Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers
Abstract
1. Introduction
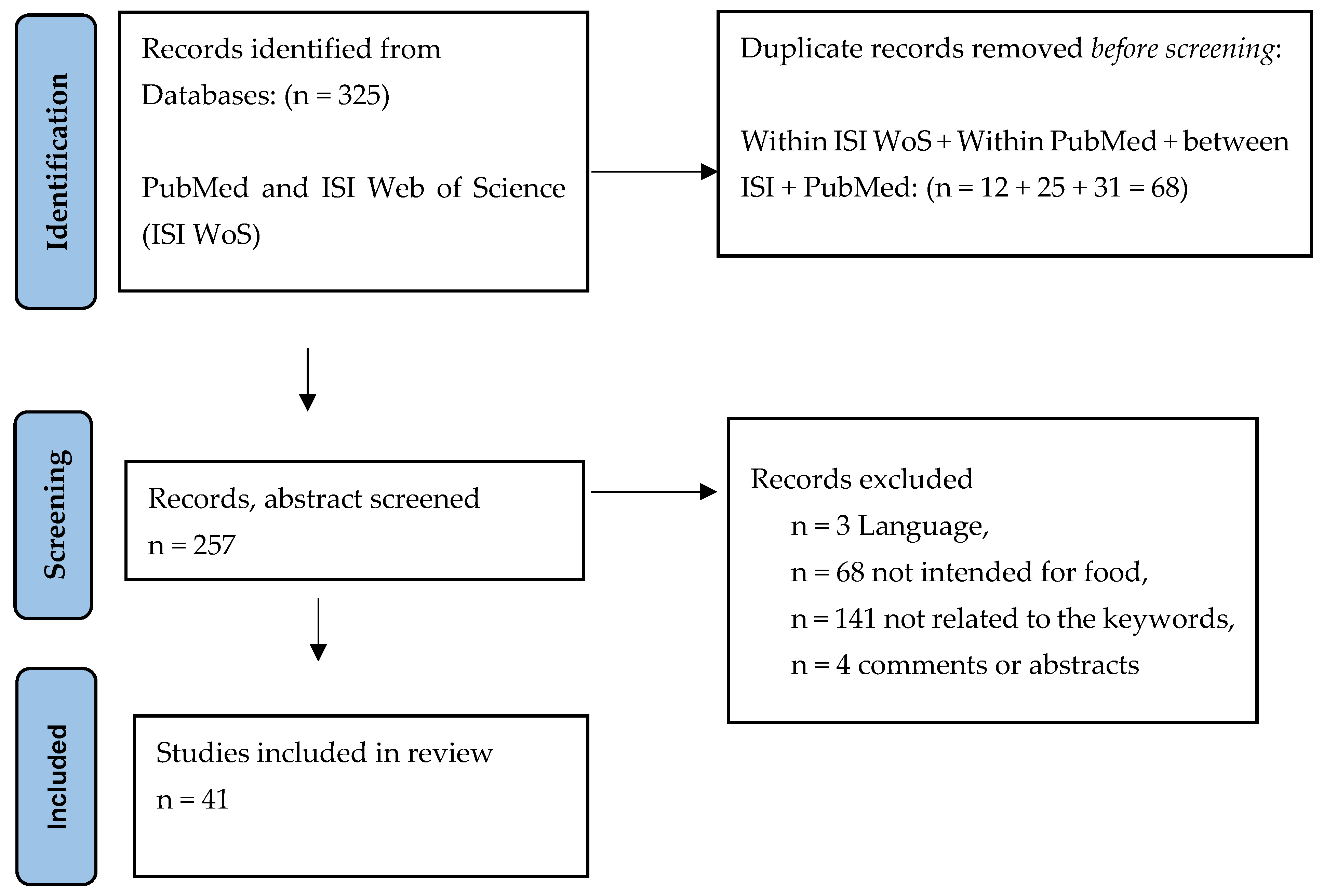
2. Data Sources and Search Strategies
Eligibility Criteria
3. Results
3.1. Proximate Composition
3.2. Lipids
3.3. Proteins
3.4. Minerals
3.4.1. Selenium
3.4.2. Iodine
3.4.3. Heavy Metals and Trace Elements
4. Hazards
4.1. Food Safety and Risk Assessments
4.2. Chemical Hazards, Heavy Metals
| Species | Geographical Region | Mercury | Cadmium | Arsenic | Lead | Paper | |
|---|---|---|---|---|---|---|---|
| Acaudina leucoprocta | n = 3 | East China Sea | 0.06 ± 0.01 | 0.05 ± 0.01 | 5.64 ± 0.24 | 1.38 ± 0.21 | Lin et al. [38] |
| Actinopyga caerulea | n = 3 | Guangzhou, China. Market samples | nd | 0.06 ± 0.01 | 3.3 ± 0.1 | 0.15 ± 0.01 | Wen & Hu [55] |
| Actinopyga mauritiana | n = 3 | Guangzhou, China. Market samples | nd | 0.05 ± 0.01 | 2.1 ± 0.1 | 0.11 ± 0.01 | Wen & Hu [55] |
| Apostichopus japonicus | n = 3 | Bohai Sea Yellow Sea. Range eight farming sites | na | 0.31–0.85 | 4.26–12.39 | 1.05–4.25 | Mohsen et al. [74] |
| n = 3 | Panshan, Bohai Sea Yellow sea | na | 0.85 ± 0.02 | 10.47 ± 0.28 | 2.18 ± 0.36 | Mohsen et al. [74] | |
| n = 3 | Lvshunkou, Bohai Sea Yellow Sea | na | 0.38 ± 0.03 | 10.88 ± 0.29 | 2.59 ± 0.21 | Mohsen et al. [74] | |
| n = 3 | Rongcheng, Weihai, Bohai Sea Yellow Sea | na | 0.36 ± 0.04 | 5.99 ± 0.11 | 1.05 ± 0.13 | Mohsen et al. [74] | |
| n = 3 | Haiyang, Yantai, Bohai Sea Yellow Sea | na | 0.82 ± 0.03 | 5.38 ± 0.83 | 1.76 ± 0.07 | Mohsen et al. [74] | |
| n = 3 | Chengyang, Qindao, Bohai Sea Yellow Sea | na | 0.57 ± 0.05 | 5.25 ± 0.42 | 2.72 ± 0.08 | Mohsen et al. [74] | |
| n = 3 | Pingdao island, Rizaho, Bohai Sea Yellow Sea | na | 0.31 ± 0.03 | 12.39 ± 0.25 | 1.56 ± 0.25 | Mohsen et al. [74] | |
| n = 3 | Tangshan, Bohai Sea Yellow Sea | na | 0.54 ± 0.02 | 9.86 ± 0.11 | 1.94 ± 0.15 | Mohsen et al. [74] | |
