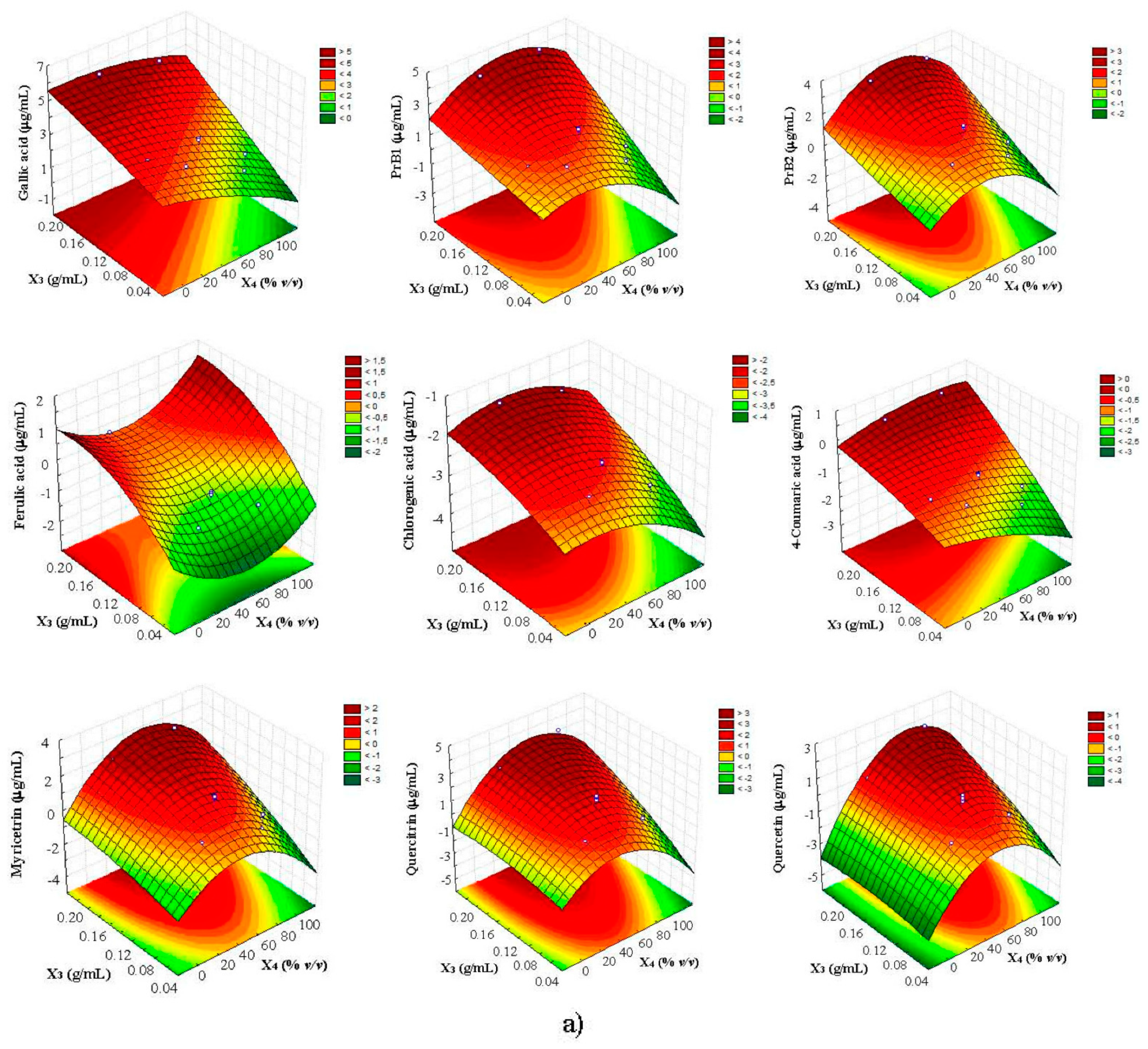
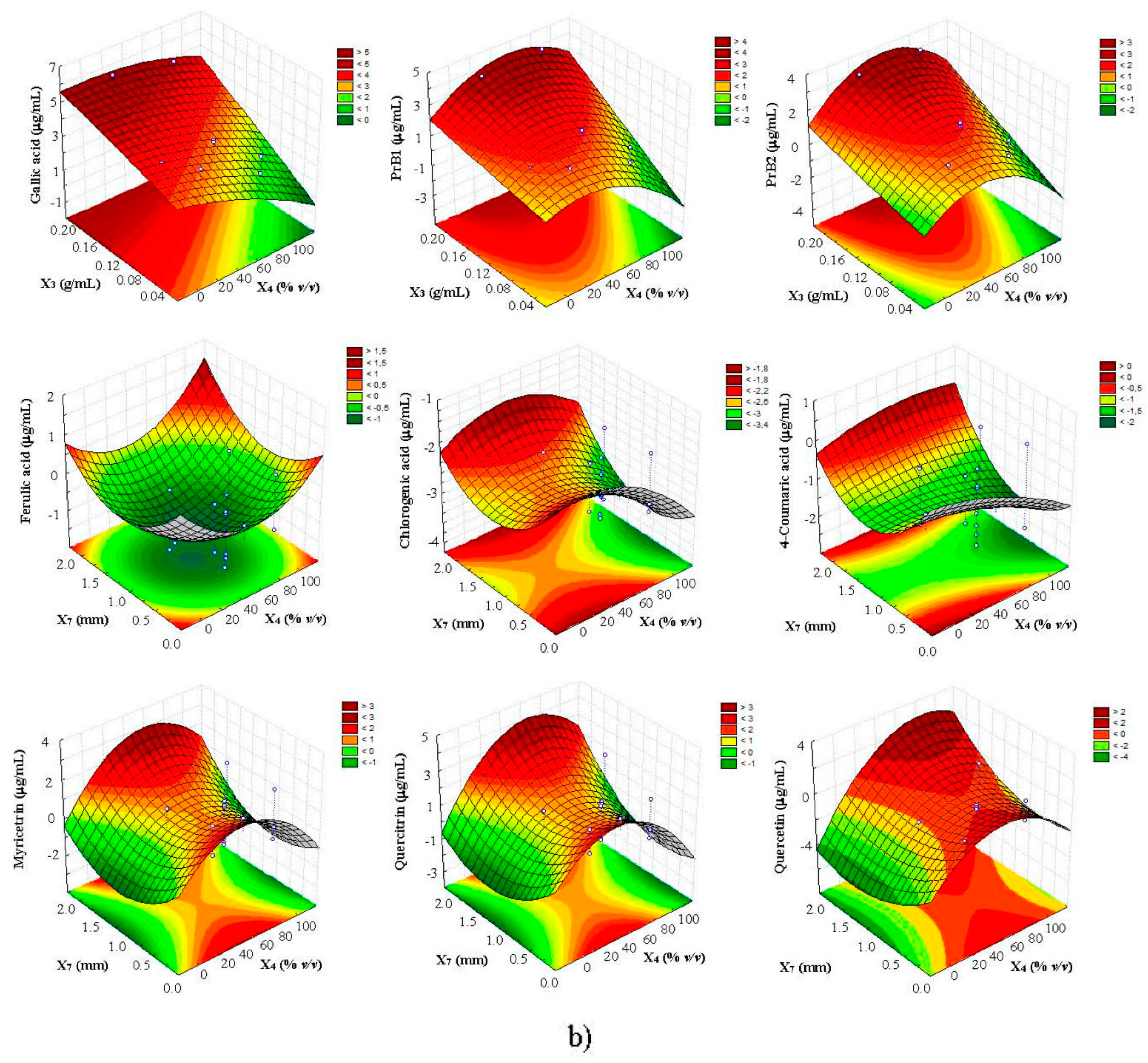
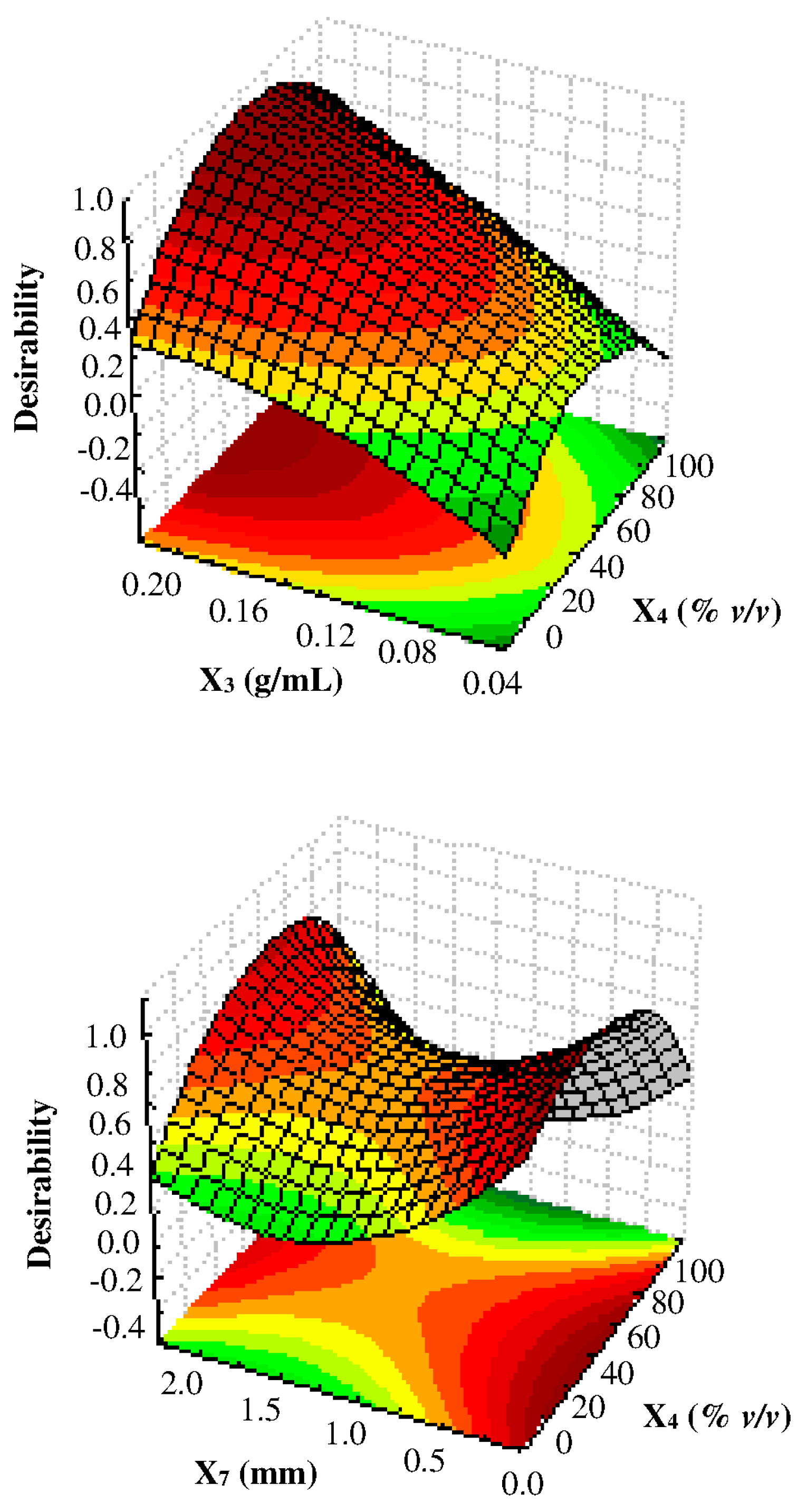
3.3. Multi-Response Optimization of UAE by CCD-RSM and D
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO (2002): Non-Wood Forest Products from Temperate Broad-Leaved Trees, Chapter 7. Available online: http://www.fao.org/3/y4351e/y4351e0b.htm (accessed on 9 November 2021).
