Comparison of Milk Odd- and Branched-Chain Fatty Acids among Human, Dairy Species and Artificial Substitutes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Univariate Analysis
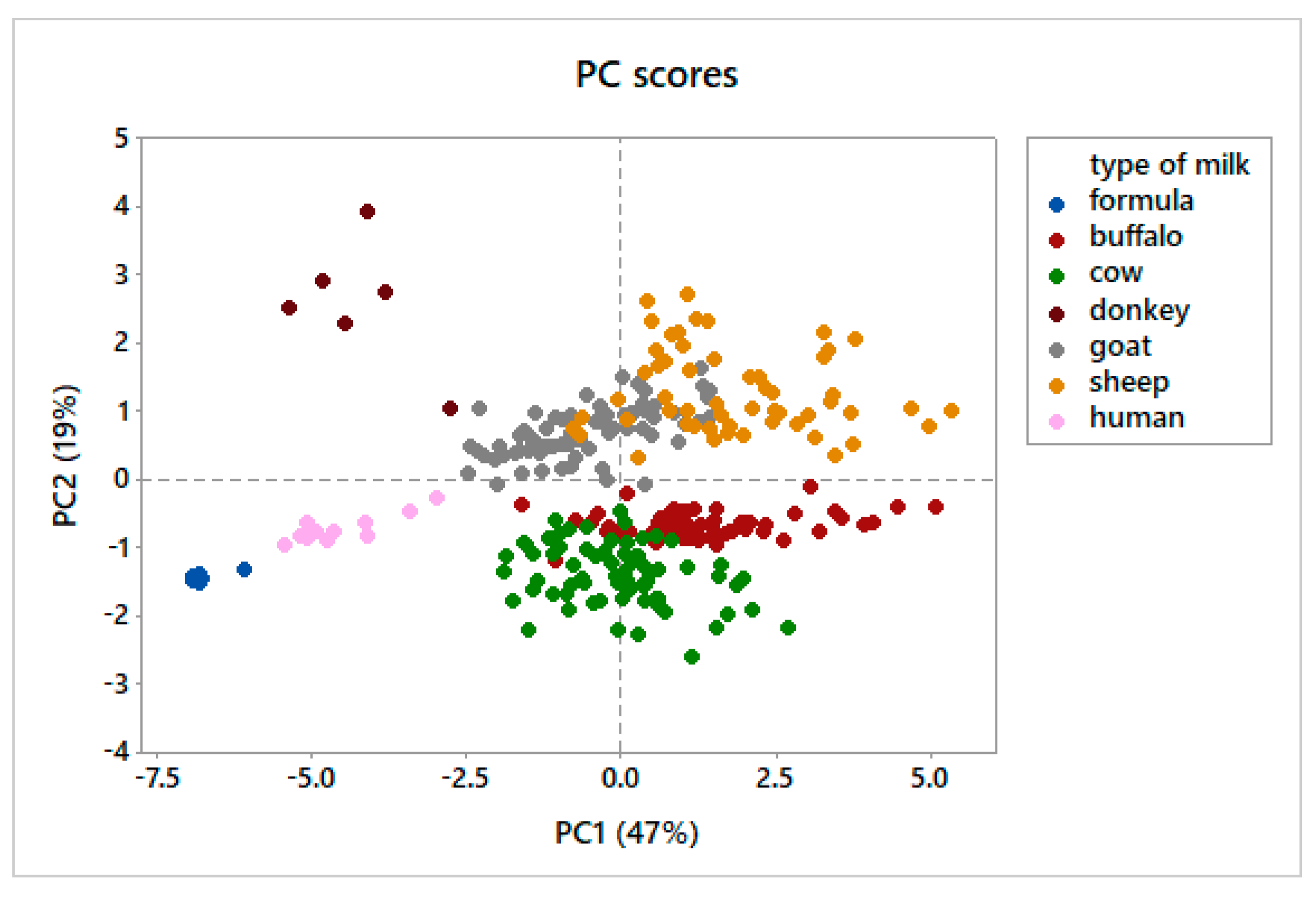
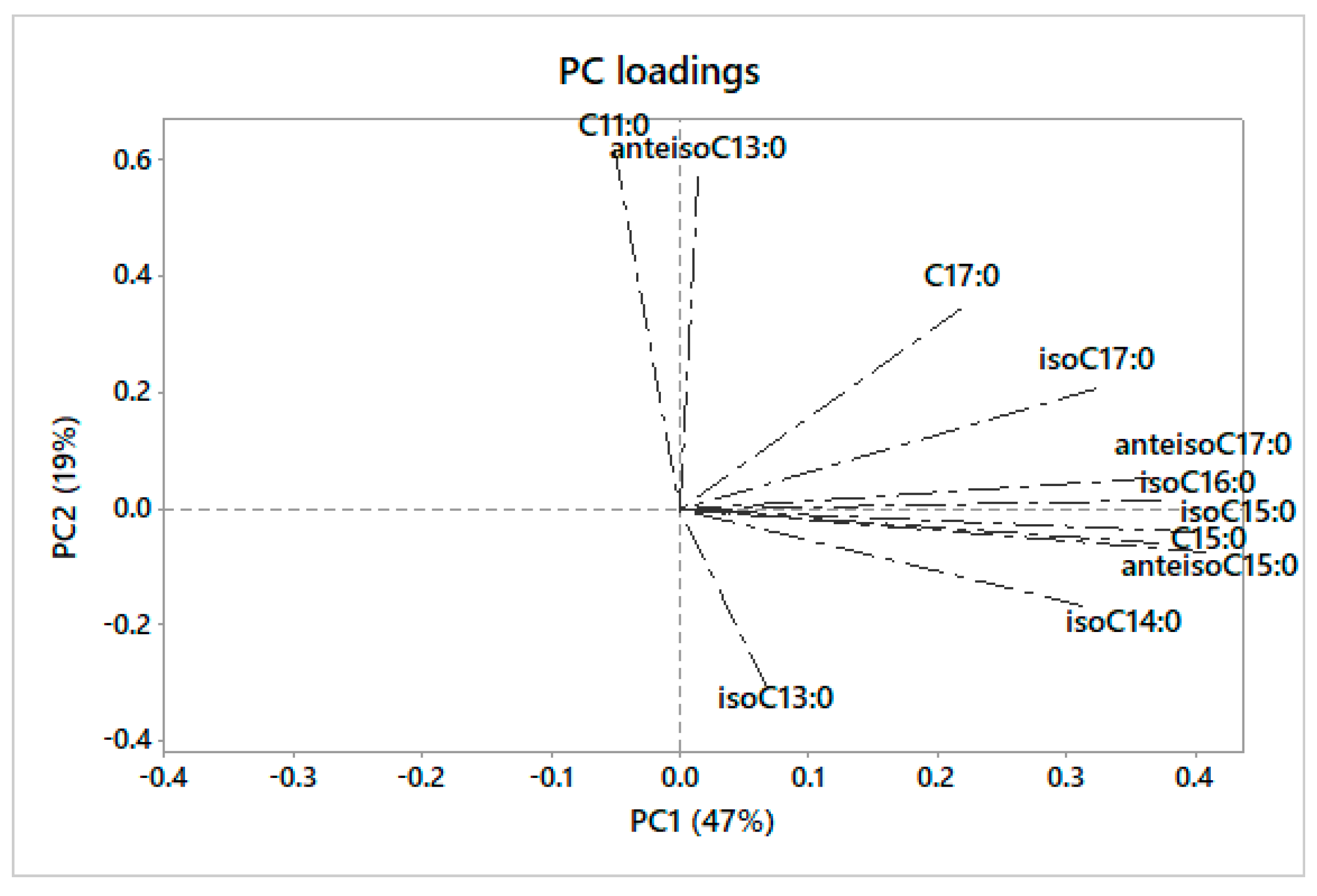
3.2. Principal Component Analysis
4. Discussion
4.1. Univariate Analysis
4.2. Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed. Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Abdoul-Aziz, S.K.A.; Zhang, Y.; Wang, J. Milk Odd and Branched Chain Fatty Acids in Dairy Cows: A Review on Dietary Factors and Its Consequences on Human Health. Animals 2021, 11, 3210. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Cesarani, A.; Pulina, G.; Battacone, G. Functional Odd- and Branched-Chain Fatty Acid in Sheep and Goat Milk and Cheeses. Dairy 2021, 2, 79–89. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2022, 105, 1687–1700. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.; Brenna, J.T. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef]
- Dingess, K.A.; Valentine, C.J.; Ollberding, N.J.; Davidson, B.S.; Woo, J.; Summer, S.; Peng, Y.M.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Ran-Ressler, R.R.; et al. Branched-chain fatty acid composition of human milk and the impact of maternal diet: The Global Exploration of Human Milk (GEHM) Study. Am. J. Clin. Nutr. 2017, 105, 177–184. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Paengkoum, P. Milk fatty acid composition, rumen microbial population and animal performance in response to diets rich in linoleic acid supplemented with Piper betle leaves in Saanen goats. Anim. Prod. Sci. 2020, 62, 1391–1401. [Google Scholar] [CrossRef]
