Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Phytochemicals and Antioxidant Activity of POP
2.2.1. Phytochemical Extraction
2.2.2. Phytochemical Content
2.2.3. Antioxidant Assays
2.3. Preparation of Pork Patties
2.4. Meat Quality Measurements
2.4.1. Physicochemical Assays
2.4.2. Oxidative Stability
2.5. ivGD of Pork Patties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Content and Antioxidant Activity of POP
3.2. Physicochemical Analysis of Meat Samples
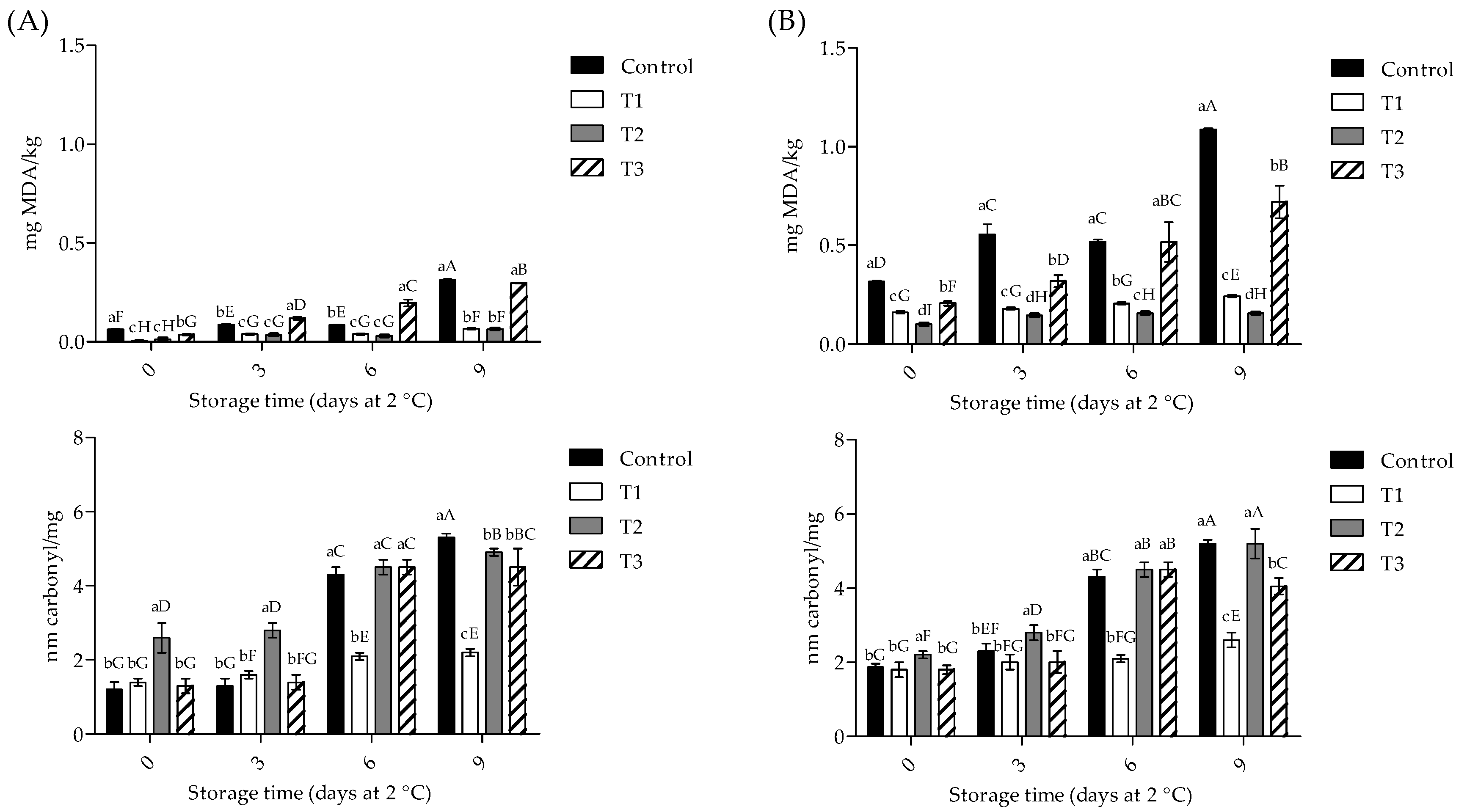
3.3. Oxidative Stability of Meat Samples
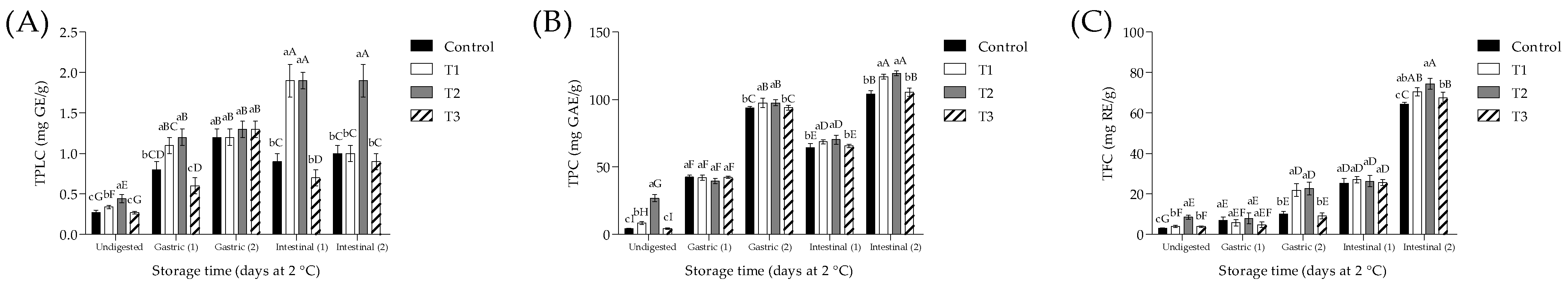
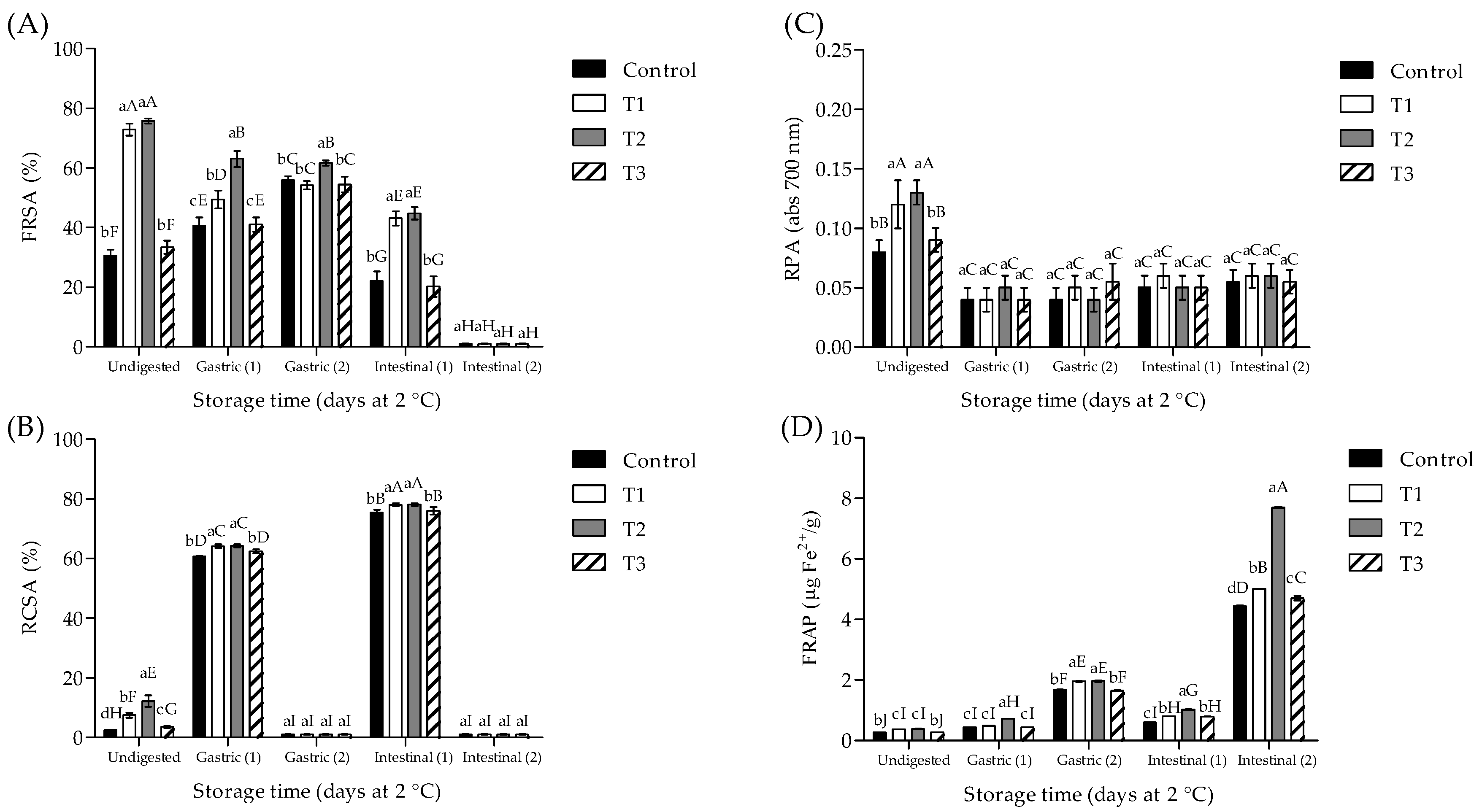
3.4. Phytochemical Content and Antioxidant Activity of Meat Samples Subjected to ivGD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, P.M.D.C.C.; Vicente, A.F.D.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Młyniec, K.; Gaweł, M.; Doboszewska, U.; Starowicz, G.; Pytka, K.; Davies, C.L.; Budziszewska, B. Essential elements in depression and anxiety. Part II. Pharmacol. Rep. 2015, 67, 187–194. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Siegrist, M.; Sütterlin, B. Importance of perceived naturalness for acceptance of food additives and cultured meat. Appetite 2017, 113, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Stojković, D.S.; Reis, F.S.; Ćirić, A.; Barros, L.; Glamočlija, J.; Ferreira, I.C.; Soković, M. Boletus aereus growing wild in Serbia: Chemical profile, in vitro biological activities, inactivation and growth control of food-poisoning bacteria in meat. J. Food Sci. Technol. 2015, 52, 7385–7392. [Google Scholar] [CrossRef]
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of enoki mushroom (Flammulina Velutipes) stem wastes as functional ingredients in goat meat nuggets. Foods 2020, 9, 432. [Google Scholar] [CrossRef]