| n = 3 | Laizhou, Bohai Sea Yellow Sea | na | 0.50 ± 0.01 | 4.26 ± 0.87 | 4.25 ± 0.23 | Mohsen et al. [74] | |
| n = 3 | North Yantai region of China | na | 0.32 ± 0.07 | na | na | Liu et al. [75] | |
| n = 63 | South Korea | 0.002 ± 0.002 | 0.05 ± 0.07 | 2.24 ± 1.82 | 0.06 ± 0.05 | Choi et al. [76] | |
| Bohadschia argus | n = 3 | Guangzhou. China. Market samples | nd | 0.03 ± 0.01 | 3.2 ± 0.1 | 0.13 ± 0.01 | Wen & Hu [55] |
| Cucumaria frondosa | n = 3 | Guangzhou Qingping, China, Canadian S2 | 0.17 ± 0.01 | 0.61 ± 0.05 | 3.59 ± 0.39 | 0.57 ± 0.04 | Song et al. [77] |
| n = 3 | Guangzhou Qingping, China, Canadian S4 | 0.15 ± 0.00 | 0.40 ± 0.37 | 3.13 ± 1.12 | 0.08 ± 0.02 | Song et al. [77] | |
| n = 3 | Guangzhou Qingping, China, Canadian S6 | 0.17 ± 0.00 | 0.43 ± 0.03 | 2.58 ± 0.14 | 0.53 ± 0.04 | Song et al. [77] | |
| n = 3 | Guangzhou Qingping, China, Canadian S7 | 0.17 ± 0.01 | 0.32 ± 0.09 | 2.52 ± 0.17 | 0.45 ± 0.04 | Song et al. [77] | |
| Eupentacta fraudatrix | n = 6 | Peter the Great Bay, Sea of Japan 2016 | na | 0.15 ± 0.01 | na | 17.08 ± 1.12 | Dolmatova et al. [78] |
| n = 5 | Peter the Great Bay, Sea of Japan 2008 | na | 0.38 ± 0.03 | na | 5.01 ± 0.66 | Dolmatova et al. [78] | |
| n = 5 | Peter the Great Bay, Sea of Japan 2008 | na | 0.24 ± 0.01 | na | nd | Dolmatova et al. [78] | |
| n = 6 | Peter the Great Bay, Sea of Japan 2016 | na | 0.36 ± 0.04 | na | nd | Dolmatova et al. [78] | |
| Holothuria floridana | n = 8 | Cispatá Bay, Colombia. Range seven sites | 0.074–0.090 | 0.016–0.026 | na | 0.030–0.038 | Marrugo-Negrete et al. [79] |
| Holothuria fuscogilva | n = 3 | Guangzhou, China. Market samples | nd | 0.03 ± 0.01 | 3.8 ± 0.1 | 0.13 ± 0.01 | Wen & Hu [55] |
| Holothuria fuscopunctata | n = 3 | Guangzhou, China. Market samples | nd | 0.03 ± 0.01 | 6.1 ± 0.1 | 0.56 ± 0.03 | Wen & Hu [55] |
