Quality Change in Camellia Oil during Intermittent Frying
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Determination of Fatty Acid Composition
2.3. Determination of Tocopherol Content
2.4. Determination of Carbonyl Compounds Value
2.5. Determination of Total Polar Compounds
2.6. Analysis of Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Fatty Acid Composition during Frying
3.2. Changes in Tocopherol Content during Frying
3.3. Evolution of Carbonyl Value and Total Polar Compounds Level during Frying
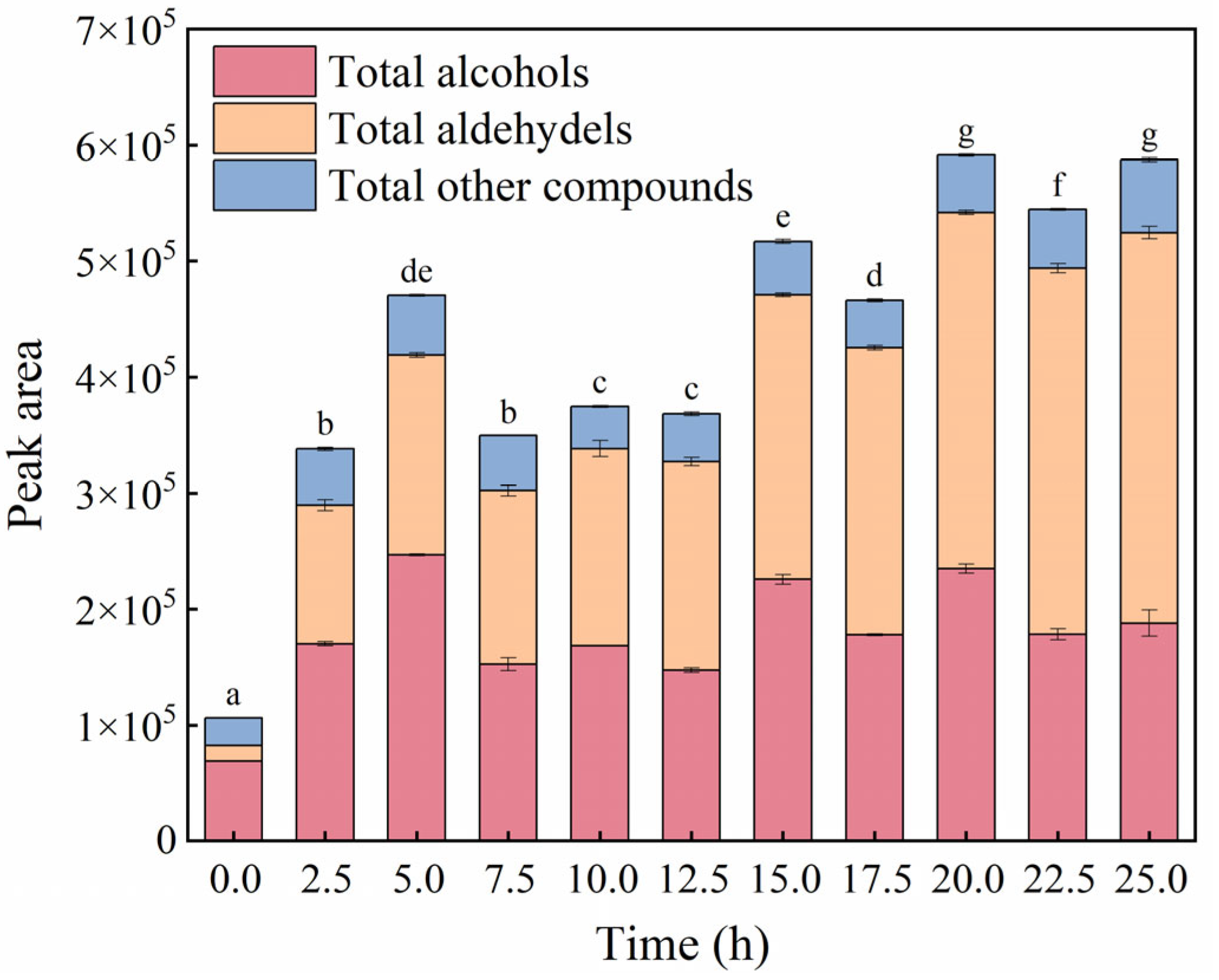
3.4. Changes in Volatile Compounds Profiles
3.5. Principal Component Analysis of Quality Change in Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Pan, Y.G.; Zheng, L.; Yang, Y.; Zheng, X.; Ai, B.; Xu, Z.; Sheng, Z. Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities. Food Sci. Nutr. 2019, 7, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X.; Zhou, Y.; Chen, J. Effects of different preheat treatments on volatile compounds of camellia (Camellia oleifera Abel.) seed oil and formation mechanism of key aroma compounds. J. Food Biochem. 2021, 45, e13649. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Tech. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J. Med. Food 2016, 19, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.Y.; Lu, Y.F.; Inbaraj, B.S.; Chen, B.H. Camelia oil and soybean-camelia oil blend enhance antioxidant activity and cardiovascular protection in hamsters. Nutrition 2018, 51–52, 86–94. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.; Wu, P.; Lu, F.; Zhu, J.; Ma, H.; Chen, Y.; Zhang, T. Camellia oil lowering blood pressure in spontaneously hypertension rats. J. Funct. Foods 2020, 70, 103915. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, M.; Chen, Y.; Yan, X.; Chen, Q.; Wu, X.; Lin, J.; Xie, M. 1H NMR combined with chemometrics for the rapid detection of adulteration in camellia oils. Food Chem. 2018, 242, 308–315. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, T.; Guo, Z.; Liao, H.; Chen, Y. Comparative study of the oxidation of cold-pressed and commercial refined camellia oil during storage with 1H and 31P NMR spectroscopy. Food Chem. 2020, 321, 126640. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.; Liu, T.; Zeng, X.; Liang, X.; Wu, X.; Fu, G. Effect of refining degree on the quality changes and lipid oxidation of camellia (camellia oleifera) oil during heating. Foods 2022, 11, 2232. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, J.; Fan, Y.W.; Zheng, T.W.; Deng, Z.Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2014, 18, 1478–1490. [Google Scholar] [CrossRef]
