The Loading of Epigallocatechin Gallate on Bovine Serum Albumin and Pullulan-Based Nanoparticles as Effective Antioxidant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
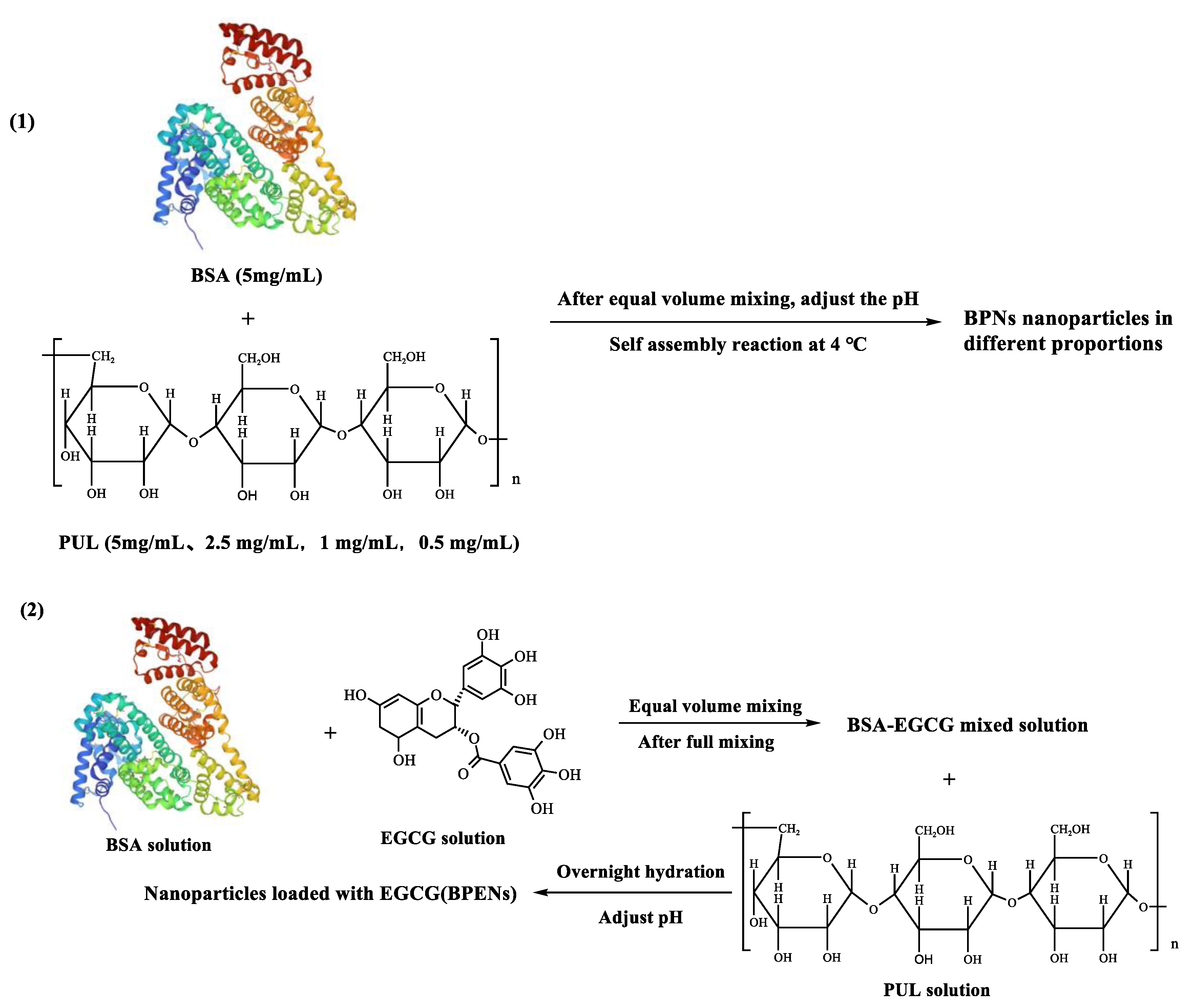
2.2. Preparation and Characterization of BPNs
2.2.1. Preparation of BPNs
2.2.2. Determination of Particle Size, PDI, and Zeta-Potential of BPNs
2.2.3. Thermal Stability
2.3. Preparation and Characterization of EGCG-Loaded BPNs (BPENs)
2.3.1. Preparation of BPENs
2.3.2. Encapsulation Efficiency and Loading Rate
2.3.3. DPPH Scavenging Assay
2.3.4. ABTS+ Scavenging Assay
2.3.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.3.6. Hydroxyl Radicals (OH) Scavenging Assay
2.3.7. In Vitro Release Behavior
2.3.8. Fourier Transform Infrared Spectra (FTIR) Analysis
2.3.9. Morphological Observation of BPNs and BPENs
2.3.10. Statistical Analysis
3. Results and Discussion
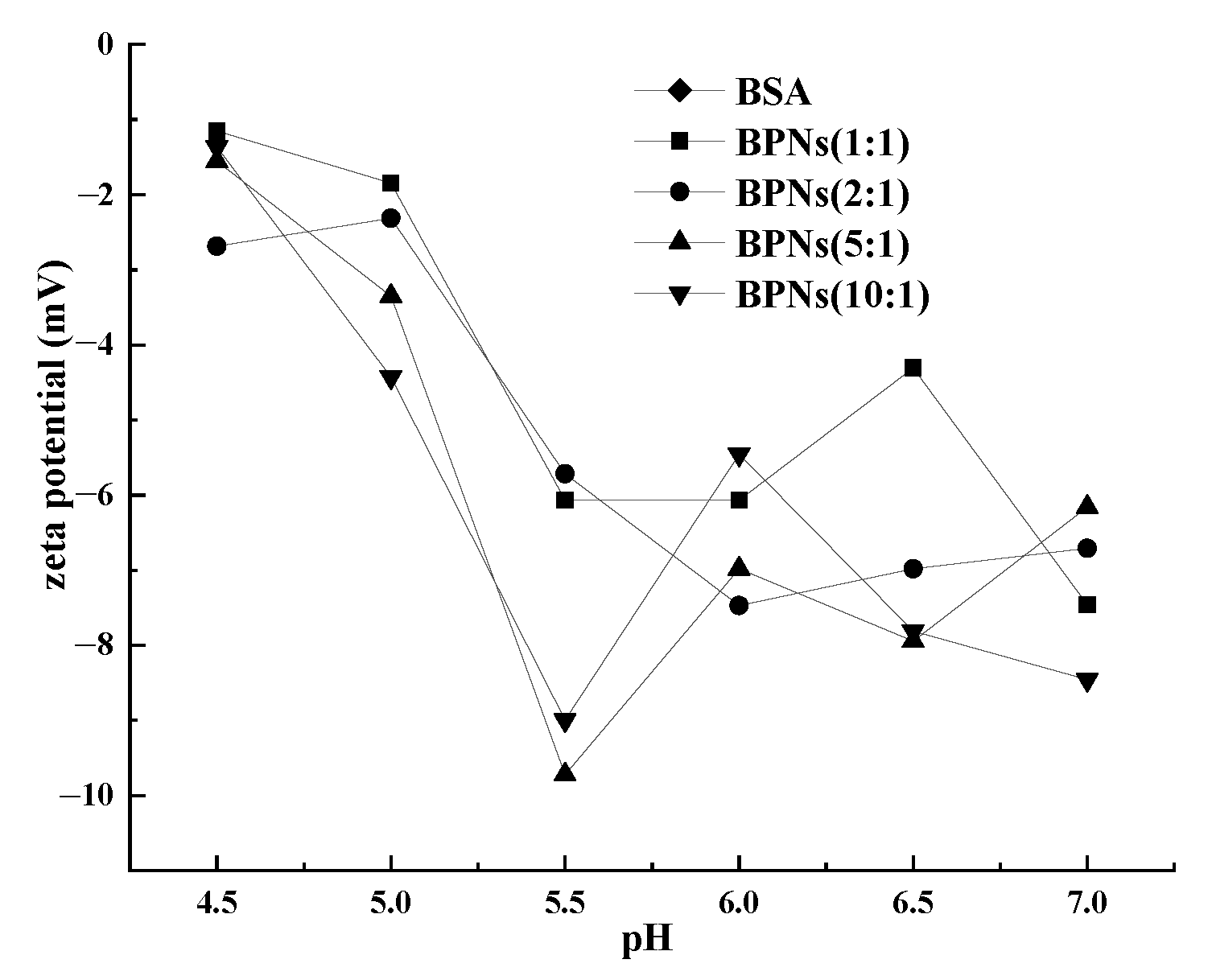
3.1. Particle Size and Zeta-Potential of BPNs
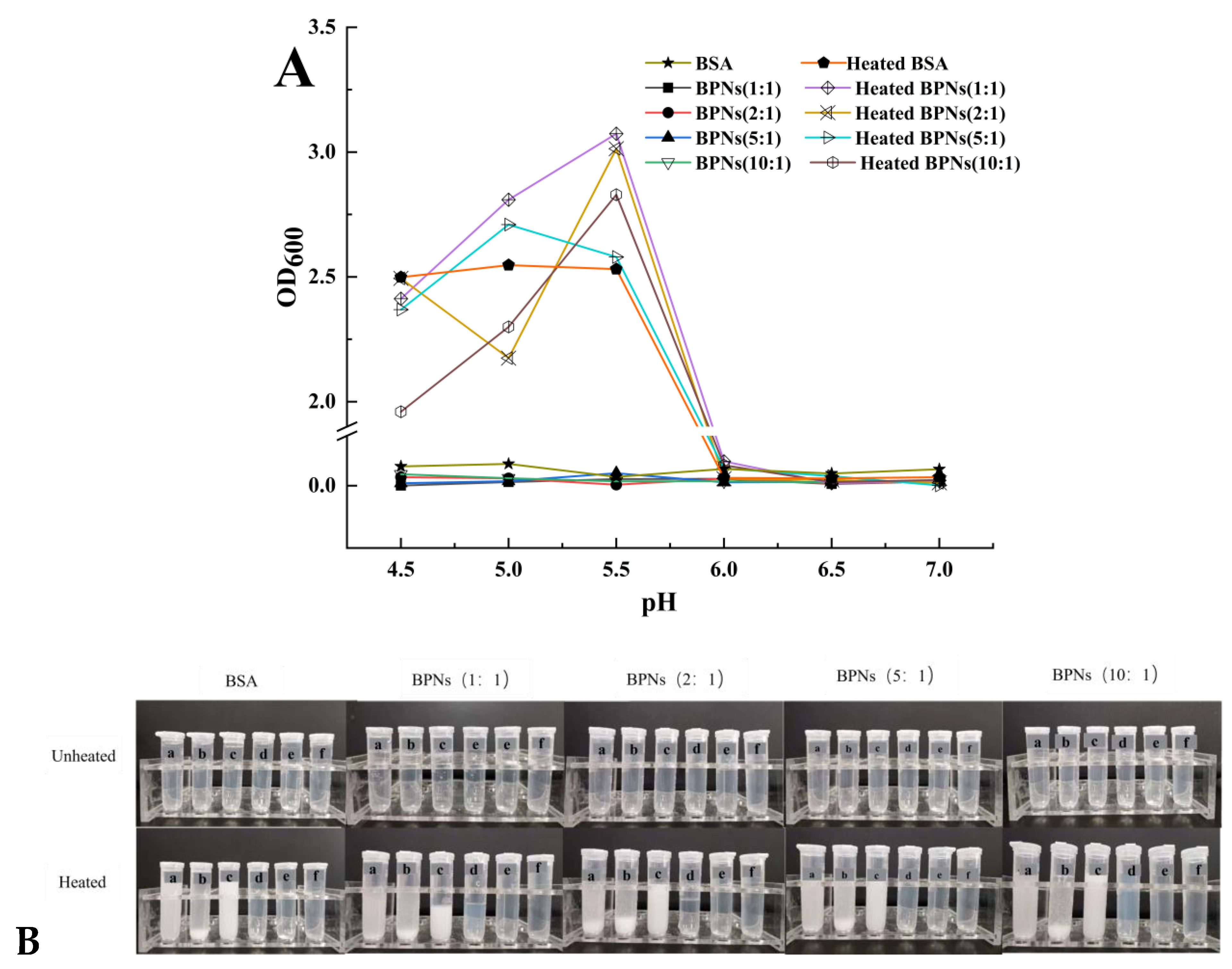
3.2. Turbidity Determination and Thermal Stability
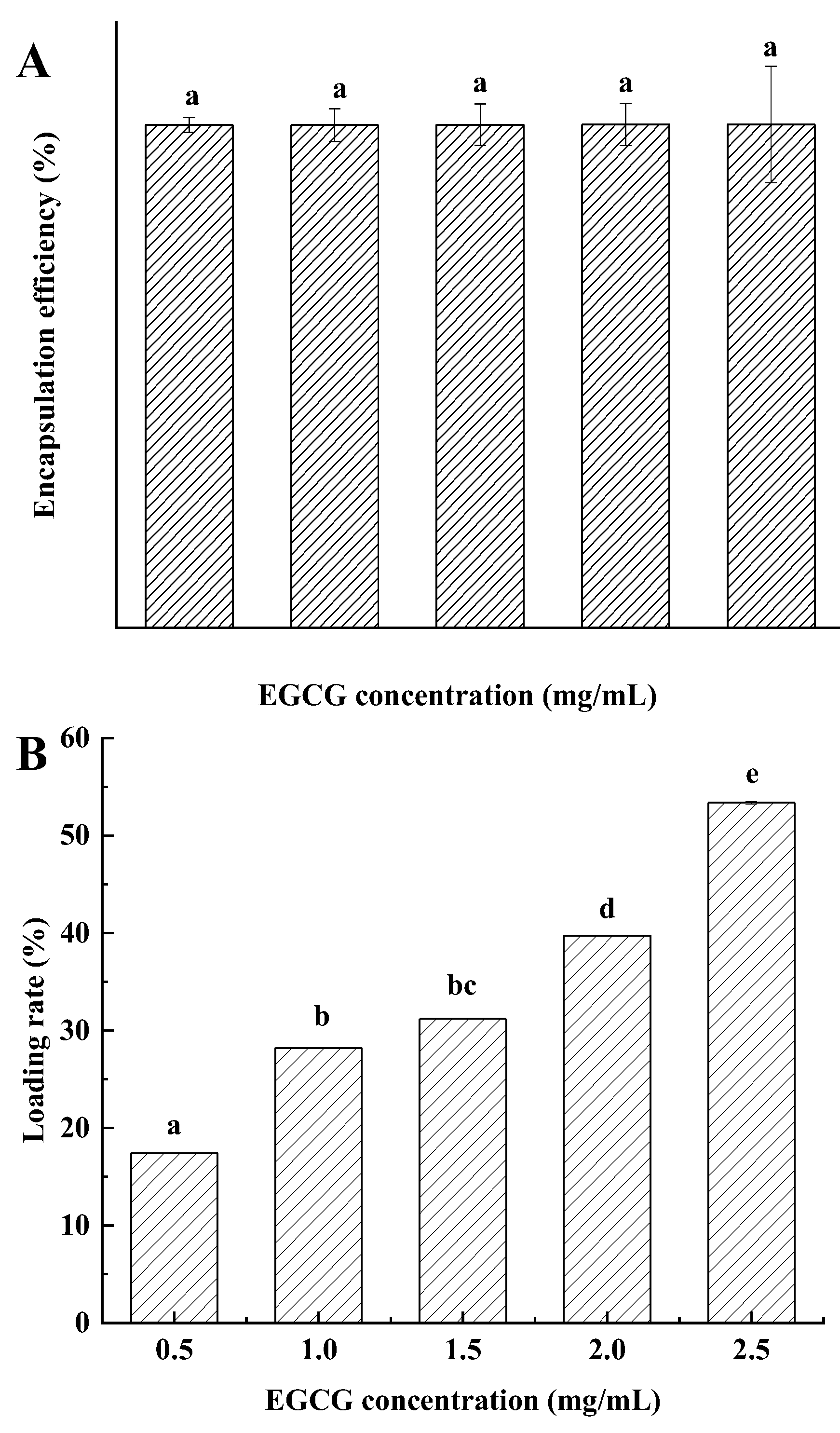
3.3. BPENs’ Encapsulation Efficiency and Loading Rate
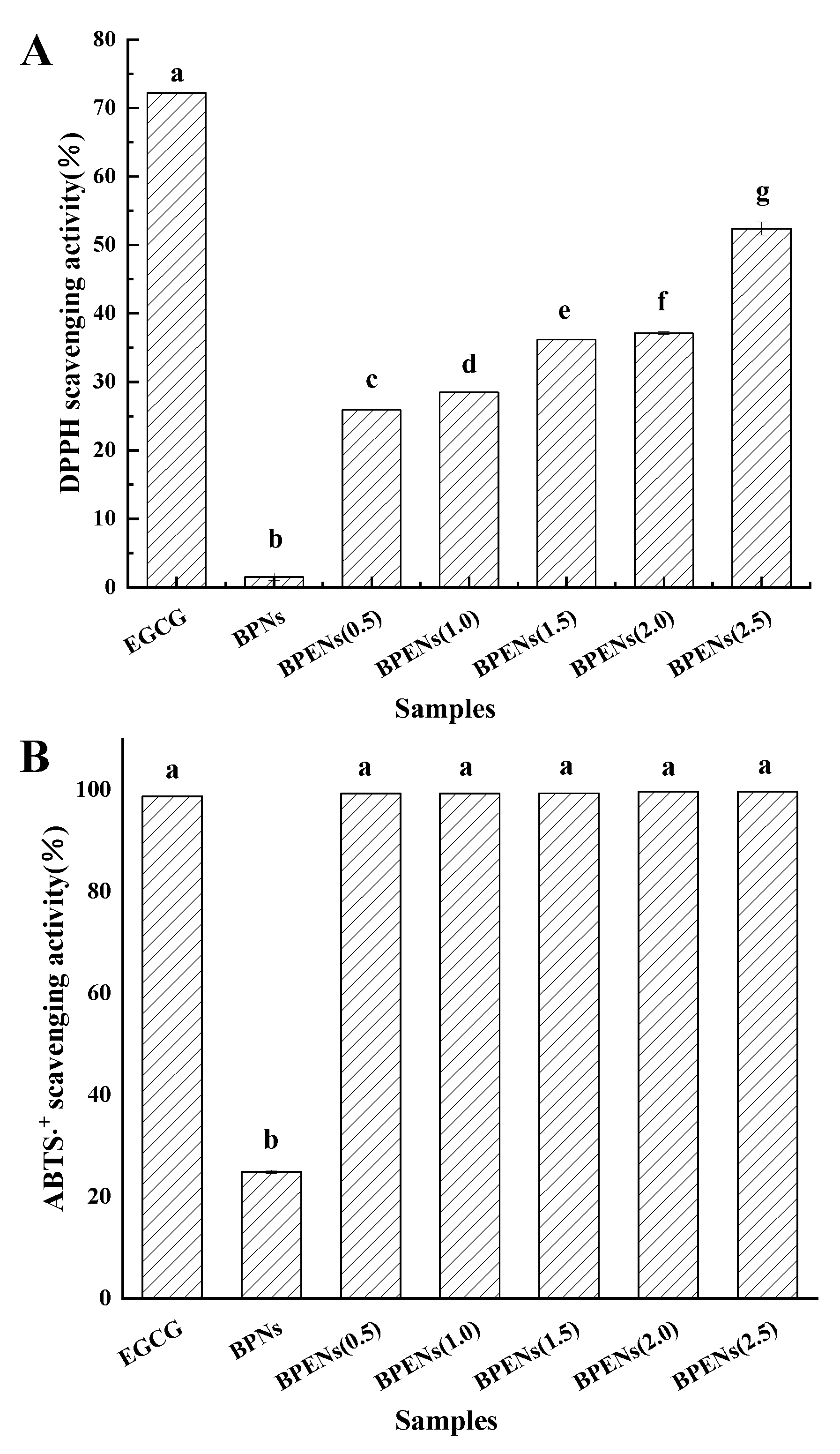
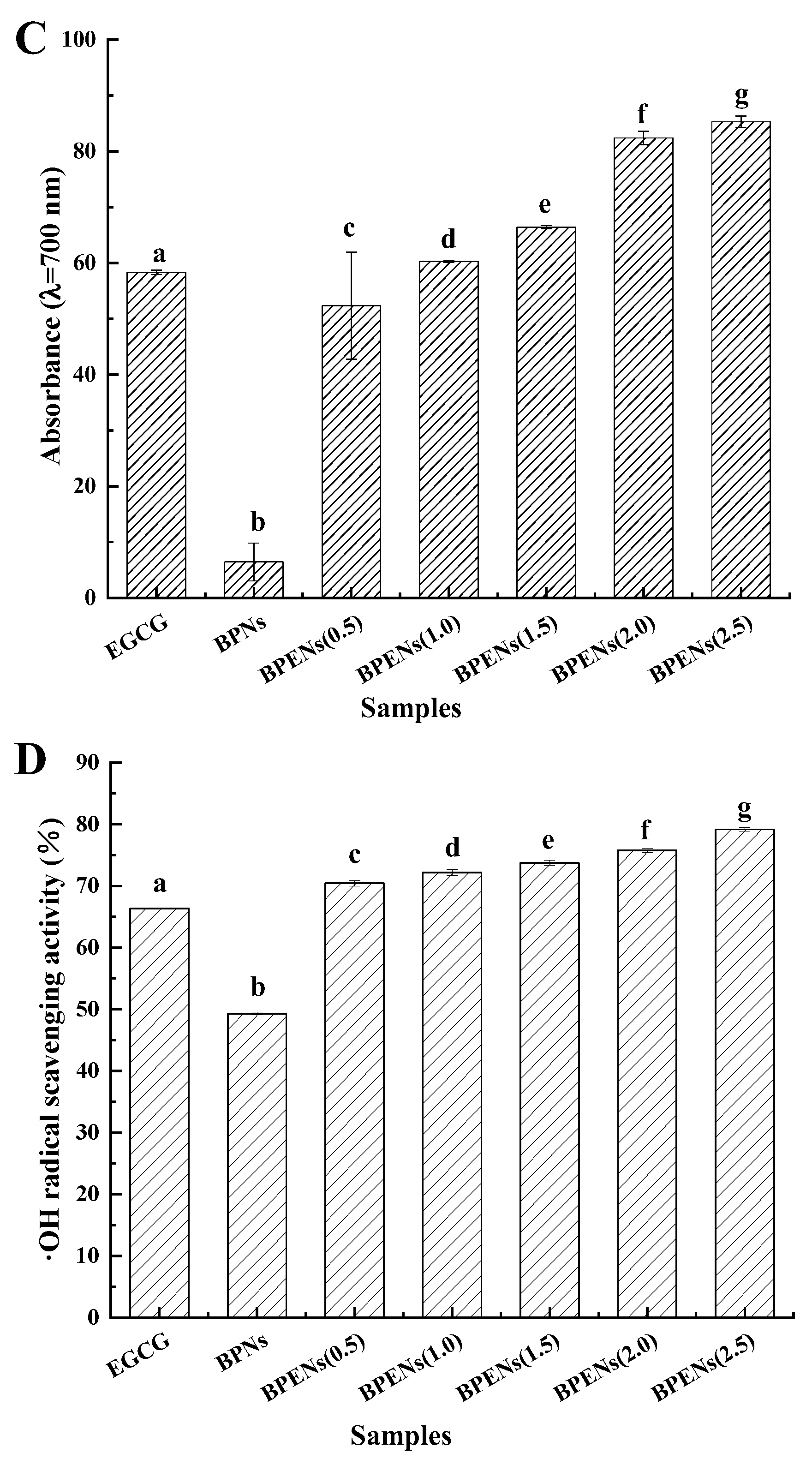
3.4. In Vitro Antioxidant Activities
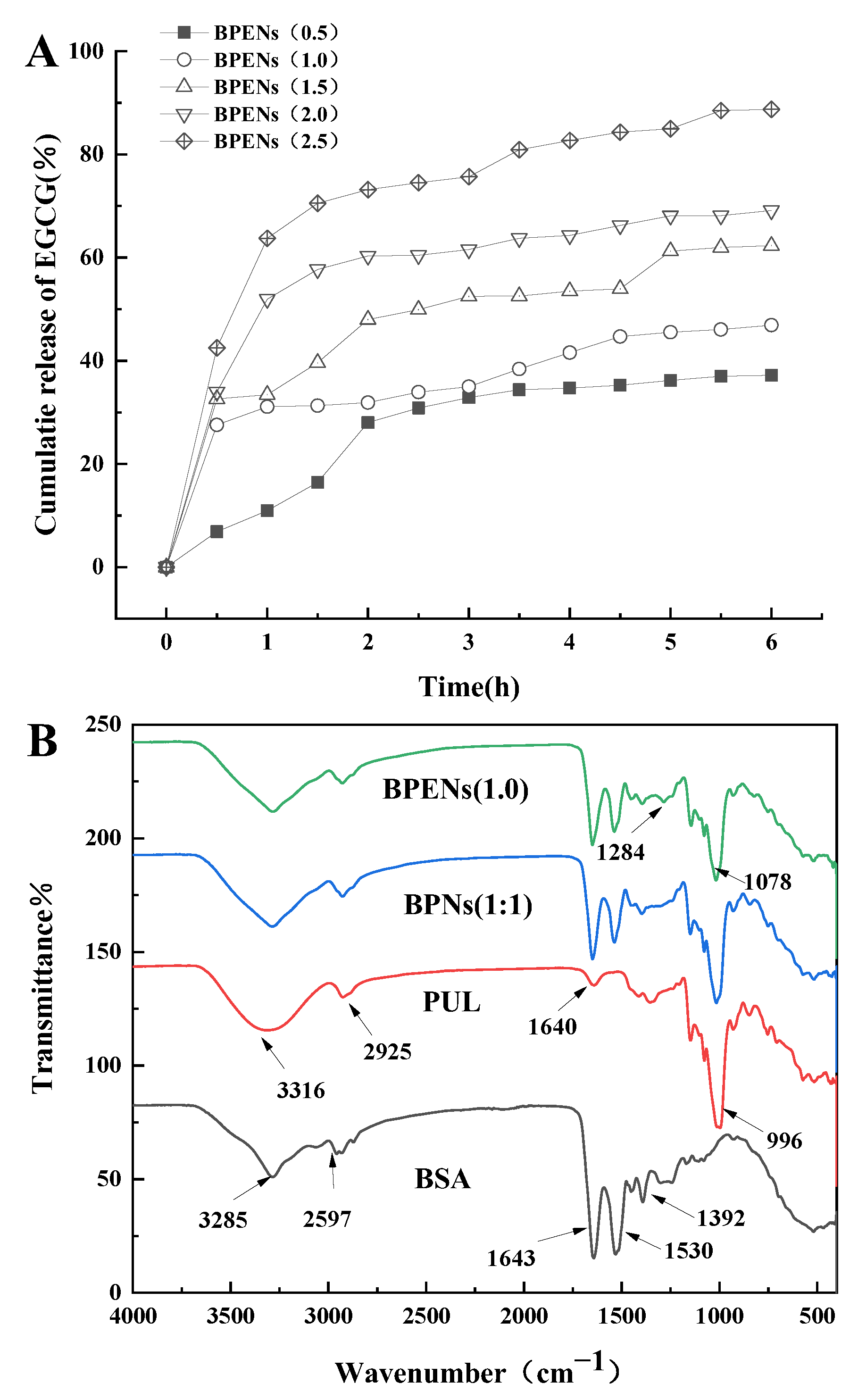
3.5. In Vitro Release Test
3.6. FTIR Analysis
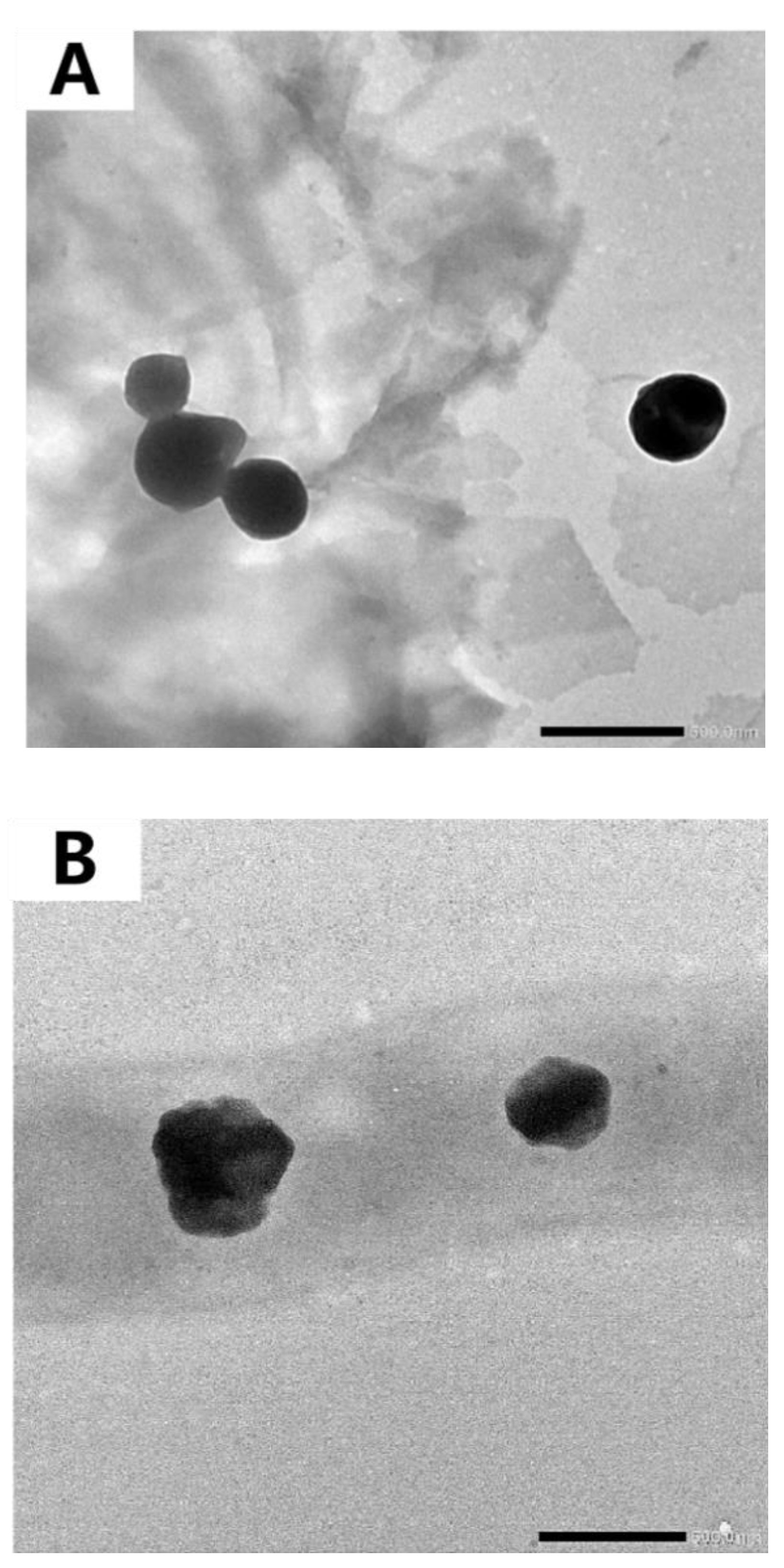
3.7. TEM Observation of Microscopic Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebrahimian, J.; Khayatkashani, M.; Soltani, N.; Yousif, Q.A.; Salavati-Niasari, M. Catechin mediated green synthesis of Au nanoparticles: Experimental and theoretical approaches to the determination HOMO-LUMO energy gap and reactivity indexes for the (+)-epicatechin (2S, 3S). Arab. J. Chem. 2022, 15, 103758. [Google Scholar] [CrossRef]