- van Rijs, P.; Fogliano, V. Roasting carob flour decreases the capacity to bind glycoconjugates of bile acids. Food Funct. 2020, 11, 5924–5932. [Google Scholar] [CrossRef]
- Ayache, S.B.; Reis, F.S.; Dias, M.I.; Pereira, C.; Glamočlija, J.; Soković, M.; Saafi, E.B.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263–129271. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Dakia, P.A.; Wathelet, B.; Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007, 102, 1368–1374. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Dugo, G. Oxygen concentration control during olive oil extraction process: A new system to emphasize the organoleptic and healthy properties of virgin olive oil. Acta Hortic. 2008, 949, 473–480. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Belkhir, M.; Akinocho, I.; Raboudi, F.; Pamies, D.; Barrajón, E.; Estevan, C.; Fattouch, S. Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT-Food Sci. Technol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Hanousek Čiča, K.; Mrvčić, J.; Srečec, S.; Filipan, K.; Blažić, M.; Stanzer, D. Physicochemical and aromatic characterization of carob macerates produced by different maceration conditions. Food Sci. Nutr. 2020, 8, 942–954. [Google Scholar] [CrossRef]
- El Ansari Zineb, B.M.; Alain, B.; Ahmed, L. Total polyphenols and gallic acid contents in domesticated carob (Ceratonia siliqua L.) pods and leaves. Ind. J. Pure Appl. Biosci. 2017, 5, 22–30. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Roselli, L.; Cicia, G.; Cavallo, C.; Del Giudice, T.; Carlucci, D.; Clodoveo, M.L.; De Gennaro, B.C. Consumers’ willingness to buy innovative traditional food products: The case of extra-virgin olive oil extracted by ultrasound. Food Res. Int. 2018, 108, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging technology to develop novel red winemaking practices: An overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and pulsed ultrasound-assisted extraction of carob’s antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason. Sonochem. 2021, 76, 105630–105641. [Google Scholar] [CrossRef]
- Hadrich, B.; Dimitrov, K.; Kriaa, K. Modelling investigation and parameters study of polyphenols extraction from carob (Ceratonia siliqua L.) using experimental factorial design. J. Food Process. Preserv. 2017, 41, e12769. [Google Scholar] [CrossRef]
- Tetik, N.; Yüksel, E. Ultrasound-assisted extraction of d-pinitol from carob pods using response surface methodology. Ultrason. Sonochem. 2014, 21, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Reche, C.; Rosselló, C.; Umaña, M.M.; Eim, V.; Simal, S. Mathematical Modelling of Ultrasound-Assisted Extraction Kinetics of Bioactive Compounds from Artichoke By-Products. Foods 2021, 10, 931. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigós, M.D.C.; Balart, R.; Torres-Giner, S. Optimization of microwave-assisted extraction of phenolic compounds with antioxidant activity from carob pods. Food Anal. Methods 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Vanaja, K.; Shobha Rani, R.H. Design of experiments: Concept and applications of Plackett Burman design. Clin. Res. Regul. Aff. 2007, 24, 1–23. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Bin Li, H. Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef] [Green Version]
- Chavan, Y.; Singhal, R.S. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol. 2013, 17, 106–113. [Google Scholar] [CrossRef]