- Wang, S.N.; Sui, X.N.; Wang, Z.J.; Qi, B.K.; Jiang, L.Z.; Li, Y.; Wang, R.; Wei, X. Improvement in thermal stability of soybean oil by blending with camellia oil during deep fat frying. Eur. J. Lipid Sci. Technol. 2015, 118, 524–531. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-chemical changes during repeated frying of cooked oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Lu, Y.; Li, P.; Qi, X.; Zhang, H.; Wang, X.; Jin, Q. Comparative analysis of the effects of novel electric field frying and conventional frying on the quality of frying oil and oil absorption of fried shrimps. Food Control 2021, 128, 108195. [Google Scholar] [CrossRef]
- Sayyad, R. Effects of deep-fat frying process on the oil quality during French fries preparation. J. Food Sci. Technol. 2017, 54, 2224–2229. [Google Scholar] [CrossRef]
- Farhoosh, R.; Tavassoli-Kafrani, M.H. Simultaneous monitoring of the conventional qualitative indicators during frying of sunflower oil. Food Chem. 2011, 125, 209–213. [Google Scholar] [CrossRef]
- Paul, S.; Mittal, G.S.; Chinnan, M.S. Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit. Rev. Food Sci. Nutr. 1997, 37, 635–662. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chen, X.; Yang, Y.; Zhang, J. Application of Fourier transform near-infrared spectroscopy to the quantification and monitoring of carbonyl value in frying oils. Anal. Methods 2014, 6, 7628–7633. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Xu, L.; Wu, G.; Ji, X.; Zhang, H.; Jin, Q.; Wang, X. Influence of prolonged deep-frying using various oils on volatile compounds formation of French fries using GC–MS, GC-O, and sensory evaluation. J. Am. Oil Chem. Soc. 2021, 98, 657–671. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Yuan, Y.; Yang, T.; Liu, S. Changes in volatiles of palm kernel oil before and after kernel roasting. LWT-Food Sci. Technol. 2016, 73, 432–441. [Google Scholar] [CrossRef]
- Liu, X.; Hoshino, N.; Wang, S.; Masui, E.; Chen, J.; Zhang, H. A novel evaluation index for predicting the degradation rate of frying oils based on their fatty acid composition. Eur. J. Lipid Sci. Technol. 2018, 120, 1700528. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Analysis of the Dynamic Decomposition of Unsaturated Fatty Acids and Tocopherols in Commercial Oils During Deep Frying. Anal. Lett. 2019, 52, 1991–2005. [Google Scholar] [CrossRef]