- Barros, L.; Barreira, J.C.; Grangeia, C.; Batista, C.; Cadavez, V.A.; Ferreira, I.C. Beef burger patties incorporated with Boletus edulis extracts: Lipid peroxidation inhibition effects. Eur. J. Lipid Sci. Technol. 2011, 113, 737–743. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Torres-Martínez, B.D.M.; Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Esqueda, M.; Rodríguez-Carpena, J.G.; Fernández-López, J.; Perez-Alvarez, J.A.; Sánchez-Escalante, A. Pleurotus genus as a potential ingredient for meat products. Foods 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Guevara, M.I.; Rangel-Vargas, E.; Lorenzo, J.M.; Bermúdez, R.; Pateiro, M.; Rodriguez, J.A.; Sanchez-Ortega, I.; Santos, E.M. Effect of the addition of edible mushroom flours (Agaricus bisporus and Pleurotus ostreatus) on physicochemical and sensory properties of cold-stored beef patties. J. Food. Process. Pres. 2020, 44, e14351. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after in vitro gastrointestinal digestion. J. Proteomics 2019, 208, 103500. [Google Scholar] [CrossRef]
- Antonini, E.; Torri, L.; Piochi, M.; Cabrino, G.; Meli, M.A.; De Bellis, R. Nutritional, antioxidant and sensory properties of functional beef burgers formulated with chia seeds and goji puree, before and after in vitro digestion. Meat Sci. 2020, 161, 108021. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Q.; Du, H.; Kimatu, B.M.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Demirata, B.; Apak, R. Total antioxidant capacity assay using optimized ferricyanide/prussian blue method. Food Anal. Methods 2010, 3, 154–168. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Robertson, A.R.; Lozano, R.D.; Alman, D.H.; Orchard, S.E.; Keitch, J.A.; Connely, R.; Graham, L.A.; Acree, W.L.; John, R.S.; Hoban, R.F.; et al. CIE Recommendations on uniform color spaces, color-difference equations, and metric color terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar]
