Citric Acid Induces the Increase in Lenthionine Content in Shiitake Mushroom, Lentinula edodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Material Culture
2.2. Citric Acid Treatment
2.3. Determination of Mycelium Biomass
2.4. Analysis of the Activities of LEGGT and LECSL
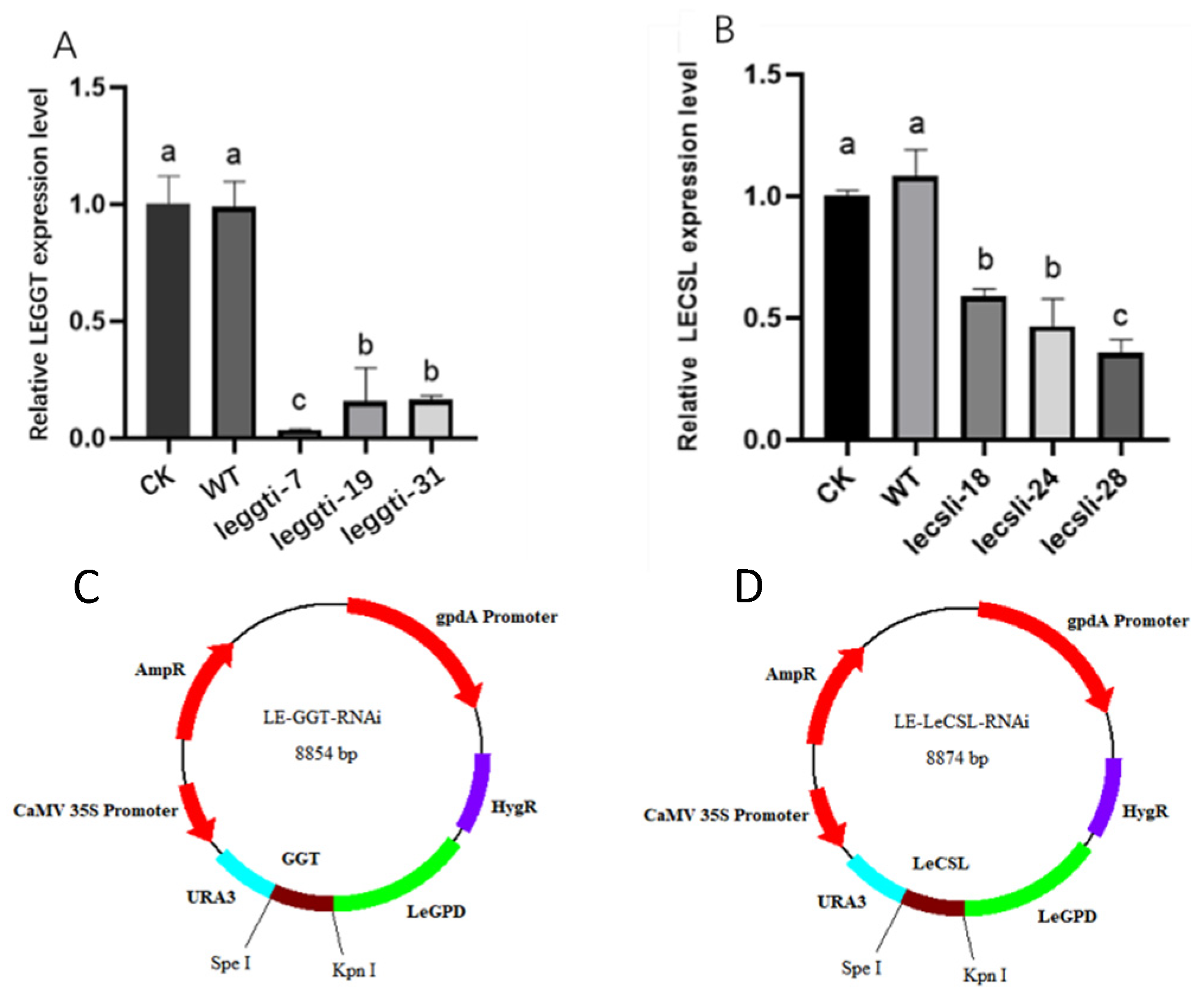
2.5. Construction of RNAi Plasmids and Strains
2.6. Real-time PCR Analysis of Gene Expression
2.7. Detection of Lenthionine Content
2.8. Experimental Parameters of Response Surface Method
- Y—lenthionine content;
- βk0—intercept;
- βki—linear effect coefficient;
- βkii—secondary effect coefficient;
- βkij—coefficient of interaction.