- Rached, I.; Barros, L.; Fernandes, I.P.; Santos-Buelga, C.; Rodrigues, A.E.; Ferchichi, A.; Barreiro, M.F.; Ferreira, I.C. Ceratonia siliqua L. hydroethanolic extract obtained by ultrasonication: Antioxidant activity, phenolic compounds profile and effects in yogurts functionalized with their free and microencapsulated forms. Food Funct. 2016, 7, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.L.C.; Bruns, R.E.; da Silva, E.G.P.; Dos Santos, W.N.L.; Quintella, C.M.; David, J.M.; de Andrade, J.B.; Breitkreitz, M.C.; Jardim, I.C.; Neto, B.B. Statistical designs and response surface techniques for the optimization of chromatographic systems. J. Chromatogr. A 2007, 1158, 2–14. [Google Scholar] [CrossRef]
- Clark, C.; Williges, R.C. Response surface methodology central-composite design modifications for human performance research. Hum. Factors 1973, 15, 295–310. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Prasad, K.N.; Kong, K.W.; Ramanan, R.N.; Azlan, A.; Ismail, A. Selection of experimental domain using two-level factorial design to determine extract yield, antioxidant capacity, phenolics, and flavonoids from Mangifera pajang Kosterm. Sep. Sci. Technol. 2012, 47, 2417–2423. [Google Scholar]
- de Sousa Sabino, L.B.; Alves Filho, E.G.; Fernandes, F.A.N.; de Brito, E.S.; da Silva Júnior, I.J. Optimization of pressurized liquid extraction and ultrasound methods for recovery of anthocyanins present in jambolan fruit (Syzygium cumini L.). Food Bioprod. Process. 2021, 127, 77–89. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
| Factor | Symbol | Factor Levels | |
|---|---|---|---|
| Low (−1) | High (+1) | ||
| Extraction time (min) | X1 | 5 | 60 |
| Extraction temperature (°C) | X2 | 15 | 50 |
| Solid–solvent ratio (g/mL) | X3 | 0.05 | 0.2 |
| Solvent concentration (% ethanol, v/v) | X4 | 0 | 100 |
| Sonication frequency (kHz) | X5 | 37 | 80 |
| Sonication power (W) | X6 | 30 | 100 |
| Particle size (mm) | X7 | 0.3 | 2 |
| Run | X1 (min) | X2 (°C) | X3 (g/mL) | X4 (% Ethanol v/v) | X5 (kHz) | X6 (W) | X7 (mm) | Gallic Acid (μg/mL) | 4-Coumaric Acid (μg/mL) | Myricitrin (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 12(C) | 32.5 | 32.5 | 0.125 | 50 | 60 | 60 | 1.0 | 32.40 | 0.66 | 8.59 |
| 3 | 5.0 | 50.0 | 0.2 | 100 | 80 | 30 | 2.0 | 9.88 | 0.37 | 1.60 |
| 1 | 5.0 | 15.0 | 0.2 | 0 | 80 | 100 | 0.3 | 77.90 | 1.44 | 1.94 |
| 5 | 5.0 | 15.0 | 0.05 | 0 | 37 | 30 | 2.0 | 9.27 | 0.21 | 0.44 |
| 6 | 60.0 | 15.0 | 0.05 | 100 | 80 | 30 | 0.3 | 0.65 | 0.036 | 0.17 |
| 9(C) | 32.5 | 32.5 | 0.125 | 50 | 60 | 60 | 1.0 | 30.40 | 0.90 | 7.66 |
| 11(C) | 32.5 | 32.5 | 0.125 | 50 | 60 | 60 | 1.0 | 22.20 | 0.78 | 4.52 |
| 7 | 5.0 | 50.0 | 0.05 | 100 | 37 | 100 | 0.3 | 3.67 | 0.17 | 0.67 |
| 10(C) | 32.5 | 32.5 | 0.125 | 50 | 60 | 60 | 1.0 | 26.00 | 0.48 | 6.69 |
| 4 | 60.0 | 50.0 | 0.2 | 0 | 37 | 30 | 0.3 | 86.50 | 2.29 | 2.94 |
| 2 | 60.0 | 15.0 | 0.2 | 100 | 37 | 100 | 2.0 | 15.20 | 0.56 | 2.86 |
| 8 | 60.0 | 50.0 | 0.05 | 0 | 80 | 100 | 2.0 | 11.60 | 0.24 | 0.59 |
| Run | X3 (g/mL) | X4 (% Ethanol v/v) | X7 (mm) | Gallic Acid (μg/mL) | PrB1 (μg/mL) | PrB2 (μg/mL) | Ferulic Acid (μg/mL) | ChloroGenic Acid (μg/mL) | 4-Coumaric Acid (μg/mL) | Myricitrin (μg/mL) | Quercitrin (μg/mL) | Quercetin (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20(C) | 0.08 | 50 | 0.5 | 7.1 | 2.2 | 1.8 | 0.2 | 0.02 | 0.2 | 1.4 | 2.2 | 0.7 |