- Makmur, M.; Zain, M.; Sholikin, M.M.; Suharlina; Jayanegara, A. Modulatory effects of dietary tannins on polyunsaturated fatty acid biohydrogenation in the rumen: A meta-analysis. Heliyon 2022, 8, e09828. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Ma, T.; Xu, Y.; Chen, G.; Chen, Y.; Villot, C.; Renaud, D.L.; Steele, M.A.; Guan, L.L. Characterization of fecal branched-chain fatty acid profiles and their associations with fecal microbiota in diarrheic and healthy dairy calves. J. Dairy Sci. 2021, 104, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study. PLOS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.; Brenna, J.T.; Dvorak, B. Branched Chain Fatty Acids Reduce the Incidence of Necrotizing Enterocolitis and Alter Gastrointestinal Microbial Ecology in a Neonatal Rat Model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [PubMed]
- Vahmani, P.; Salazar, V.; Rolland, D.C.; Gzyl, K.E.; Dugan, M.E.R. Iso- but Not Anteiso-Branched Chain Fatty Acids Exert Growth-Inhibiting and Apoptosis-Inducing Effects in MCF-7 Cells. J. Agric. Food Chem. 2019, 67, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Santaren, I.D.; Watkins, S.M.; Liese, A.D.; Wagenknecht, L.E.; Rewers, M.J.; Haffner, S.M.; Lorenzo, C.; Hanley, A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef]
- Su, X.; Magkos, F.; Zhou, D.; Eagon, J.C.; Fabbrini, E.; Okunade, A.L.; Klein, S. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity 2015, 23, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Śledziński, T.; Mika, A. Branched-Chain Fatty Acids Alter the Expression of Genes Responsible for Lipid Synthesis and Inflammation in Human Adipose Cells. Nutrients 2022, 14, 2310. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Domenech, F.R.; Rueda, M.C.; De La Fuente, M.; Schiavone, A.; Marín, A.L.M. Odd- and Branched-Chain Fatty Acids in Lamb Meat as Potential Indicators of Fattening Diet Characteristics. Foods 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.; Park, H.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef]