- Xi—coded variable;
- Ui—actual value of variable;
- Ui0—actual value of variable center point;
- △Ui—gradient of variable.
2.9. Statistical Analysis
3. Results
3.1. Effect of Different Concentrations of Citric Acid on Lenthionine and Biomass
3.2. Effect of Citric Acid on Lenthionine and Biomass at Different Times
3.3. Response Surface Methodology to Optimize Citric Acid Treatment Conditions
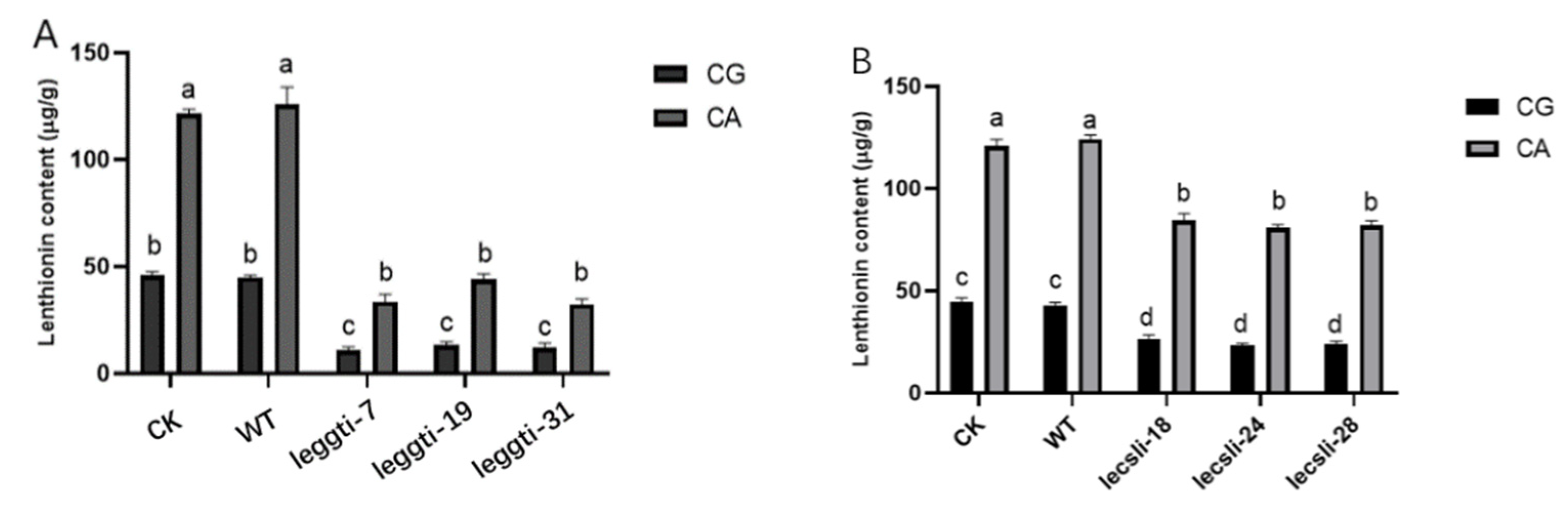
3.4. Effect of Citric Acid on the Expression Levels of LEGGT and LECSL
3.5. LEGGT and LECSL Genes Play an Important Role in the Citric-Acid-Induced Biosynthesis of Lenthionine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B.K.S. Lentinus edodes: A Macrofungus with Pharmacological Activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Hatvani, N. Antibacterial effect of the culture fluid of Lentinus edodes mycelium grown in submerged liquid culture. Int. J. Antimicrob. Agents 2001, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef] [PubMed]
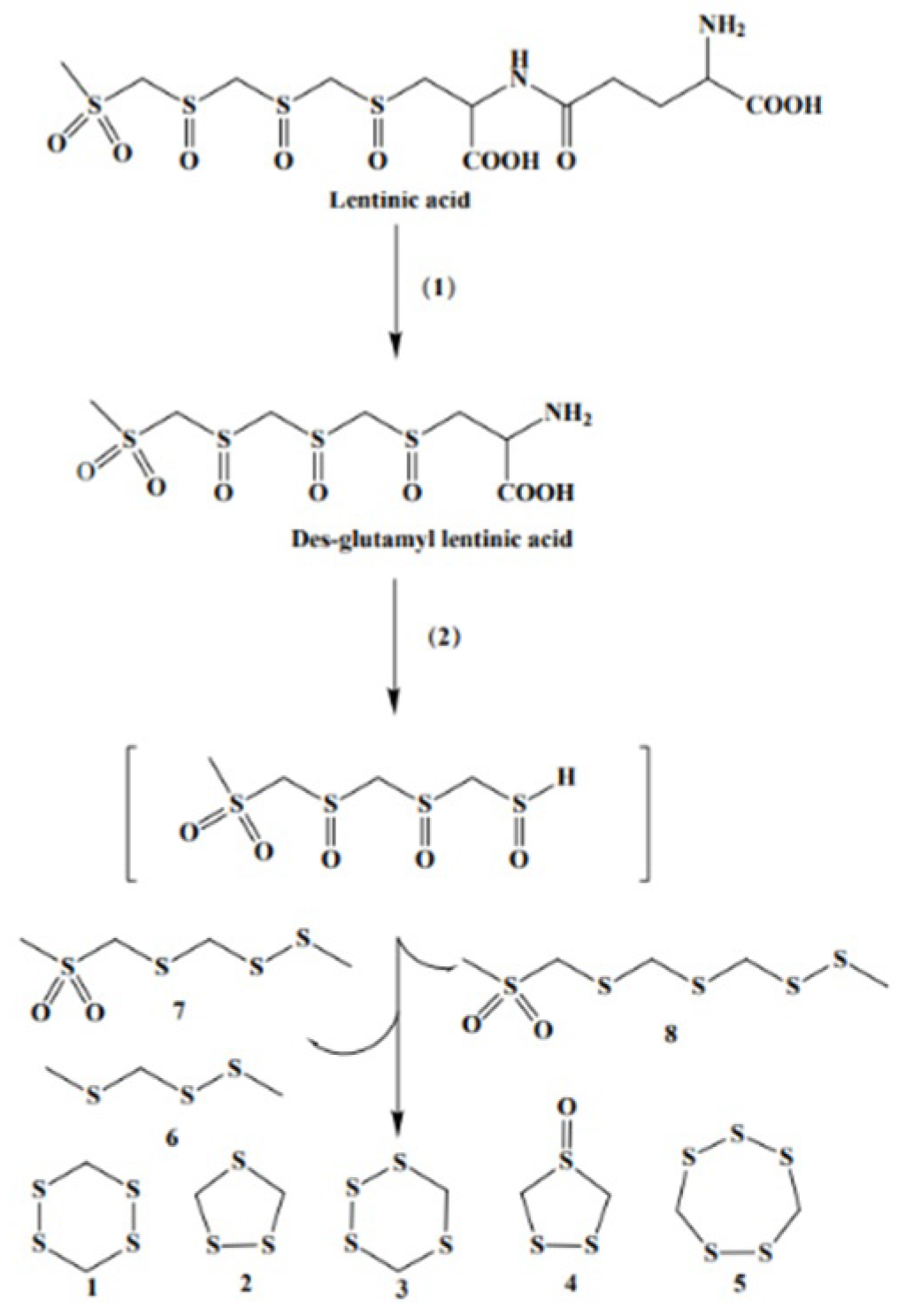
- Morita, K.; Kobayashi, S. Isolation, structure, and synthesis of lenthionine and its analogs. Chem. Pharm. Bull. 1967, 15, 988–993. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, X.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of drying process on the volatile and non-volatile flavor compounds of Lentinula edodes. Foods 2021, 10, 2836. [Google Scholar] [CrossRef]
- Sasaki, H.; Sakai, T.; Aoyagi, Y.; Sugahara, T. Changes in gamma-glutamyl-transferase activity and lenthionine content during rehydration and heating of dried shiitake mushroom. J. Jpn. Soc. Food Sci. Technol. -Nippon. Shokuhin Kagaku Kogaku Kaishi 1993, 40, 107–112. [Google Scholar] [CrossRef]
- Yasumoto, K.; Iwami, K.; Mitsuda, H. Enzyme-catalized evolution of lenthionine from lentinic acid. Agric. Biol. Chem. 1971, 35, 2070–2080. [Google Scholar] [CrossRef]
- Yasumoto, K.; Iwami, K.; Mitsuda, H.; Nakamura, K. Enzymic formation of Shiitake aroma from non-volatile precursor(s)-lenthionine from lentinic acid. Mushroom Sci. 1976, 9, 338–371. [Google Scholar] [CrossRef][Green Version]