- Song, H.; Wang, Q.; He, A.; Li, S.; Guan, X.; Hu, Y.; Feng, S. Antioxidant activity, storage stability and in vitro release of ep-igallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022, 388, 132903. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, T.; Li, J.; Liu, L.; Dou, X.; Zhang, X. Development and evaluation of tea polyphenols loaded water in oil emulsion with zein as stabilizer. J. Drug Deliv. Sci. Technol. 2020, 56, 101528. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Suh, J.H. Multi-omics approach in tea polyphenol research regarding tea plant growth, development and tea processing: Current technologies and perspectives. Food Sci. Hum. Wellness 2022, 11, 524–536. [Google Scholar] [CrossRef]
- Han, Y.; Jia, F.; Bai, S.; Xiao, Y.; Meng, X.; Jiang, L. Effect of operating conditions on size of catechin/β-cyclodextrin nanopar-ticles prepared by nanoprecipitation and characterization of their physicochemical properties. LWT 2022, 153, 112447. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanopar-ticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J. Food Eng. 2008, 87, 505–513. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of tea catechins with chitosan nanoparticles. Food Hydrocoll. 2018, 84, 561–570. [Google Scholar] [CrossRef]
- Laha, B.; Maiti, S. Design of Core-Shell Stearyl Pullulan Nanostructures for Drug Delivery. Mater. Today Proc. 2019, 11, 620–627. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, X.; Song, L.; Jing, H. Interaction mode and nanoparticle formation of bovine serum albumin and anthocyanin in three buffer solutions. J. Lumin. 2014, 155, 244–250. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, Z.; Li, Y.; Chen, L.; Liu, Y.; Xu, Y.; Meng, K.; Cheng, L.; Sun, J. Preparation and characterization of thermosen-sitive hydrogel system for dual sustained-release of chlorhexidine and bovine serum albumin. Mater. Lett. 2021, 300, 130121. [Google Scholar] [CrossRef]
- Rostamnezhad, F.; Hossein Fatemi, M. Comprehensive investigation of binding of some polycyclic aromatic hydrocarbons with bovine serum albumin: Spectroscopic and molecular docking studies. Bioorg. Chem. 2022, 120, 105656. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of chitosan modified halloysite on the physical and functional properties of pullulan/chitosan biofilm integrated with rutin. Appl. Clay Sci. 2021, 211, 106205. [Google Scholar] [CrossRef]
- Yuzbasioglu, D.; Mamur, S.; Avuloglu-Yilmaz, E.; Erikel, E.; Celebi-Keskin, A.; Unal, F. Evaluation of the genotoxic and anti-genotoxic effects of exopolysaccharide pullulan in human lymphocytes in vitro. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2021, 870–871, 503391. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef]
- Silva, N.H.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Li, D.; Wu, G.; Zhang, H. Preparation, structure and stability of protein-pterostilbene nanocomplexes coated by soybean polysaccharide and maltodextrin. Food Biosci. 2022, 49, 101899. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Wan, Y.; Wang, L.; Zhou, T. Preparation, characterization and releasing property of antibacterial nano-capsules composed of ε-PL-EGCG and sodium alginate-chitosan. Int. J. Biol. Macromol. 2022, 204, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.-W.; Yu, S.-H.; Ho, Y.-C.; Huang, B.-Q.; Tsai, G.-J.; Hsieh, H.-Y.; Sung, H.-W.; Mi, F.-L. Characterization of tea cat-echins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Yun, D.; Yong, H.; Liu, J. Antioxidant packaging films developed based on chitosan grafted with different catechins: Characterization and application in retarding corn oil oxidation. Food Hydrocoll. 2022, 133, 107970. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Cui, Q.; Cheng, J.; Killpartrik, A.; Kemp, A.H.; Guo, M. Characterization, antioxidant capacity, and bioac-cessibility of Coenzyme Q10 loaded whey protein nanoparticles. LWT 2022, 160, 113258. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Yin, R.; Pang, J.; Cong, Y.; Yang, S. Water molecule attachment mode on the dried polysaccharide influences its free radical scavenging ability. Process Biochem. 2020, 91, 15–22. [Google Scholar] [CrossRef]
- Jan, N.; Madni, A.; Rahim, M.A.; Khan, N.U.; Jamshaid, T.; Khan, A.; Jabar, A.; Khan, S.; Shah, H. In vitro anti-leukemic assessment and sustained release behaviour of cytarabine loaded biodegradable polymer based nanoparticles. Life Sci. 2021, 267, 118971. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, S.; Liu, S.; Li, C. Modification of coconut residue fiber and its bile salt adsorption mechanism: Action mode of insoluble dietary fibers probed by microrheology. Food Hydrocoll. 