- Jenkins, B.; Seyssel, K.; Chiu, S.; Pan, P.-H.; Lin, S.-Y.; Stanley, E.; Ament, Z.; West, J.; Summerhill, K.; Griffin, J.; et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Sci. Rep. 2017, 7, 44845. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Luo, G.; Xin, H.; Zhang, Y.; Li, G. Relations of Ruminal Fermentation Parameters and Microbial Matters to Odd- and Branched-Chain Fatty Acids in Rumen Fluid of Dairy Cows at Different Milk Stages. Animals 2019, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Bertino, E.; Agosti, M.; Peila, C.; Corridori, M.; Pintus, R.; Fanos, V. The Donkey Milk in Infant Nutrition. Nutrients 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Sarti, L.; Martini, M.; Brajon, G.; Barni, S.; Salari, F.; Altomonte, I.; Ragona, G.; Mori, F.; Pucci, N.; Muscas, G.; et al. Donkey’s Milk in the Management of Children with Cow’s Milk protein allergy: Nutritional and hygienic aspects. Ital. J. Pediatr. 2019, 45, 102. [Google Scholar] [CrossRef]
- Nudda, A.; McGuire, M.; Battacone, G.; Pulina, G. Seasonal Variation in Conjugated Linoleic Acid and Vaccenic Acid in Milk Fat of Sheep and its Transfer to Cheese and Ricotta. J. Dairy Sci. 2005, 88, 1311–1319. [Google Scholar] [CrossRef]
- Correddu, F.; Gaspa, G.; Pulina, G.; Nudda, A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016, 99, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Standard 182:1999; Milk Fat. Preparation of Fatty Acid Methyl Esters. FIL-IDF International Dairy Federation: Brussels, Belgium, 1999.