- Iwami, K.; Yasumoto, K.; Nakamura, K.; Mitsuda, H. Reactivity of lentinus γ-Glutamyltransferase with lentinic acid as the principal endogenous substrate. Agric. Biol. Chem. 1975, 39, 1941–1946. [Google Scholar] [CrossRef][Green Version]
- Wang, R.; Guan, L.I.; Yan, L.I. Effects of inducers on relevant resistant substance in colored cotton. Acta Gossypii Sin. 2005, 15, 256–270. [Google Scholar] [CrossRef]
- Cao, P.F.; Wu, C.G.; Dang, Z.H.; Shi, L.; Jiang, A.L.; Ren, A.; Zhao, M.W. Effects of exogenous salicylic acid on ganoderic acid biosynthesis and the expression of key genes in the ganoderic acid biosynthesis pathway in the Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Li, X.-B.; Miao, Z.-G.; Shi, L.; Jaing, A.-L.; Zhao, M.-W. Transcript and metabolite alterations increase ganoderic acid content in Ganoderma lucidum using acetic acid as an inducer. Biotechnol. Lett. 2014, 36, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Huang, X.Y.; Sun, J.A.; Wei, D.Z.; Shen, Y.L. Effects of different organic acids on 2,3-butanediol production by Serratia marcescens. Ind. Microbiol. 2012, 3, 4926–4942. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Liu Aimin, C.J.; Zhang, J.; Fang, Q.; Wang, M.; Zha, Q. Effect of organic acid and amino acid on the mycelial growth of agaricus bisporus 2796. Chin. Agric. Sci. Bull. 2010, 26, 46–51. [Google Scholar] [CrossRef]
- Zhang, T.J.; Shi, L.; Chen, D.D.; Liu, R.; Shi, D.K.; Wu, C.G.; Sun, Z.H.; Ren, A.; Zhao, M.W. 14-3-3 proteins are involved in growth, hyphal branching, ganoderic acid biosynthesis, and response to abiotic stress in Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2018, 102, 1769–1782. [Google Scholar] [CrossRef]
- Kyoden, K.I. Properties of γ -Glutamyltransferase from Lentinus. Agric. Biol. Chem. 1975, 10, 1933–1940. [Google Scholar]
- Li, J.; Song, J.; Huang, J.; Wu, N.; Zhang, L.; Jiang, T. Study on key enzymes of endogenous formaldehyde metabolism and it's content in shiitake mushrooms (Lentinus Edodes). J. Chin. Inst. Food Sci. Technol. 2013, 13, 213–218. [Google Scholar] [CrossRef]
- Mu, D.; Shi, L.; Ren, A.; Li, M.; Wu, F.; Jiang, A.; Zhao, M. The development and application of a multiple gene co-silencing system using endogenous URA3 as a reporter gene in Ganoderma lucidum. PLoS ONE 2012, 7, e43737. [Google Scholar] [CrossRef]