2023, 136, 108221. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Wu, A.; Wang, X.; Liang, Y.; Dai, X.; Cao, Y.; Li, X. Delivery of silybin using a zein-pullulan nanocomplex: Fabrication, characterization, in vitro release properties and antioxidant capacity. Colloids Surf. B Biointerfaces 2022, 217, 112682. [Google Scholar] [CrossRef]
- Karami, K.; Jamshidian, N.; Hajiaghasi, A.; Amirghofran, Z. BSA nanoparticles as controlled release carriers for isophethalaldoxime palladacycle complex; synthesis, characterization, in vitro evaluation, cytotoxicity and release kinetics analysis. New J. Chem. 2020, 44, 4394–4405. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, W.; Chen, D.; Yang, Y.; Sun, T.; Chen, H.; Zhang, J. Electrospun nanofibers containing chitosan-stabilized bovine serum albumin nanoparticles for bone regeneration. Colloids Surf. B: Biointerfaces 2022, 217, 112680. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, Q. Microemulsions as nanoreactors to produce whey protein nanoparticles with enhanced heat stability by thermal pretreatment. Food Chem. 2010, 119, 1318–1325. [Google Scholar] [CrossRef]
- Seo, J.A.; Hedoux, A.; Guinet, Y.; Paccou, L.; Affouard, F.; Lerbret, A.; Descamps, M. Thermal Denaturation of Be-ta-Lactoglobulin and Stabilization Mechanism by Trehalose Analyzed from Raman Spectroscopy Investigations. J. Phys. Chem. B 2010, 114, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; Benevides, C.A.; Rodrigues, G.C.P.; Tenório, R.P. Thermal Denaturation and γ-Irradiation effects on the Crack Patterns of Bovine Serum Albumin (BSA) Dry Droplets. Colloid Interface Sci. Commun. 2019, 28, 15–19. [Google Scholar] [CrossRef]