- Sutton, D.S.; Ellis, M.; Lan, Y.; McKeith, F.K.; Wilson, E.R. Influence of slaughter weight and stress gene genotype on the water-holding capacity and protein gel characteristics of three porcine muscles. Meat Sci. 1977, 46, 173–180. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Reinbach, H.C.; Meinert, L.; Ballabio, D.; Aaslyng, M.D.; Bredie, W.L.P.; Olsen, K.; Møller, P. Interactions between oral burn, meat flavor and texture in chili spiced pork patties evaluated by time-intensity. Food Qual. Prefer. 2007, 18, 909–919. [Google Scholar] [CrossRef]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Alpha-tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Simonetti, A.; Gambacorta, E.; Perna, A. Antioxidative and antihypertensive activities of pig meat before and after cooking and in vitro gastrointestinal digestion: Comparison between Italian autochthonous pig Suino Nero Lucano and a modern crossbred pig. Food Chem. 2016, 212, 590–595. [Google Scholar] [CrossRef] [PubMed]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, T.; Yan, J.; Liu, J. Technology optimization for polysaccharides (POP) extraction from the fruiting bodies of Pleurotus ostreatus by Box–Behnken statistical design. Carbohydr. Polym. 2010, 80, 242–247. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Cri. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Torres-Martínez, B.D.M.; Vargas-Sánchez, R.D.; Ibarra-Arias, F.J.; Ibarra-Torres, E.V.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Effect of extraction solvent on chemical composition, physicochemical and biological properties of edible mushrooms extracts. TIP. Rev. Espec. Cienc. Quím. Biol. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- El-Refai, A.; El-Zeiny, A.R.; Abd Rabo, E.A.A. Quality attributes of mushroom-beef patties as a functional meat product. J. Hyg. Eng. Des. 2014, 6, 49–62. [Google Scholar]