- JOCS. Standard Methods for the Analysis of Fats, Oils and Related Materials; Japan Oil Chemists’ Society: Tokyo, Japan, 2013. [Google Scholar]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Real-Time Model for Carbonyl Value as a Function of Total Polar Compounds in Oil during Frying. Anal. Lett. 2021, 54, 2813–2825. [Google Scholar] [CrossRef]
- Battaloglu, R. Calculation of retention indices of essential oils with the aid of the Van den Dool and Kratz equation and Bézier curves. Math. Meth. Appl. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, T.; Chen, Y.; Luo, S.; Leng, T.; Wang, Y.; Guo, C.; Xie, M. Prediction of fatty acid composition in camellia oil by 1H NMR combined with PLS regression. Food Chem. 2019, 279, 339–346. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; del Mar Contreras, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- Romero, A.; Sánchez-Muniz, F.J.; Cuesta, C. Deep fat frying of frozen foods in sunflower oil. Fatty acid composition in fryer oil and frozen prefried potatoes. J. Sci. Food Agric. 2000, 80, 2135–2141. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil adulteration detection using fatty acid ratios and tocopherol compositions with chemometrics. Food Control 2022, 133, 108565. [Google Scholar] [CrossRef]
- Afaf, K.E.; Lars-Åke, A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Saari Csallany, A. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Kamal-Eldin, A.; Marinova, E.M.; Toneva, A.G. Kinetics of antioxidant action of α-and γ-toco-pherols in sunflower and soybean triacylglycerols. Euro. J. Lipid Sci. Tech. 2002, 104, 262–270. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B.R. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.; Shen, Q.; Saleh, A.S. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015, 52, 7683–7696. [Google Scholar] [CrossRef]
- da Silva, G.; Bozzelli, J.W. Enthalpies of Formation, Bond Dissociation Energies, and Molecular Structures of the n-Aldehydes (Acetaldehyde, Propanal, Butanal, Pentanal, Hexanal, and Heptanal) and Their Radicals. J. Phys. Chem. A 2006, 110, 13058–13067. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Tamogami, S.; Chen, J.; Zhang, H. An evaluation model for the quality of frying oil using key aldehyde detected by HS-GC/MS. Foods 2022, 11, 2413. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q.; Huang, F. Unraveling of the Aroma-Active Compounds in Virgin Camellia Oil (Camellia oleifera Abel) Using Gas Chromatography-Mass Spectrometry-Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef]