- Fan, Y.T.; Yi, J.; Zhang, Y.Z.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanopar-ticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Z.; Wang, X.; Wang, Y.; Tang, Q.; Huang, Q.; Xue, C. Fabrication and characterization of core-shell glia-din/tremella polysaccharide nanoparticles for curcumin delivery: Encapsulation efficiency, physicochemical stability and bio-accessibility. Curr. Res. Food Sci. 2022, 5, 288–297. [Google Scholar] [CrossRef]
- Hu, H.; Yong, H.; Yao, X.; Yun, D.; Huang, J.; Liu, J. Highly efficient synthesis and characterization of starch aldehyde-catechin conjugate with potent antioxidant activity. Int. J. Biol. Macromol. 2021, 173, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, Q.; Chen, Y.; Mao, L.; Gao, Y.; Yuan, F. Utilization of β-lactoglobulin- (−)-Epigallocatechin- 3-gallate(EGCG) composite colloidal nanoparticles as stabilizers for lutein pickering emulsion. Food Hydrocoll. 2020, 98, 105293. [Google Scholar] [CrossRef]
- Chen, W.; Lv, R.; Muhammad, A.I.; Guo, M.; Ding, T.; Ye, X.; Liu, D. Fabrication of (−)-epigallocatechin-3-gallate carrier based on glycosylated whey protein isolate obtained by ultrasound Maillard reaction. Ultrason. Sonochem. 2019, 58, 104678. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Soler, C.; Lopez-Rubio, A. Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll. 2016, 61, 128–138. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhuang, G.; Li, S.-J. Multiple on-line screening and identification methods for hydroxyl radical scavengers in Yudanshen. J. Pharm. Biomed. Anal. 2018, 156, 278–283. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z. Fabrication of heat-treated soybean protein isolate-EGCG complex nanoparticle as a functional carrier for curcumin. LWT 2022, 159, 113059. [Google Scholar] [CrossRef]
- Kaith, B.S.; Sharma, K.; Kumar, V.; Kalia, S.; Swart, H.C. Fabrication and characterization of gum ghatti-polymethacrylic acid based electrically conductive hydrogels. Synth. Met. 2014, 187, 61–67. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, K.; Patel, S. Bovine Serum Albumin Nanoparticles for the Efficient Delivery of Berberine: Preparation, Characterization and In vitro biological studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar] [CrossRef]
- Dionísio, M.; Cordeiro, C.; Remuñán-López, C.; Seijo, B.; Rosa da Costa, A.M.; Grenha, A. Pullulan-based nanoparticles as carriers for transmucosal protein delivery. Eur. J. Pharm. Sci. 2013, 50, 102–113. [Google Scholar] [CrossRef]
- Bera, H.; Abosheasha, M.A.; Ito, Y.; Ueda, M. Hypoxia-responsive pullulan-based nanoparticles as erlotinib carriers. Int. J. Biol. Macromol. 2021, 191, 764–774. [Google Scholar] [CrossRef]
- Das, R.P.; Singh, B.G.; Kunwar, A.; Ramani, M.V.; Subbaraju, G.V.; Hassan, P.A.; Priyadarsini, K.I. Tuning the binding, release and cytotoxicity of hydrophobic drug by Bovine Serum Albumin nanoparticles: Influence of particle size. Colloids Surf. B Biointerfaces 2017, 158, 682–688. [Google Scholar] [CrossRef]
| pH | 1:1 | 2:1 | 5:1 | 10:1 | ||||
|---|---|---|---|---|---|---|---|---|
| Particle Size (nm) | PDI | Particle Size (nm) | PDI | Particle Size (nm) | PDI | Particle Size (nm) | PDI | |