| Holothuria leucospilota | n = 30 | Qeshm Island, Persian Gulf | na | 0.29 ± 0.14 | na | 21.39 ± 2.52 | Mohammadizadeh et al. [80] |
| Range | Qeshm Island, Persian Gulf. Range three sites | na | 0.16–0.45 | na | 19.09–23.24 | Mohammadizadeh et al. [80] | |
| Holothuria mexicana | n = 3 | Guangzhou, China. Market samples | nd | 0.04 ± 0.01 | 2.0 ± 0.1 | 0.69 ± 0.06 | Wen & Hu [55] |
| Holothuria polii | n = 19 | Gulf of Cagliari, Foxi, Sardinia, Italy | 0.023 | 0.03 | 22.9 | 0.88 | Montero et al. [30] |
| n = 5 | Southern Adriatic Sea, Italy, Pooled samples of 2 kg | na | 0.07 ± 0.0 | 33.30 ± 0.85 | 0.65 ± 0.03 | Sicuro et al. [42] | |
| n = 500 | Southern Adriatic Sea, Italy | 0.96 ± 0.022 | 0.04 ± 0.01 | na | 1.26 ± 0.12 | Storelli et al. [37] | |
| Holothuria scabra | n = 3 | Qeshm Island, Persian Gulf | na | 0.15 ± 0.02 | na | 1.92 ± 0.49 | Mohammadizadeh et al. [80] |
| Range | Qeshm Island, Persian Gulf. Range three sites | na | 0.13–0.17 | na | 1.52–2.55 | Mohammadizadeh et al. [80] | |
| n = 3 | Guangzhou, China. Market samples | nd | 0.04 ± 0.01 | 1.1 ± 0.1 | 0.56 ± 0.03 | Wen & Hu [55] | |
| Holoturia tubulosa | n = 15 | Gulf of Cagliari, Giorgino, Sardinia, Italy | 0.043 | 0.02 | 18 | 0.44 | Montero et al. [30] |
| Range | Dardanelles Strait, Turkey. Range three sites | na | 0.04–1.66 | na | 0.48–5.80 | Turk Culha et al. [81] | |
| n = 5 | Southern Adriatic Sea, Italy. Pooled samples of 2 kg | na | 0.07 ± 0.0 | 22.35 ± 0.08 | 1.16 ± 0.04 | Sicuro et al. [42] | |
| Stichopus chloronotus | n = 3 | Guangzhou, China. Market samples | nd | 0.06 ± 0.01 | 1.7 ± 0.1 | 0.12 ± 0.01 | Wen & Hu [55] |
| Stichopus herrmanni | n = 3 | Guangzhou, China. Market samples | nd | 0.03 ± 0.01 | 2.5 ± 0.1 | 0.52 ± 0.03 | Wen & Hu [55] |