- Xu, L.; Mei, X.; Chang, J.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Comparative characterization of key odorants of French fries and oils at the break-in, optimum, and degrading frying stages. Food Chem. 2022, 368, 130581. [Google Scholar] [CrossRef]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, A.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- O'Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Aranda, J.J.; Nichols, P.D.; David Waite, T.; Hallegraeff, G.M. Strain variability in fatty acid composition of Chattonella marina (Raphidophyceae) and its relation to differing ichthyotoxicity toward rainbow trout gill cells. J. Phycol. 2013, 49, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, C.; Wang, C.; Chen, H.; Liu, Y.; Li, S.; Lin, D.; Wu, D.; Qin, W. Evaluation of the non-aldehyde volatile compounds formed during deep-fat frying process. Food Chem. 2018, 243, 151–161. [Google Scholar] [CrossRef]
- Min, D.; Callison, A.; Lee, H. Singlet oxygen oxidation for 2-pentylfuran and 2-pentenyfuran formation in soybean oil. J. Food Sci. 2003, 68, 1175–1178. [Google Scholar] [CrossRef]
- Tan, Z.W.; Yu, A.N. Volatiles from the Maillard reaction of L-ascorbic acid with L-glutamic acid/L-aspartic acid at different reaction times and temperatures. Asia-Pac. J. Chem. Eng. 2012, 7, 563–571. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Matthäus, B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Euro. J. Lipid Sci. Tech. 2006, 108, 200–211. [Google Scholar] [CrossRef]
- Shuang, G.; Jun, W.; Yongwei, W. Early discrimination and growth tracking of Aspergillus spp. contamination in rice kernels using electronic nose. Food Chem. 2019, 292, 325–335. [Google Scholar] [CrossRef]


| Time (h) | Fatty Acid (%) | ||||||
|---|---|---|---|---|---|---|---|
| Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Linolenic Acid | PUFA | TUFA | |
| 0.0 | 7.02 ± 0.08 a | 1.53 ± 0.02 b | 78.42 ± 0.18 a | 11.13 ± 0.05 c | 1.91 ± 0.03 a | 13.04 ± 0.08 b | 91.45 ± 0.10 a |
| 2.5 | 7.12 ± 0.02 a | 1.50 ± 0.01 a | 78.74 ± 0.04 a | 10.83 ± 0.02 d | 1.81 ± 0.00 b | 12.64 ± 0.02 c | 91.38 ± 0.03 a |
| 5.0 | 7.13 ± 0.25 a | 1.64 ± 0.00 cd | 77.83 ± 0.29 b | 11.49 ± 0.05 a | 1.91 ± 0.02 a | 13.40 ± 0.07 a | 91.23 ± 0.25 a |
| 7.5 | 7.93 ± 0.53 b | 1.66 ± 0.02 d | 77.34 ± 0.43 de | 11.22 ± 0.08 b | 1.85 ± 0.00 b | 13.07 ± 0.08 b | 90.41 ± 0.51 b |