- Lei, X.; Gao, S.; Feng, X.; Huang, Z.; Bian, Y.; Huang, W.; Liu, Y. Effects of GGT and CS lyase on the generation of endogenous formaldehyde in Lentinula edodes at different growth stages. Molecules 2019, 24, 4203. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fang, X.; Li, M.; Mu, D.; Ren, A.; Tan, Q.; Zhao, M. Development of a simple and efficient transformation system for the basidiomycetous medicinal fungus Ganoderma lucidum. World J. Microbiol. Biotechnol. 2012, 28, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chegeni, A.; Bahrami, A.; Khani, M.; Ghafari, M.D.; Sabet, N.M. The effects of two different organic acids: Citric acid and oxalic acid on poly (gamma) glutamate biosenthesis by flavobacterium sp. Iran. J. Public Health 2016, 45, 90. [Google Scholar] [CrossRef]
- De Kok, L.J.; Castro, A.; Durenkamp, M.; Stuiver, C.E.E.; Westerman, S.; Yang, L.; Stulen, I. Sulphur in Plant Physiology. In Sulfur Fertilizers: Demand Production and Use; International Fertiliser Society: Colchester, UK, 2002. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, D.; Zhang, X.; Zhou, C.; Zhou, P.; Zhi, Y. Medium optimization for spore production of a straw-cellulose degrading actinomyces strain under solid-state fermentation using response surface method. Sustainability 2020, 12, 8893. [Google Scholar] [CrossRef]
- Hiraide, M.; Nakashima, T.; Fujiwara, T. The smell and odorous components of dried shiitake mushroom, Lentinula edodes VI: Increase in odorous compounds of dried shiitake mushroom cultivated on bed logs. J. Wood Sci. 2010, 56, 483–487. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Onuma, M.; Sugino, Y.; Nakabayashi, R.; Imai, S.; Tsuneyoshi, T.; Sumi, S.-I.; Saito, K. Identification of a flavin-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J. 2015, 83, 941–951. [Google Scholar] [CrossRef]
- Sneeden, E.Y.; Harris, H.H.; Pickering, I.J.; Prince, R.C.; Johnson, S.; Li, X.J.; Block, E.; George, G.N. The sulfur chemistry of shiitake mushroom. J. Am. Chem. Soc. 2004, 126, 458–459. [Google Scholar] [CrossRef]





| Run | A: Concentration (μM) | B: Time (d) | Lenthionine (μg/g) |
|---|---|---|---|
| 1 | 400 | 15 | 127.42 |