- Vetter, W.; Gaul, S.; Thurnhofer, S.; Mayer, K. Stable carbon isotope ratios of methyl-branched fatty acids are different to those of straight-chain fatty acids in dairy products. Anal. Bioanal. Chem. 2007, 389, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Rodríguez-Pino, V.; Juárez, M.; de la Fuente, M. Optimization of milk odd and branched-chain fatty acids analysis by gas chromatography using an extremely polar stationary phase. Food Chem. 2017, 231, 11–18. [Google Scholar] [CrossRef] [PubMed]
- SAS. User’s Guide: Statistics; Version 8.2; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Gantner, V.; Mijić, P.; Baban, M.; Škrtić, Z.; Turalija, A. The overall and fat composition of milk of various species. Mljekarstvo 2015, 65, 223–231. [Google Scholar] [CrossRef]
- Valle, E.; Pozzo, L.; Giribaldi, M.; Bergero, D.; Gennero, M.S.; Dezzutto, D.; McLean, A.; Borreani, G.; Coppa, M.; Cavallarin, L. Effect of farming system on donkey milk composition. J. Sci. Food Agric. 2018, 98, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Le Huërou-Luron, I.; Lemaire, M.; Blat, S. Health benefits of dairy lipids and MFGM in infant formula. OCL 2018, 25, D306. [Google Scholar] [CrossRef]
- Demmelmair, H.; Koletzko, B. Lipids in human milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 57–68. [Google Scholar] [CrossRef]
- Daly, S.E.; Di Rosso, A.; Owens, R.A.; Hartmann, P. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp. Physiol. 1993, 78, 741–755. [Google Scholar] [CrossRef]
- Devle, H.; Vetti, I.; Naess-Andresen, C.F.; Rukke, E.; Vegarud, G.; Ekeberg, D. A comparative study of fatty acid profiles in ruminant and non-ruminant milk. Eur. J. Lipid Sci. Technol. 2012, 114, 1036–1043. [Google Scholar] [CrossRef]
- Mollica, M.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef]
- Carta, S.; Tsiplakou, E.; Mitsiopoulou, C.; Pulina, G.; Nudda, A. Cocoa husks fed to lactating dairy ewes affect milk fatty acid profile and oxidative status of blood and milk. Small Rumin. Res. 2022, 207, 106599. [Google Scholar] [CrossRef]
- Carta, S.; Tsiplakou, E.; Nicolussi, P.; Pulina, G.; Nudda, A. Effects of spent coffee grounds on production traits, haematological parameters, and antioxidant activity of blood and milk in dairy goats. Animal 2022, 16, 100501. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E.; Chouinard, P.; Lebeuf, Y.; Rico, D.; Gervais, R. Effect of lipid supplementation on milk odd- and branched-chain fatty acids in dairy cows. J. Dairy Sci. 2016, 99, 6311–6323. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Stocco, G.; Mele, M.; Schiavon, S.; Bittante, G.; Cecchinato, A. Factors affecting variations in the detailed fatty acid profile of Mediterranean buffalo milk determined by 2-dimensional gas chromatography. J. Dairy Sci. 2017, 100, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Carta, S.; Mazza, A.; Nudda, A.; Rassu, S.P.G. Effect of extruded linseed on sarda donkey milk quality. Ital. J. Anim. Sci. 2022, 21, 1200–1210. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I. Update on food sources and biological activity of odd-chain, branched and cyclic fatty acids—A review. Trends Food Sci. Technol. 2021, 119, 514–529. [Google Scholar] [CrossRef]
- Sellem, L.; Jackson, K.G.; Givens, I.D.; Lovegrove, J.A. Can individual fatty acids be used as functional biomarkers of dairy fat consumption in relation to cardiometabolic health? A narrative review. Br. J. Nutr. 2022, 128, 2373–2386. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. Int. Rev. J. 2016, 7, 730–734. [Google Scholar] [CrossRef]
- Xin, H.; Xu, Y.; Chen, Y.; Chen, G.; Steele, M.A. Odd-chain and branched-chain fatty acid concentrations in bovine colostrum and transition milk and their stability under heating and freezing treatments. J. Dairy Sci. 2020, 103, 11483–11489. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Gervais, R.; Rahman, M.; Gadeyne, F.; Gorniak, M.; Doreau, M.; Fievez, V. Postruminal synthesis modifies the odd- and branched-chain fatty acid profile from the duodenum to milk. J. Dairy Sci. 2015, 98, 4829–4840. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; De la Fuente, M.Á. Metabolic origin and bioactive properties of odd and branched-chain fatty acids in ruminants’ milk. Review. Rev. Mex. Cienc. Pecu. 2020, 11, 1174–1191. [Google Scholar] [CrossRef]
- Berger, A.; Fleith, M.; Crozier, G. Nutritional Implications of Replacing Bovine Milk Fat With Vegetable Oil in Infant Formulas. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of Fatty Acids in Human, Cow, Buffalo, Goat, Yak, and Camel Milk Using an Improved One-Step GC-FID Method. Food Anal. Methods 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- Malissiova, E.; Arsenos, G.; Papademas, P.; Fletouris, D.; Manouras, A.; Aspri, M.; Nikolopoulou, A.; Giannopoulou, A.; Arvanitoyannis, I.S. Assessment of donkey milk chemical, microbiological and sensory attributes in Greece and Cyprus. Int. J. Dairy Technol. 2016, 69, 143–146. [Google Scholar] [CrossRef]
- Martemucci, G.; D’Alessandro, A.G. Fat content, energy value and fatty acid profile of donkey milk during lactation and implications for human nutrition. Lipids Health Dis. 2012, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, I.; Salari, F.; Licitra, R.; Martini, M. Donkey and human milk: Insights into their compositional similarities. Int. Dairy J. 2019, 89, 111–118. [Google Scholar] [CrossRef]