| 10.0 | 8.22 ± 0.19 bc | 1.64 ± 0.04 c | 77.69 ± 0.34 bcd | 10.74 ± 0.11 e | 1.71 ± 0.00 c | 12.45 ± 0.11 d | 90.14 ± 0.23 bc |
| 12.5 | 8.32 ± 0.15 c | 1.74 ± 0.01 e | 77.24 ± 0.03 e | 10.90 ± 0.06 d | 1.80 ± 0.07 b | 12.70 ± 0.13 c | 89.94 ± 0.15 cd |
| 15.0 | 8.48 ± 0.19 c | 1.79 ± 0.01 f | 77.49 ± 0.15 bcde | 10.59 ± 0.06 f | 1.66 ± 0.01 d | 12.24 ± 0.06 e | 89.73 ± 0.19 d |
| 17.5 | 9.03 ± 0.21 d | 1.80 ± 0.01 f | 77.34 ± 0.26 de | 10.26 ± 0.05 g | 1.57 ± 0.01 e | 11.83 ± 0.04 f | 89.17 ± 0.22 e |
| 20.0 | 9.34 ± 0.22 de | 1.85 ± 0.00 g | 77.40 ± 0.13 cde | 9.93 ± 0.08 h | 1.47 ± 0.04 f | 11.40 ± 0.11 g | 88.81 ± 0.23 f |
| 22.5 | 9.41 ± 0.15 e | 1.90 ± 0.00 h | 77.73 ± 0.17 bc | 9.56 ± 0.03 i | 1.40 ± 0.01 g | 10.96 ± 0.02 h | 88.69 ± 0.15 f |
| 25.0 | 9.98 ± 0.38 f | 1.93 ± 0.01 i | 77.58 ± 0.41 bcde | 9.20 ± 0.01 j | 1.30 ± 0.03 h | 10.51 ± 0.04 i | 88.09 ± 0.37 g |
| Fatty Acid | Slope | R2 |
|---|---|---|
| Palmitic acid | 1.58 | 0.965 |
| Stearic acid | 1.07 | 0.957 |
| Oleic acid | −0.06 | 0.284 |
| Linoleic acid | −0.51 | 0.724 |
| Linolenic acid | −1.07 | 0.863 |
| PUFA | −0.59 | 0.762 |
| TUFA | −0.14 | 0.974 |
| Time (h) | Tocopherol (mg/100 g) | Loss Rate (%) | ||||
|---|---|---|---|---|---|---|
| α | β | γ | δ | Total | ||
| 0.0 | 8.19 ± 0.37 a | 0.06 ± 0.00 a | 4.39 ± 0.20 a | 0.13 ± 0.01 ab | 12.77 ± 0.58 a | 0.00 |
| 2.5 | 7.57 ± 0.41 b | 0.04 ± 0.00 b | 3.98 ± 0.22 b | 0.12 ± 0.01 ab | 11.71 ± 0.62 b | 8.34 |
| 5.0 | 5.87 ± 0.18 c | 0.03 ± 0.00 c | 3.11 ± 0.10 c | 0.12 ± 0.00 ab | 9.13 ± 0.28 c | 28.41 |
| 7.5 | 3.48 ± 0.02 d | 0.02 ± 0.00 d | 2.10 ± 0.00 d | 0.12 ± 0.00 a | 5.72 ± 0.11 d | 55.19 |
| 10.0 | 1.65 ± 0.01 e | 0.01 ± 0.00 e | 1.17 ± 0.04 e | 0.11 ± 0.01 bc | 2.94 ± 0.06 e | 77.05 |
| 12.5 | 0.19 ± 0.01 f | 0.01 ± 0.00 f | 0.57 ± 0.02 f | 0.10 ± 0.01 bc | 0.87 ± 0.03 f | 93.23 |
| 15.0 | 0.08 ± 0.00 f | - | 0.23 ± 0.02 g | 0.09 ± 0.00 bcd | 0.40 ± 0.01 g | 96.89 |
| 17.5 | 0.08 ± 0.00 f | - | 0.06 ± 0.00 h | 0.08 ± 0.00 bcd | 0.22 ± 0.00 g | 98.35 |
| 20.0 | 0.07 ± 0.00 f | - | 0.05 ± 0.00 h | 0.06 ± 0.00 cd | 0.18 ± 0.00 g | 98.51 |
| 22.5 | 0.07 ± 0.00 f | - | 0.05 ± 0.00 h | 0.06 ± 0.00 cd | 0.18 ± 0.00 g | 98.60 |
| 25.0 | 0.06 ± 0.00 f | - | 0.04 ± 0.00 h | 0.05 ± 0.00 d | 0.15 ± 0.00 g | 98.84 |
| No. | RT (min) | RI | RIr | Volatile Compounds | Peak Area of Volatile Compounds Identified in Oil Samples with Different Heating Times | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 2.5 | 5.0 | 7.5 | 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 | 25.0 | |||||