| Thelenota ananas | n = 3 | Guangzhou, China. Market samples | nd | 0.03 ± 0.01 | 5.1 ± 0.1 | 0.55 ± 0.03 | Wen & Hu [55] |
| Thelenota anax | n = 3 | Guangzhou, China. Market samples | nd | 0.04 ± 0.01 | 2.8 ± 0.1 | 0.25 ± 0.02 | Wen & Hu [55] |
| Recovered | Recovered | |||
|---|---|---|---|---|
| Species | Body Part and Location | Total As | iAs | Total As |
| Cucumaria frondosa | Body wall NS | 6.0 ± 1.1 | 1.4 ± 0.17 | 2.0 ± 0.27 |
| Body wall NL | 5.2 ± 0.8 | 1.7 ± 0.41 | 2.3 ± 0.34 | |
| Process Body wall NS | 4.3 ± 1.6 | 0.63 ± 0.24 | 0.93 ± 0.17 | |
| Apostichopus californicus | Muscle BC | 36.0 ± 3.5 | 0.017 ± 0.012 | 4.3 ± 1.1 |
| Skin BC | 28.0 ± 0.5 | 0.042 ± 0.054 | 0.85 ± 0.20 | |
| Apostichopus japonicus | Body wall Liaoning | 5.0 ± 0.1 | 0.069 | 1 |
| Body wall Shandong | 7.1 ± 0.04 | 0.13 | 1.9 | |
| Body wall Fujian | 7.7 ± 0.09 | 0.12 | 1.1 |
4.2.1. Mercury
4.2.2. Cadmium
4.2.3. Arsenic
4.2.4. Lead
4.2.5. Removal of Heavy Metals from Body Wall of Sea Cucumbers
4.3. Persistent Organic Pollutants (POPs)
4.3.1. Dioxins and PCBs
4.3.2. Polycyclic Aromatic Hydrocarbons
4.3.3. Poly- and Perfluorinated Compounds (PFAS)
4.3.4. Lindane
4.4. Microbiological Hazards
Antibiotics
4.5. Physical Hazards
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 13 September 2022).
- Alava, J.J.; Cheung, W.W.L.; Ross, P.S.; Sumaila, U.R. Climate change–contaminant interactions in marine food webs: Toward a conceptual framework. Glob. Chang. Biol. 2017, 23, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Caldeira, K.; Chopin, T.; Gaines, S.; Haugan, P.; Hemer, M.; Howard, J.; Konar, M.; Krause-Jensen, D.; Lindstad, E.; et al. The Ocean as a Solution to Climate Change: Five Opportunities for Action. Available online: https://www.wri.org/events/2019/10/ocean-solution-climate-change-5-opportunities-action (accessed on 12 September 2022).