- Rosli, W.; Solihah, M.A. Nutritional composition and sensory properties of oyster mushroom-based patties packed with biodegradable packaging. Sains Malays. 2014, 43, 65–71. [Google Scholar]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R.S. Novel trends in development of dietary fiber rich meat products—A critical review. J. Food Sci Technol. 2015, 52, 633–647. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lee, J.; Jo, K.; Jo, C.; Song, M.; Jung, S. Application of winter mushroom powder as an alternative to phosphates in emulsion-type sausages. Meat Sci. 2018, 143, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Özünlü, O.; Ergezer, H. Possibilities of using dried oyster mushroom (Pleurotus ostreatus) in the production of beef salami. J. Process. Preserv. 2021, 45, e15117. [Google Scholar] [CrossRef]
- Jung, D.Y.; Lee, H.J.; Shin, D.J.; Kim, C.H.; Jo, C. Mechanism of improving emulsion stability of emulsion-type sausage with oyster mushroom (Pleurotus ostreatus) powder as a phosphate replacement. Meat Sci. 2022, 194, 108993. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. 2020, 141, 111401. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. From balsamic to healthy: Traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem. Toxicol. 2010, 48, 2097–2102. [Google Scholar] [CrossRef]
| Item | Test | Water | Ethanol | 1:1 | BHT |
|---|---|---|---|---|---|
| Phytochemical content | TPLC (mg GE/g) | 13.0 ± 1.0 b | 15.0 ± 1.0 a | 10.0 ± 1.0 c | |
| TPC (mg GAE/g) | 35.1 ± 0.6 c | 39.0 ± 1.0 b | 40.1 ± 0.6 b | ||
| TFC (mg RE/g) | 18.0 ± 1.2 b | 20.1 ± 1.0 a | 15.8 ± 0.3 c | ||
| Antioxidant activity | FRSA (%) | 91.7 ± 1.7 a | 92.0 ± 1.5 a | 90.9 ± 0.7 a | 70.9 ± 1.3 b |
| RCSA (%) | 55.7 ± 0.9 c | 58.5 ± 1.0 b | 56.4 ± 0.5 c | 65.8 ± 0.7 a | |