| Alcohol | |||||||||||||||
| 1 | 1.72 | <600 | 489 | Ethanol | 56180 ± 114 f | 7788 ± 648 a | 8181 ± 151 a | 8374 ± 270 a | 8523 ± 565 ab | 10320 ± 307 b | 12870 ± 647 c | 12890 ± 223 c | 15041 ± 753 d | 16125 ± 795 e | 16402 ± 1027 e |
| 2 | 1.78 | <600 | 621 | 2-Methylpropan-1-ol | 12804 ± 43 a | 152038 ± 5117 f | 224495 ± 2207 h | 131626 ± 3668 c | 146445 ± 7544 ef | 121075 ± 3456 b | 192245 ± 962 g | 143751 ± 2456 de | 194646 ± 1219 g | 137312 ± 5779 cd | 147082 ± 4947 ef |
| 3 | 3.10 | 714 | 686 | Pent-1-en-3-ol | - | 6959 ± 66 a | 9081 ± 45 c | 6804 ± 197 a | 7327 ± 91 a | 8243 ± 708 b | 10943 ± 212 de | 10706 ± 212 d | 12497 ± 627 f | 12146 ± 295 f | 11285 ± 143 e |
| 4 | 5.03 | 795 | 764 | Pentan-1-ol | - | 2771 ± 101 a | 3985 ± 165 b | 4543 ± 189 b | 4655 ± 250 b | 5840 ± 420 c | 7408 ± 456 d | 8355 ± 293 e | 9730 ± 65 f | 10033 ± 81 f | 10371 ± 687 f |
| 5 | 13.60 | 997 | 965 | Heptan-1-ol | - | 328 ± 13 a | 546 ± 28 b | 753 ± 37 bc | 728 ± 29 c | 971 ± 4 d | 1217 ± 47 de | 1356 ± 53 de | 1661 ± 119 f | 1515 ± 32 f | 1423 ± 47 ef |
| 6 | 17.90 | 1097 | 1070 | Octan-1-ol | - | 228 ± 1 a | 433 ± 43 b | 482 ± 52 ab | 772 ± 25 c | 908 ± 39 d | 897 ± 23 d | 920 ± 76 d | 1239 ± 94 e | 1139 ± 79 e | 1330 ± 40 e |
| Aldehyde | |||||||||||||||
| 7 | 2.03 | 619 | 601 | Butanal | 907 ± 27 abc | 1380 ± 137 bc | 885 ± 68 abc | 868 ± 95 abc | 657 ± 10 a | 881 ± 69 abc | 1208 ± 7 bc | 852 ± 44 ab | 1309 ± 180 bc | 1115 ± 166 abc | 1521 ± 57 c |
| 8 | 2.70 | 692 | 657 | (E)-But-2-enal | - | 2446 ± 10 a | 3728 ± 161 b | 2710 ± 185 a | 3405 ± 134 b | 4275 ± 106 c | 6762 ± 450 d | 7383 ± 45 e | 9070 ± 272 g | 8695 ± 35 f | 8697 ± 298 f |
| 9 | 2.88 | 704 | 664 | 2-Methylbutanal | 5145 ± 112 a | 8754 ± 142 e | 8810 ± 374 e | 8056 ± 437 d | 5652 ± 301 a | 6167 ± 126 c | 7106 ± 234 c | 5365 ± 231 a | 6435 ± 514 bc | 5826 ± 389 ab | 8315 ± 344 de |
| 10 | 3.34 | 724 | 701 | Pentanal | 6017 ± 55 a | 51617 ± 1306 b | 80296 ± 424 g | 62084 ± 905 c | 70645 ± 380 e | 66498 ± 349 d | 93133 ± 677 i | 77233 ± 523 f | 101482 ± 392 j | 84322 ± 329 h | 94284 ± 2926 i |
| 11 | 4.65 | 779 | 759 | (E)-Pent-2-enal | - | 1172 ± 121 a | 1739 ± 159 b | 1775 ± 46 b | 2062 ± 164 b | 2270 ± 20 c | 3673 ± 126 d | 4807 ± 162 e | 6357 ± 397 f | 7313 ± 12 g | 7387 ± 142 g |