| 2 | 400 | 15 | 127.42 |
| 3 | 258.579 | 15 | 52.5612 |
| 4 | 300 | 20 | 78.2731 |
| 5 | 400 | 15 | 127.42 |
| 6 | 541.421 | 15 | 57.3184 |
| 7 | 541.421 | 15 | 57.3184 |
| 8 | 400 | 15 | 127.42 |
| 9 | 400 | 7.92893 | 48.9913 |
| 10 | 400 | 22.0711 | 73.5658 |
| 11 | 500 | 20 | 87.6737 |
| 12 | 500 | 10 | 83.6007 |
| 13 | 300 | 10 | 61.356 |
| 14 | 400 | 7.92893 | 48.9913 |
| 15 | 500 | 20 | 87.6737 |
| 16 | 300 | 10 | 61.356 |
| 17 | 258.579 | 15 | 52.5612 |
| 18 | 400 | 15 | 127.42 |
| 19 | 500 | 10 | 83.6007 |
| 20 | 400 | 22.0711 | 73.5658 |
| 21 | 300 | 20 | 78.2731 |
| Source | Sum of Squares | DF | Mean squares | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 14,795.45 | 5 | 2959.09 | 25.48 | <0.0001 |
| Residual | 1741.84 | 15 | 116.12 | ||
| Lack of fit | 1741.84 | 3 | 580.61 | ||
| Pure error | 0.0000 | 12 | 0.0000 | ||
| Cor total | 16,537.29 | 20 |
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 127.42 | 1 | 4.82 | 117.15 | 137.69 | |
| A: time | 4.80 | 1 | 2.69 | −0.9455 | 10.54 | 1.0000 |
| B: concentration | 6.97 | 1 | 2.69 | 1.23 | 12.71 | 1.0000 |
| AB | −3.21 | 1 | 3.81 | −11.33 | 4.91 | 1.0000 |
| A2 | −31.34 | 1 | 3.35 | −38.48 | -24.19 | 1.14 |
| B2 | −28.17 | 1 | 3.35 | −35.32 | -21.02 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Han, D.; Qiao, J.; Zhou, X.; Yu, H.; Shi, L. Citric Acid Induces the Increase in Lenthionine Content in Shiitake Mushroom, Lentinula edodes. Foods 2022, 11, 4110. https://doi.org/10.3390/foods11244110
Hong M, Han D, Qiao J, Zhou X, Yu H, Shi L. Citric Acid Induces the Increase in Lenthionine Content in Shiitake Mushroom, Lentinula edodes. Foods. 2022; 11(24):4110. https://doi.org/10.3390/foods11244110
Chicago/Turabian StyleHong, Mengting, Dan Han, Jinjin Qiao, Xiaolin Zhou, Hanshou Yu, and Liang Shi. 2022. "Citric Acid Induces the Increase in Lenthionine Content in Shiitake Mushroom, Lentinula edodes" Foods 11, no. 24: 4110. https://doi.org/10.3390/foods11244110
APA StyleHong, M., Han, D., Qiao, J., Zhou, X., Yu, H., & Shi, L. (2022). Citric Acid Induces the Increase in Lenthionine Content in Shiitake Mushroom, Lentinula edodes. Foods, 11(24), 4110. https://doi.org/10.3390/foods11244110