| RPA (abs) | 0.40 ± 0.1 a | 0.45 ± 0.1 b | 0.41 ± 0.2 a | 0.80 ± 0.1 c | |
| FRAP (µg Fe2+/g) | 0.65 ± 0.1 c | 0.68 ± 0.1 b | 0.61 ± 0.1 d | 0.74 ± 0.1 a |
| Treatment | Moisture | Fat | Protein | Ash | Carbohydrate |
|---|---|---|---|---|---|
| POP | 0.9 ± 0.6 | 9.2 ± 0.9 | 8.4 ± 0.1 | 5.2 ± 0.1 | 76.0 ± 0.9 |
| Control | 70.3 ± 1.3 a | 9.6 ± 0.8 | 18.0 ± 1.2 | 1.8 ± 0.3 | 0.7 ± 0.2 b |
| T1 | 68.8 ± 0.1 b | 9.5 ± 0.7 | 19.2 ± 1.3 | 1.8 ± 0.3 | 1.2 ± 0.3 a |
| T2 | 65.4 ± 0.4 c | 10.0 ± 1.2 | 20.1 ± 1.6 | 2.2 ± 0.5 | 1.3 ± 0.3 a |
| T3 | 68.4 ± 1.2 ab | 10.1 ± 0.2 | 19.0 ± 1.3 | 2.0 ± 0.1 | 0.9 ± 0.2 b |
| Item | Meat | Day | Treatments | |||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | |||
| pH | Raw | 0 | 5.8 ± 0.1 aA | 5.8 ± 0.2 aA | 5.7 ± 0.1 aA | 5.9 ± 0.1 aA |
| 3 | 5.8 ± 0.1 aA | 5.7 ± 0.1 aA | 5.7 ± 0.1 aA | 5.6 ± 0.1 aB | ||
| 6 | 5.7 ± 0.1 aAB | 5.7 ± 0.1 aA | 5.7 ± 0.1 aA | 5.6 ± 0.1 aB | ||
| 9 | 5.4 ± 0.1 bB | 5.7 ± 0.1 bA | 5.7 ± 0.1 bA | 5.6 ± 0.1 abB | ||
| Cooked | 0 | 6.1 ± 0.1 aA | 6.1 ± 0.1 aA | 6.1 ± 0.1 aA | 6.0 ± 0.1 aA | |
| 3 | 6.1 ± 0.1 aA | 5.9 ± 0.1 aAB | 5.9 ± 0.1 aA | 5.9 ± 0.1 aA | ||
| 6 | 6.1 ± 0.1 aA | 5.9 ± 0.1 aAB | 5.9 ± 0.1 aA | 5.9 ± 0.1 aA | ||
| 9 | 5.8 ± 0.1 aB | 5.8 ± 0.1 aB | 5.9 ± 0.1 aA | 5.9 ± 0.1 aA | ||
| WHC (%) | Raw | 0 | 90.1 ± 2.2 aA | 90.4 ± 2.0 aA | 92.7 ± 0.5 aA | 89.7 ± 0.4 aA |
| 3 | 90.0 ± 1.3 aA | 91.9 ± 0.8 aA | 92.3 ± 1.1 aA | 89.0 ± 1.5 aA | ||
| 6 | 91.0 ± 2.0 aA | 90.8 ± 1.5 aA | 92.2 ± 0.7 aA | 85.3 ± 1.3 bB | ||
| 9 | 85.7 ± 1.1 bB | 91.9 ± 1.4 aA | 92.9 ± 0.4 aA | 84.1 ± 0.3 bB | ||
| Cooked | 0 | 85.7 ± 1.9 bA | 89.2 ± 1.9 bA | 93.9 ± 1.4 aA | 89.4 ± 1.4 bA | |
| 3 | 85.7 ± 1.9 bA | 88.1 ± 1.5 bA | 94.3 ± 1.8 aA | 87.5 ± 1.5 bAB | ||
| 6 | 85.7 ± 1.6 aA | 86.1 ± 1.6 aA | 84.5 ± 2.0 aB | 85.1 ± 1.7 aB | ||