| 12 | 6.14 | 824 | 802 | Hexanal | 1477 ± 96 a | 31899 ± 205 b | 45520 ± 365 d | 39555 ± 1714 c | 48037 ± 550 d | 56662 ± 152 e | 84428 ± 2094 f | 99801 ± 2237 g | 128433 ± 2339 h | 153853 ± 1838 i | 163206 ± 5931 j |
| 13 | 8.30 | 877 | 864 | (E)-Hex-2-enal | - | 885 ± 50 a | 1340 ± 70 b | 1412 ± 93 b | 1471 ± 146 bc | 1647 ± 109 cd | 1891 ± 96 de | 2078 ± 149 ef | 2239 ± 282 f | 2366 ± 56 f | 2375 ± 57 f |
| 14 | 10.45 | 927 | 903 | Heptanal | - | 1943 ± 195 a | 2819 ± 79 ab | 3221 ± 263 c | 2992 ± 121 bc | 3581 ± 196 c | 4424 ± 390 d | 5018 ± 225 e | 6314 ± 126 f | 6419 ± 288 f | 7223 ± 427 g |
| 15 | 12.90 | 981 | 956 | (E)-Hept-2-enal | - | 4173 ± 127 a | 5878 ± 313 b | 6040 ± 530 b | 7206 ± 240 c | 8782 ± 263 d | 10015 ± 18 e | 10215 ± 192 e | 11303 ± 392 f | 11387 ± 329 f | 10985 ± 771 f |
| 16 | 15.00 | 1029 | 1003 | Octanal | - | 2150 ± 191 a | 2276 ± 188 ab | 2426 ± 404 ab | 2954 ± 157 bc | 3517 ± 270 c | 4702 ± 231 d | 4877 ± 291 de | 5448 ± 142 ef | 5603 ± 235 fg | 5432 ± 65 g |
| 17 | 15.30 | 1036 | 1015 | (2E,4E)-Hepta-2,4-dienal | - | 5223 ± 464 a | 6817 ± 234 cd | 6820 ± 234 cd | 6896 ± 389 cd | 6202 ± 77 b | 7383 ± 163 d | 6866 ± 77 cd | 6538 ± 543 bc | 5595 ± 235 a | 5519 ± 223 a |
| 18 | 17.30 | 1083 | 1064 | (E)-Oct-2-enal | - | 702 ± 47 a | 810 ± 58 ab | 847 ± 185 bc | 1283 ± 61 d | 1219 ± 107 cd | 1286 ± 135 cd | 1353 ± 63 d | 1593 ± 118 e | 1671 ± 39 ef | 1773 ± 108 f |
| 19 | 19.30 | 1132 | 1104 | Nonanal | - | 5285 ± 230 a | 6712 ± 459 b | 7251 ± 636 b | 8761 ± 236 cd | 8318 ± 676 c | 8893 ± 130 cd | 9012 ± 693 cd | 8734 ± 244 cd | 10273 ± 652 d | 9294 ± 34 d |
| 20 | 21.50 | 1187 | 1165 | (E)-Non-2-enal | - | - | 441 ± 18 a | 528 ± 20 ab | 664 ± 77 cd | 599 ± 46 bc | 724 ± 22 cd | 916 ± 10 de | 854 ± 9 ef | 1125 ± 64 f | 1026 ± 57 ef |
| 21 | 25.50 | 1294 | 1263 | (E)-Dec-2-enal | - | 665 ± 48 a | 1304 ± 67 b | 1841 ± 19 c | 2272 ± 95 c | 2833 ± 243 d | 3191 ± 153 e | 3908 ± 82 g | 3949 ± 78 g | 3699 ± 69 f | 3537 ± 156 f |
| 22 | 26.70 | 1327 | 1316 | (2E,4E)-Deca-2,4-dienal | - | 292 ± 27 a | 479 ± 104 b | 561 ± 48 b | 679 ± 40 bc | 791 ± 51 cd | 824 ± 40 cd | 797 ± 143 cd | 979 ± 83 d | 869 ± 64 cd | 799 ± 70 cd |
| 23 | 27.50 | 1350 | 1360 | (E)-Undec-2-enal | - | 646 ± 0 a | 2019 ± 130 b | 2785 ± 172 c | 3161 ± 159 cd | 3773 ± 254 e | 3702 ± 243 e | 4449 ± 268 f | 3871 ± 227 e | 3424 ± 363 e | 3146 ± 180 de |
| 24 | 29.10 | 1396 | 1420 | (2E,4E)-Undeca-2,4-dienal | - | - | 402 ± 18 a | 678 ± 17 b | 1152 ± 96 c | 1762 ± 106 d | 1928 ± 177 e | 2332 ± 90 e | 2185 ± 129 e | 2117 ± 1 e | 1969 ± 62 e |