| 3 | 0.20 | 20 | 0.3 | 70.5 | 15.2 | 12.7 | 1.8 | 0.21 | 1.5 | 7.7 | 12.2 | 1.6 |
| 19(C) | 0.08 | 50 | 0.5 | 6.1 | 2.0 | 1.7 | 0.2 | 0.02 | 0.17 | 1.2 | 2.3 | 0.5 |
| 11 | 0.04 | 50 | 0.5 | 4.5 | 1.6 | 1.5 | 0.17 | 0.02 | 0.12 | 1.1 | 1.9 | 0.4 |
| 5 | 0.05 | 80 | 0.3 | 11.6 | 3.5 | 3.2 | 0.4 | 0.03 | 0.4 | 2.4 | 2.9 | 1.0 |
| 17(C) | 0.08 | 50 | 0.5 | 11.3 | 3.6 | 2.9 | 0.4 | 0.04 | 0.30 | 2.6 | 4.6 | 0.6 |
| 15(C) | 0.08 | 50 | 0.5 | 21.7 | 7.5 | 6.7 | 0.7 | 0.08 | 0.6 | 5.8 | 13.3 | 2.8 |
| 18(C) | 0.08 | 50 | 0.5 | 22.7 | 7.3 | 6.5 | 0.7 | 0.08 | 0.6 | 6.3 | 11.9 | 2.5 |
| 13 | 0.08 | 50 | 0.3 | 21.7 | 7.1 | 6.9 | 0.7 | 0.08 | 0.6 | 6.0 | 10.5 | 2.1 |
| 1 | 0.05 | 20 | 0.3 | 19.9 | 4.5 | 3.7 | 0.5 | 0.06 | 0.5 | 2.2 | 3.5 | 0.8 |
| 8 | 0.2 | 80 | 1.0 | 40.2 | 16.3 | 10.0 | 1.7 | 0.13 | 1.3 | 10.3 | 9.3 | 5.2 |
| 7 | 0.2 | 80 | 0.3 | 50.2 | 13.6 | 12.9 | 1.9 | 0.14 | 1.7 | 10.8 | 13.7 | 3.4 |
| 2 | 0.05 | 20 | 1.0 | 9.1 | 2.2 | 1.7 | 0.2 | 0.03 | 0.2 | 0.91 | 1.6 | 0.13 |
| 6 | 0.05 | 80 | 1.0 | 5.5 | 1.7 | 1.6 | 0.2 | 0.013 | 0.19 | 1.1 | 1.2 | 0.4 |
| 14 | 0.08 | 50 | 2.0 | 21.5 | 7.4 | 7.0 | 0.7 | 0.08 | 0.6 | 6.0 | 13.4 | 2.5 |
| 10 | 0.08 | 100 | 0.5 | 2.4 | 0.4 | 0.5 | 0.8 | 0.011 | 0.10 | 0.4 | 0.5 | 0.3 |
| 9 | 0.08 | 0 | 0.5 | 23.1 | 4.2 | 2.0 | 0.6 | 0.05 | 0.5 | 1.6 | 1.5 | 0.01 |
| 12 | 0.21 | 50 | 0.5 | 34.9 | 10.2 | 9.4 | 1.2 | 0.13 | 1.0 | 7.3 | 10.7 | 2.3 |
| 16(C) | 0.08 | 50 | 0.5 | 12.8 | 3.6 | 3.2 | 0.4 | 0.04 | 0.4 | 2.0 | 1.7 | 0.7 |
| 4 | 0.2 | 20 | 1.0 | 45.5 | 10.0 | 7.4 | 1.2 | 0.13 | 0.9 | 4.8 | 7.1 | 0.7 |
| Compound | Equation | R2 | R2adj | Lack of Fit (P) |
|---|---|---|---|---|
| Gallic acid | 4.35 + 11.68X3 + 0.38X72 | 0.8418 | 0.7993 | 0.6462 |
| Procyanidin B1 | 2.50 + 7.13X3 − 0.04X42 + 0.38X72 | 0.8249 | 0.7674 | 0.6102 |
| Procyanidin B2 | 1.35 + 7.38X3 − 0.05X42 + 0.38X72 | 0.8259 | 0.7693 | 0.8053 |
| Ferulic acid | −1.04 + 22.89X3 + 0.21X72 | 0.8624 | 0.8386 | 0.9947 |
| Chlorogenic acid | −2.38 + 8.28X3 − 0.01X42 + 0.15X72 | 0.8733 | 0.8592 | 0.9719 |
| 4-Coumaric acid | −0.45 + 8.22X3 + 0.25X72 | 0.8075 | 0.7343 | 0.6034 |
| Myricitrin | 0.38 + 11.5X3 − 0.05X42 + 0.34X72 | 0.8058 | 0.7310 | 0.8848 |
| Quercitrin | 1.18 + 9.98X3 − 0.06X42 + 0.36X72 | 0.7697 | 0.6624 | 0.9149 |
| Quercetin | −1.03 + 6.81X3 + 0.56X4 − 0.06X42 + 0.33X72 | 0.8271 | 0.7714 | 0.9617 |
| Compound | Experimental (μg/mL) | Predicted (μg/mL) |
|---|---|---|
| Gallic acid | 56.6 ± 1.5 | 61.5 |
| Procyanidin B1 | 14.1 ± 1.2 | 15.3 |
| Procyanidin B2 | 13.8 ± 0.8 | 14.7 |
| Ferulic acid | 1.39 ± 0.11 | 1.51 |
| Chlorogenic acid | 0.21 ± 0.04 | 0.20 |
| 4-Coumaric acid | 1.47 ± 0.13 | 1.61 |
| Myricitrin | 10.1 ± 1.6 | 11.0 |
| Quercitrin | 14.8 ± 1.9 | 16.3 |
| Quercetin | 2.61 ± 0.17 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods 2022, 11, 284. https://doi.org/10.3390/foods11030284
Clodoveo ML, Crupi P, Muraglia M, Corbo F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods. 2022; 11(3):284. https://doi.org/10.3390/foods11030284
Chicago/Turabian StyleClodoveo, Maria Lisa, Pasquale Crupi, Marilena Muraglia, and Filomena Corbo. 2022. "Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters" Foods 11, no. 3: 284. https://doi.org/10.3390/foods11030284
APA StyleClodoveo, M. L., Crupi, P., Muraglia, M., & Corbo, F. (2022). Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods, 11(3), 284. https://doi.org/10.3390/foods11030284