| 9 | 79.2 ± 2.3 bB | 86.3 ± 1.4 aA | 85.2 ± 2.3 aB | 82.1 ± 1.8 aB | ||
| CLW (%) | Raw | 0 | -- | -- | -- | -- |
| 3 | -- | -- | -- | -- | ||
| 6 | -- | -- | -- | -- | ||
| 9 | -- | -- | -- | -- | ||
| Cooked | 0 | 16.0 ± 1.2 aB | 14.9 ± 1.4 abB | 11.9 ± 1.9 bB | 16.0 ± 0.7 aB | |
| 3 | 22.5 ± 1.2 aA | 16.6 ± 1.3 bB | 14.0 ± 1.6 bB | 21.9 ± 1.9 aA | ||
| 6 | 21.6 ± 1.5 aA | 19.3 ± 0.7 bA | 16.3 ± 0.4 cA | 22.8 ± 1.3 aA | ||
| 9 | 23.8 ± 2.3 aA | 19.4 ± 0.7 bA | 17.1 ± 0.7 bA | 25.2 ± 1.7 aA | ||
| Texture (kg–f) | Raw | 0 | 1.1 ± 0.3 aB | 1.0 ± 0.3 aA | 1.1 ± 0.3 aA | 1.2 ± 0.2 aB |
| 3 | 1.1 ± 0.1 aB | 1.1 ± 0.2 aA | 1.1 ± 0.3 aA | 1.1 ± 0.3 aB | ||
| 6 | 1.0 ± 0.2 aB | 1.1 ± 0.1 aA | 1.2 ± 0.2 aA | 1.2 ± 0.2 aB | ||
| 9 | 1.6 ± 0.1 aA | 1.2 ± 0.2 bA | 1.2 ± 0.2 bA | 1.7 ± 0.2 aA | ||
| Cooked | 0 | 0.9 ± 0.1 aB | 1.1 ± 0.1 aA | 1.0 ± 0.2 aA | 0.9 ± 0.1 aB | |
| 3 | 0.9 ± 0.1 aB | 1.0 ± 0.2 aA | 1.1 ± 0.2 aA | 1.2 ± 0.1 abA | ||
| 6 | 1.0 ± 0.1 aB | 1.0 ± 0.2 aA | 1.1 ± 0.2 aA | 1.2 ± 0.1 aA | ||
| 9 | 1.4 ± 0.1 bA | 1.0 ± 0.2 aA | 1.1 ± 0.2 aA | 1.4 ± 0.1 bA | ||
| Item | Meat | Day | Treatments | |||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | |||
| L* | Raw | 0 | 49.3 ± 2.3 aA | 48.6 ± 1.5 aA | 44.7 ± 2.1 bA | 48.0 ± 2.5 aA |
| 3 | 49.6 ± 2.5 aA | 48.1 ± 2.5 aA | 45.4 ± 1.2 bA | 51.4 ± 1.9 aA | ||
| 6 | 52.4 ± 2.5 aAB | 50.0 ± 2.3 aA | 45.0 ± 1.1 bA | 51.3 ± 2.1 aA | ||
| 9 | 55.0 ± 1.5 aA | 50.1 ± 1.4 bA | 45.9 ± 2.2 cA | 52.1 ± 1.8 bA | ||
| Cooked | 0 | 52.4 ± 2.0 aB | 47.9 ± 2.5 aA | 49.3 ± 2.5 aA | 52.6 ± 2.8 aB | |
| 3 | 59.4 ± 2.3 aA | 51.6 ± 2.5 bcA | 48.9 ± 2.5 cA | 54.4 ± 2.2 bB | ||
| 6 | 59.3 ± 2.5 aA | 50.5 ± 3.0 bcA | 49.0 ± 2.7 cA | 55.3 ± 2.5 bAB | ||
| 9 | 60.3 ± 1.6 aA | 53.7 ± 2.7 bA | 53.0 ± 2.8 bA | 59.0 ± 2.7 aA | ||
| a* | Raw | 0 | 7.9 ± 0.9 bA | 10.1 ± 1.5 aA | 11.6 ± 1.0 aA | 7.3 ± 1.1 bA |