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The environmental cost of animal source foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Jensen, I.J.; Walquist, M.; Liaset, B.; Elvevoll, E.O.; Eilertsen, K.E. Dietary intake of cod and scallop reduces atherosclerotic burden in female apolipoprotein E-deficient mice fed a Western-type high fat diet for 13 weeks. Nutr. Metab. 2016, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.A.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- SAPEA, Science Advice for Policy by European Academies. Food from the Oceans: How Can More Food and Biomass Be Obtained from the Oceans in a Way that Does Not Deprive Future Generations of Their Benefits? SAPEA: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Koehn, J.Z.; Allison, E.H.; Golden, C.D.; Hilborn, R. The role of seafood in sustainable diets. Environ. Res. Lett. 2022, 17, 035003. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, S.; Qin, D.; Chen, Z.; Wang, J.; Bai, S.; Mou, Z. Heavy Metals in Sea Cucumber Juveniles from Coastal Areas of Bohai and Yellow Seas, North China. Bull. Environ. Contam. Toxicol. 2015, 94, 577–582. [Google Scholar] [CrossRef]
- Han, Q.; Keesing, J.K.; Liu, D. A Review of Sea Cucumber Aquaculture, Ranching, and Stock Enhancement in China. Rev. Fish. Sci. Aquac. 2016, 24, 326–341. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: http://www.marinespecies.org (accessed on 11 May 2021).
- Purcell, S.W.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World; FAO: Rome, Italy, 2012. [Google Scholar]
- Rahman, M.A.; Chowdhury, S.H.; Hasan, M.J.; Rahman, M.H.; Yeasmin, S.M.; Farjana, N.; Molla, M.H.R.; Parvez, M.S. Status, Prospects and Market Potentials of the Sea Cucumber Fisheries with Special Reference on Their Proper Utilization and Trade. Annu. Res. Rev. Biol. 2020, 35, 84–101. [Google Scholar] [CrossRef]
- Baker-Médard, M.; Ohl, K.N. Sea cucumber management strategies: Challenges and opportunities in a developing country context. Environ. Conserv. 2019, 46, 267–277. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture (SOFIA). In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020; Volume 32, p. 244. [Google Scholar] [CrossRef]
- Purcell, S.W.; Williamson, D.H.; Ngaluafe, P. Chinese market prices of beche-de-mer: Implications for fisheries and aquaculture. Mar. Policy 2018, 91, 58–65. [Google Scholar] [CrossRef]
- Fabinyi, M.; Barclay, K.; Eriksson, H. Chinese Trader Perceptions on Sourcing and Consumption of Endangered Seafood. Front. Mar. Sci. 2017, 4, 181. [Google Scholar] [CrossRef]
- Liang, Q.; Ahmed, F.; Zhang, M.; Sperou, N.; Franco, C.M.M.; Feng, Q.; Zhang, W. In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012–2021. Front. Mar. Sci. 2022, 9, 917857. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.W. Value, Market Preferences and Trade of Beche-De-Mer from Pacific Island Sea Cucumbers. PLoS ONE 2014, 9, e95075. [Google Scholar] [CrossRef] [PubMed]
- Kinch, J.; Purcell, S.; Uthicke, S.; Friedman, K. Papua New Guinea: A hot spot of sea cucumber fisheries in the Western Pacific. In Sea Cucumbers: A Global Review of Fisheries and Trade; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; United Nations Food and Agriculture Organisation (FAO): Rome, Italy, 2008; pp. 57–80. [Google Scholar]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef]
- Özer, N.P.; Mol, S.; Varlık, C. Effect of the Handling Procedures on the Chemical Composition of Sea Cucumber. Turk. J. Fish. Aquat. Sci. 2004, 4, 71–74. [Google Scholar]
- Künili, I.E.; Çolakoğlu, F.A. Chemical and Nutritional Characteristics of Holothuria tubulosa (Gmelin, 1788); A Seasonally Comparative Study. J. Aquat. Food Prod. Technol. 2019, 28, 716–728. [Google Scholar] [CrossRef]