- Correddu, F.; Cesarani, A.; Dimauro, C.; Gaspa, G.; Macciotta, N. Principal component and multivariate factor analysis of detailed sheep milk fatty acid profile. J. Dairy Sci. 2021, 104, 5079–5094. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Murgia, M.A.; Mangia, N.P.; Lunesu, M.F.; Cesarani, A.; Deiana, P.; Pulina, G.; Nudda, A. Effect of altitude of flock location, season of milk production and ripening time on the fatty acid profile of Pecorino Sardo cheese. Int. Dairy J. 2021, 113, 104895. [Google Scholar] [CrossRef]
- Conte, G.; Palombo, V.; Serra, A.; Correddu, F.; D’Andrea, M.; Macciotta, N.P.P.; Mele, M. Study of the Fatty Acid Profile of Milk in Different Sheep Breeds: Evaluation by Multivariate Factorial Analysis. Animals 2022, 12, 722. [Google Scholar] [CrossRef]
- Fievez, V.; Vlaeminck, B.; Dhanoa, M.; Dewhurst, R. Use of Principal Component Analysis to Investigate the Origin of Heptadecenoic and Conjugated Linoleic Acids in Milk. J. Dairy Sci. 2003, 86, 4047–4053. [Google Scholar] [CrossRef]
| Cow | Buffalo | Sheep | Goat | Donkey | Human | Formula Milk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Fat content, % | 3.746 d | 0.621 | 7.581 a | 1.421 | 6.050 b | 1.386 | 5.415 bc | 3.070 | 0.390 e | 0.207 | 1.076 e | 0.708 | 3.004 cde | 1.210 |
| Individual FA | ||||||||||||||
| C11:0 | 0.085 d | 0.039 | 0.024 e | 0.014 | 0.286 b | 0.088 | 0.186 c | 0.042 | 0.895 a | 0.398 | 0.010 de | 0.009 | 0.004 de | 0.006 |
| C15:0 | 1.063 b | 0.273 | 1.153 ab | 0.176 | 1.178 a | 0.171 | 0.751 c | 0.168 | 0.374 d | 0.085 | 0.375 d | 0.101 | 0.056 e | 0.043 |
| C17:0 | 0.455 c | 0.105 | 0.506 b | 0.076 | 0.760 a | 0.104 | 0.791 a | 0.079 | 0.353 d | 0.109 | 0.407 cd | 0.080 | 0.078 e | 0.025 |
| isoC13:0 | 0.091 a | 0.047 | 0.020 bc | 0.009 | 0.026 b | 0.010 | 0.025 b | 0.005 | 0.026 bc | 0.017 | nd | - | nd | - |
| isoC14:0 | 0.120 b | 0.055 | 0.189 a | 0.04 | 0.118 bc | 0.033 | 0.099 cd | 0.031 | 0.063 de | 0.041 | 0.020 ef | 0.009 | 0.002 f | 0.005 |
| isoC15:0 | 0.218 b | 0.052 | 0.318 a | 0.058 | 0.293 a | 0.079 | 0.185 c | 0.042 | 0.087 d | 0.039 | 0.054 d | 0.029 | 0.008 d | 0.015 |
| isoC16:0 | 0.233 c | 0.070 | 0.391 a | 0.086 | 0.332 b | 0.059 | 0.245 c | 0.083 | 0.140 d | 0.041 | 0.082 de | 0.031 | 0.007 e | 0.015 |
| isoC17:0 | 0.253 c | 0.066 | 0.241 c | 0.042 | 0.415 a | 0.079 | 0.311 b | 0.094 | 0.058 de | 0.035 | 0.127 d | 0.055 | 0.006 e | 0.013 |
| AnteisoC13:0 | nd | - | 0.036 b | 0.007 | 0.043 b | 0.013 | 0.022 c | 0.007 | 0.154 a | 0.121 | nd | - | nd | - |
| AnteisoC15:0 | 0.444 b | 0.106 | 0.540 a | 0.096 | 0.562 a | 0.127 | 0.329 c | 0.060 | 0.072 d | 0.031 | 0.086 d | 0.040 | 0.011 d | 0.019 |
| AnteisoC17:0 | 0.415 b | 0.102 | 0.365 c | 0.067 | 0.500 a | 0.086 | 0.388 bc | 0.078 | 0.138 d | 0.055 | 0.181 d | 0.048 | 0.005 e | 0.012 |