| 3 | 6.8 ± 1.2 bAB | 10.0 ± 1.5 aA | 10.6 ± 0.8 aA | 6.5 ± 0.8 bAB | ||
| 6 | 5.1 ± 1.1 bBC | 8.9 ± 1.5 aA | 10.0 ± 0.9 aA | 6.4 ± 0.7 bAB | ||
| 9 | 4.8 ± 0.6 bC | 8.3 ± 1.0 aA | 9.9 ± 0.9 aA | 5.1 ± 1.0 bB | ||
| Cooked | 0 | 4.9 ± 1.1 bA | 8.2 ± 1.3 aA | 8.2 ± 1.4 aA | 7.6 ± 1.5 abA | |
| 3 | 4.1 ± 1.3 bA | 7.8 ± 1.4 aA | 8.0 ± 1.1 aA | 6.0 ± 1.3 aAB | ||
| 6 | 3.5 ± 0.7 bA | 7.0 ± 1.0 aA | 7.2 ± 1.0 aA | 6.1 ± 0.8 aAB | ||
| 9 | 2.7 ± 0.8 bA | 6.1 ± 1.2 aA | 6.5 ± 1.2 aA | 4.4 ± 1.1 abB | ||
| b* | Raw | 0 | 14.2 ± 1.3 bA | 19.2 ± 1.1 aA | 21.9 ± 1.5 aA | 14.1 ± 1.2 bA |
| 3 | 13.6 ± 1.5 bA | 18.9 ± 1.4 aA | 20.5 ± 1.1 aA | 12.6 ± 0.6 bA | ||
| 6 | 13.0 ± 1.5 bA | 19.0 ± 1.5 aA | 20.6 ± 1.1 aA | 13.6 ± 0.6 bA | ||
| 9 | 12.4 ± 0.5 bA | 18.9 ± 1.6 aA | 20.1 ± 1.6 aA | 13.1 ± 1.5 bA | ||
| Cooked | 0 | 19.2 ± 2.3 | 21.2 ± 2.4 | 22.4 ± 1.8 | 22.2 ± 1.4 | |
| 3 | 20.0 ± 1.3 | 23.4 ± 1.2 | 21.9 ± 1.3 | 22.0 ± 2.4 | ||
| 6 | 19.6 ± 1.9 | 22.0 ± 1.9 | 20.9 ± 1.2 | 20.5 ± 1.1 | ||
| 9 | 20.8 ± 1.7 | 21.5 ± 1.8 | 20.7 ± 1.4 | 22.2 ± 1.0 | ||
| C* | Raw | 0 | 15.9 ± 1.2 bA | 21.5 ± 1.4 aA | 24.0 ± 1.9 aA | 15.8 ± 1.4 bA |
| 3 | 15.2 ± 1.5 bA | 21.4 ± 2.5 aA | 23.1 ± 1.2 aA | 15.2 ± 0.6 bA | ||
| 6 | 14.0 ± 1.5 bA | 21.0 ± 2.2 aA | 22.9 ± 1.3 aA | 14.4 ± 1.4 bA | ||
| 9 | 12.2 ± 0.5 bB | 21.0 ± 1.4 aA | 22.3 ± 1.8 aA | 14.3 ± 1.4 bA | ||
| Cooked | 0 | 20.4 ± 1.4 bA | 25.0 ± 1.4 aA | 24.0 ± 0.9 aA | 24.9 ± 1.0 aA | |
| 3 | 20.5 ± 1.5 bA | 24.7 ± 1.4 aA | 23.4 ± 1.4 aA | 24.1 ± 1.1 aA | ||
| 6 | 19.0 ± 1.5 bA | 23.1 ± 1.0 aAB | 21.8 ± 1.4 aA | 23.7 ± 1.1 aAB | ||
| 9 | 19.0 ± 1.6 aA | 21.8 ± 1.0 aB | 21.8 ± 1.3 aA | 21.0 ± 1.3 aB | ||
| h* | Raw | 0 | 64.0 ± 2.0 aB | 62.8 ± 1.9 aA | 61.1 ± 1.4 aB | 62.8 ± 2.2 aA |
| 3 | 63.4 ± 2.4 aB | 62.2 ± 2.4 aA | 62.6 ± 1.5 aAB | 63.5 ± 2.3 aA | ||
| 6 | 70.4 ± 2.5 aA | 65.1 ± 2.2 bA | 63.0 ± 1.5 bAB | 64.6 ± 2.5 bA | ||