| Other volatile compounds | |||||||||||||||
| 25 | 1.89 | 604 | 559 | Methyl acetate | 3737 ± 121 d | 6276 ± 330 h | 5754 ± 166 g | 6162 ± 269 h | 2735 ± 122 b | 4323 ± 286 e | 4749 ± 242 f | 2082 ± 98 a | 1952 ± 71 a | 1793 ± 4 a | 3327 ± 140 c |
| 26 | 2.34 | 653 | 612 | Ethyl acetate | 10241 ± 89 c | 17939 ± 456 i | 16614 ± 90 h | 15317 ± 49 g | 9964 ± 413 c | 11501 ± 188 d | 12369 ± 403 e | 9206 ± 753 b | 9775 ± 177 bc | 8461 ± 332 a | 13005 ± 428 f |
| 27 | 2.11 | 628 | 600 | Acetic acid | - | - | 1354 ± 451 ab | 568 ± 80 a | 1973 ± 134 b | 1198 ± 67 ab | 2085 ± 220 b | 4878 ± 642 c | 8106 ± 144 d | 11466 ± 404 e | 11731 ± 117 e |
| 28 | 2.23 | 641 | Hexane | 2247 ± 96 a | 7311 ± 119 b | 9417 ± 296 cd | 8766 ± 293 c | 7998 ± 113 b | 8321 ± 387 b | 9968 ± 709 d | 11057 ± 383 e | 14223 ± 557 f | 14014 ± 167 f | 16806 ± 597 g | |
| 29 | 2.98 | 709 | Heptane | 6742 ± 29 ab | 11206 ± 610 f | 11317 ± 830 f | 10119 ± 237 e | 6750 ± 321 ab | 8508 ± 674 c | 8820 ± 420 cd | 6001 ± 300 a | 6948 ± 307 b | 6358 ± 335 ab | 9446 ± 552 de | |
| 30 | 11.80 | 957 | 941 | Butylcyclopentane | - | 1300 ± 89 a | 1735 ± 124 c | 1473 ± 101 b | 1852 ± 106 cd | 2106 ± 143 cd | 2272 ± 121 e | 1888 ± 93 cd | 2283 ± 169 e | 2035 ± 44 de | 2023 ± 143 d |
| 31 | 14.40 | 1015 | 993 | 2-Pentylfuran | - | 2404 ± 205 a | 2824 ± 60 bc | 2601 ± 149 ab | 2640 ± 273 ab | 2694 ± 56 ab | 3058 ± 216 c | 3128 ± 65 bc | 3438 ± 339 d | 3992 ± 116 e | 4328 ± 89 e |
| 32 | 14.65 | 1021 | 1001 | 2-Ethyl-6-methylpyrazine | - | 1359 ± 115 a | 1698 ± 105 b | 1787 ± 46 b | 1638 ± 60 b | 1741 ± 112 b | 1799 ± 176 b | 1743 ± 116 b | 1837 ± 180 b | 1542 ± 100 b | 1534 ± 84 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, S.; Yu, Y.; Zhang, X.; Chen, J.; Zhang, H. Quality Change in Camellia Oil during Intermittent Frying. Foods 2022, 11, 4047. https://doi.org/10.3390/foods11244047
Liu X, Wang S, Yu Y, Zhang X, Chen J, Zhang H. Quality Change in Camellia Oil during Intermittent Frying. Foods. 2022; 11(24):4047. https://doi.org/10.3390/foods11244047
Chicago/Turabian StyleLiu, Xiaofang, Shuo Wang, Yong Yu, Xu Zhang, Jieyu Chen, and Han Zhang. 2022. "Quality Change in Camellia Oil during Intermittent Frying" Foods 11, no. 24: 4047. https://doi.org/10.3390/foods11244047
APA StyleLiu, X., Wang, S., Yu, Y., Zhang, X., Chen, J., & Zhang, H. (2022). Quality Change in Camellia Oil during Intermittent Frying. Foods, 11(24), 4047. https://doi.org/10.3390/foods11244047