| 4.5 | 3415 a | 0.369 | 3825 a | 0.516 | 5588 a | 0.521 | 46245 a | 0.375 |
| 5.0 | 2196 b | 0.392 | 33526 b | 0.372 | 3259 b | 0.350 | 64448 b | 0.330 |
| 5.5 | 1808 c | 0.369 | 2261 c | 0.218 | 25121 c | 0.122 | 62057 bc | 0.312 |
| 6.0 | 13913 d | 0.355 | 44732 d | 0.341 | 60656 ad | 0.300 | 22421 d | 0.321 |
| 6.5 | 25111 e | 0.265 | 48837 de | 0.190 | 6703 e | 0.402 | 25713 de | 0.434 |
| 7.0 | 34728 af | 0.449 | 36218 abf | 0.546 | 201162 f | 0.503 | 74950 f | 0.303 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, X.; Zhang, M.; He, H.; Liang, B.; Sun, C.; Li, X.; Ji, C. The Loading of Epigallocatechin Gallate on Bovine Serum Albumin and Pullulan-Based Nanoparticles as Effective Antioxidant. Foods 2022, 11, 4074. https://doi.org/10.3390/foods11244074
Li Z, Wang X, Zhang M, He H, Liang B, Sun C, Li X, Ji C. The Loading of Epigallocatechin Gallate on Bovine Serum Albumin and Pullulan-Based Nanoparticles as Effective Antioxidant. Foods. 2022; 11(24):4074. https://doi.org/10.3390/foods11244074
Chicago/Turabian StyleLi, Zikun, Xiaohan Wang, Man Zhang, Hongjun He, Bin Liang, Chanchan Sun, Xiulian Li, and Changjian Ji. 2022. "The Loading of Epigallocatechin Gallate on Bovine Serum Albumin and Pullulan-Based Nanoparticles as Effective Antioxidant" Foods 11, no. 24: 4074. https://doi.org/10.3390/foods11244074
APA StyleLi, Z., Wang, X., Zhang, M., He, H., Liang, B., Sun, C., Li, X., & Ji, C. (2022). The Loading of Epigallocatechin Gallate on Bovine Serum Albumin and Pullulan-Based Nanoparticles as Effective Antioxidant. Foods, 11(24), 4074. https://doi.org/10.3390/foods11244074