| 9 | 70.0 ± 1.3 aA | 66.4 ± 1.8 bcA | 63.0 ± 1.1 cA | 67.6 ± 2.0 ab | ||
| Cooked | 0 | 73.2 ± 2.5 aB | 71.8 ± 2.5 aA | 71.4 ± 2.4 aA | 72.1 ± 2.3 aB | |
| 3 | 78.6 ± 2.3 aA | 71.5 ± 2.5 bA | 70.1 ± 2.4 bA | 74.9 ± 2.1 abAB | ||
| 6 | 81.4 ± 2.5 aA | 72.3 ± 2.5 bA | 73.2 ± 2.4 bA | 75.8 ± 1.4 bAB | ||
| 9 | 80.6 ± 1.3 aA | 74.3 ± 2.6 bA | 72.0 ± 1.0 bA | 77.1 ± 2.6 aA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; González-Ávila, M.; Rodríguez-Carpena, J.G.; Huerta-Leidenz, N.; Pérez-Alvarez, J.A.; Fernández-López, J.; Sánchez-Escalante, A. Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and In Vitro Gastrointestinal Digestion. Foods 2022, 11, 4075. https://doi.org/10.3390/foods11244075
Torres-Martínez BdM, Vargas-Sánchez RD, Torrescano-Urrutia GR, González-Ávila M, Rodríguez-Carpena JG, Huerta-Leidenz N, Pérez-Alvarez JA, Fernández-López J, Sánchez-Escalante A. Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and In Vitro Gastrointestinal Digestion. Foods. 2022; 11(24):4075. https://doi.org/10.3390/foods11244075
Chicago/Turabian StyleTorres-Martínez, Brisa del Mar, Rey David Vargas-Sánchez, Gastón Ramón Torrescano-Urrutia, Marisela González-Ávila, Javier Germán Rodríguez-Carpena, Nelson Huerta-Leidenz, José Angel Pérez-Alvarez, Juana Fernández-López, and Armida Sánchez-Escalante. 2022. "Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and In Vitro Gastrointestinal Digestion" Foods 11, no. 24: 4075. https://doi.org/10.3390/foods11244075
APA StyleTorres-Martínez, B. d. M., Vargas-Sánchez, R. D., Torrescano-Urrutia, G. R., González-Ávila, M., Rodríguez-Carpena, J. G., Huerta-Leidenz, N., Pérez-Alvarez, J. A., Fernández-López, J., & Sánchez-Escalante, A. (2022). Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and In Vitro Gastrointestinal Digestion. Foods, 11(24), 4075. https://doi.org/10.3390/foods11244075