| Groups of FA 1 | ||||||||||||||
| linear-OCFA | 1.603 b | 0.362 | 1.683 b | 0.222 | 2.223 a | 0.282 | 1.729 b | 0.247 | 1.621 b | 0.331 | 0.792 c | 0.173 | 0.138 d | 0.069 |
| iso-BCFA | 0.916 b | 0.163 | 1.159 a | 0.203 | 1.185 a | 0.228 | 0.865 b | 0.190 | 0.374 c | 0.148 | 0.283 cd | 0.119 | 0.022 d | 0.037 |
| anteiso-BCFA | 0.858 b | 0.185 | 0.941 b | 0.159 | 1.105 a | 0.196 | 0.739 c | 0.118 | 0.363 d | 0.095 | 0.267 d | 0.086 | 0.016 e | 0.031 |
| BCFA | 1.775 c | 0.303 | 2.100 b | 0.356 | 2.290 a | 0.397 | 1.604 d | 0.286 | 0.738 e | 0.185 | 0.550 e | 0.202 | 0.038 f | 0.066 |
| OBCFA | 3.378 c | 0.494 | 3.783 b | 0.538 | 4.513 a | 0.577 | 3.333 c | 0.499 | 2.359 d | 0.424 | 1.342 e | 0.364 | 0.176 f | 0.133 |
| Type of Milk | |||||||
|---|---|---|---|---|---|---|---|
| Cow | Buffalo | Sheep | Goat | Donkey | Human | Formula | |
| Individual FA | |||||||
| C11:0 | 2.5 | 0.6 | 6.3 | 5.6 | 37.9 | 0.7 | 2.3 |
| C15:0 | 31.5 | 30.5 | 26.1 | 22.5 | 15.8 | 27.9 | 31.8 |
| C17:0 | 13.5 | 13.4 | 16.8 | 23.7 | 15.0 | 30.3 | 44.4 |
| isoC13:0 | 2.7 | 0.5 | 0.6 | 0.8 | 1.1 | nd | nd |
| isoC14:0 | 3.6 | 5.0 | 2.6 | 3.0 | 2.7 | 1.5 | 1.0 |
| isoC15:0 | 6.5 | 8.4 | 6.5 | 5.5 | 3.7 | 4.0 | 4.3 |
| isoC16:0 | 6.9 | 10.3 | 7.4 | 7.4 | 5.9 | 6.1 | 3.8 |
| isoC17:0 | 7.5 | 6.4 | 9.2 | 9.3 | 2.5 | 9.4 | 3.4 |
| anteisoC13:0 | nd | 0.9 | 0.9 | 0.7 | 6.5 | nd | nd |
| anteisoC15:0 | 13.1 | 14.3 | 12.5 | 9.9 | 3.0 | 6.4 | 6.0 |
| anteisoC17:0 | 12.3 | 9.6 | 11.1 | 11.6 | 5.8 | 13.5 | 3.1 |
| Groups of FA 1 | |||||||
| linear-OCFA | 47.5 | 44.5 | 49.3 | 51.9 | 68.7 | 59.0 | 78.5 |
| BCFA | 52.5 | 55.5 | 50.7 | 48.1 | 31.3 | 41.0 | 21.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta, S.; Correddu, F.; Battacone, G.; Pulina, G.; Nudda, A. Comparison of Milk Odd- and Branched-Chain Fatty Acids among Human, Dairy Species and Artificial Substitutes. Foods 2022, 11, 4118. https://doi.org/10.3390/foods11244118
Carta S, Correddu F, Battacone G, Pulina G, Nudda A. Comparison of Milk Odd- and Branched-Chain Fatty Acids among Human, Dairy Species and Artificial Substitutes. Foods. 2022; 11(24):4118. https://doi.org/10.3390/foods11244118
Chicago/Turabian StyleCarta, Silvia, Fabio Correddu, Gianni Battacone, Giuseppe Pulina, and Anna Nudda. 2022. "Comparison of Milk Odd- and Branched-Chain Fatty Acids among Human, Dairy Species and Artificial Substitutes" Foods 11, no. 24: 4118. https://doi.org/10.3390/foods11244118
APA StyleCarta, S., Correddu, F., Battacone, G., Pulina, G., & Nudda, A. (2022). Comparison of Milk Odd- and Branched-Chain Fatty Acids among Human, Dairy Species and Artificial Substitutes. Foods, 11(24), 4118. https://doi.org/10.3390/foods11244118