- Rasyid, A.; Yasman, Y.; Putra, M.Y. Current prospects of nutraceutical and pharmaceutical use of sea cucumbers. Pharmacia 2021, 68, 561–572. [Google Scholar] [CrossRef]
- Rasyid, A. Nutritional Value and Heavy Metals Contents of the Dried Sea Cucumber Stichopus vastus from Salemo Island, Indonesia. J. Ilmu Dan Teknol. Kelaut. Trop. 2017, 9, 739–746. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Montero, N.; Atzori, M.; Marras, B.; Bettoschi, A.; Nurchis, P.; Coroneo, V.; Sanna, C.; Schintu, M. Trace metal levels in the edible tissues of sea cucumbers (Holothuria tubulosa and Holothuria polii) from Sardinia (Western Mediterranean). Ital. J. Food Saf. 2021, 10, 9576. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, J.; Moity, N.; Andrade-Vera, S.; Reyes, H. Overexploitation and More Than a Decade of Failed Management Leads to No Recovery of the Galápagos Sea Cucumber Fishery. Front. Mar. Sci. 2020, 7, 554314. [Google Scholar] [CrossRef]
- Gianasi, B.L.; Hamel, J.-F.; Montgomery, E.M.; Sun, J.; Mercier, A. Current Knowledge on the Biology, Ecology, and Commercial Exploitation of the Sea Cucumber Cucumaria frondosa. Rev. Fish. Sci. Aquac. 2021, 29, 582–653. [Google Scholar] [CrossRef]
- Conand, C.; Claereboudt, M.; Dissayanake, C.; Ebrahim, A.; Fernando, S.; Godvinden, R.; Lavitra, T.; Léopold, M.; Mmbaga, T.; Mulochau, T.; et al. Review of fisheries and management of sea cucumbers in the Indian Ocean. West. Indian Ocean J. Mar. Sci. 2022, 21, 125–148. [Google Scholar] [CrossRef]
- Mohsen, M.; Yang, H. Introduction to Sea Cucumbers. In Sea Cucumbers; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. Oceanogr. Mar. Biol. 2016, 54, 367–386. [Google Scholar]
- Lawson, C.M.; Cullen, J.A.; Nunnally, C.C.; Rowe, G.T.; Hala, D.N. PAH and PCB body-burdens in epibenthic deep-sea invertebrates from the northern Gulf of Mexico. Mar. Pollut. Bull. 2021, 162, 111825. [Google Scholar] [CrossRef]
- Storelli, M.M.; Storelli, A.; Marcotrigiano, G.O. Heavy metals in the aquatic environment of the Southern Adriatic Sea, Italy: Macroalgae, sediments and benthic species. Environ. Int. 2001, 26, 505–509. [Google Scholar] [CrossRef]
- Lin, S.-J.; Chen, L.-F.; Jia, Y.-B.; Xiao, H.-L.; Xue, Y.-P.; Zheng, Y.-G. Distribution and Chemoenzymatic Removal of Heavy Metals in Sea Cucumber “Acaudina leucoprocta”. Food Sci. Technol. Res. 2018, 24, 223–229. [Google Scholar] [CrossRef]
- FAO/WHO. General Standard for Contaminants and Toxins in Food and Feed, CXS 193-1995, Revised 2019. Codex Alimentarus (CAC). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 10 September 2020).
- EC. Commission Regulation EU. 1881/2006/Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/2022-07-01 (accessed on 14 September 2022).
- GB 2762–2017; National Food Safety Standard Maximum Levels of Contaminants in Food. Ministry of Health of the People’s Republic of China: Beijing, China, 2017.
- Sicuro, B.; Piccinno, M.; Gai, F.; Abete, M.C.; Danieli, A.; Dapra, F.; Mioletti, S.; Vilella, S. Food Quality and Safety of Mediterranean Sea Cucumbers Holothuria tubulosa and Holothuria polii in Southern Adriatic Sea. Asian J. Anim. Vet. Adv. 2012, 7, 851–859. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Arsenic in Food. Efsa J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- FAO. FishStatJ—Software for Fishery and Aquaculture Statistical Time Series. Available online: https://www.fao.org/fishery/en/fishstatj (accessed on 20 September 2022).
- Liu, H.; Luo, J.; Ding, T.; Gu, S.; Yang, S.; Yang, M. Speciation Analysis of Trace Mercury in Sea Cucumber Species of Apostichopus japonicus Using High-Performance Liquid Chromatography Conjunction with Inductively Coupled Plasma Mass Spectrometry. Biol. Trace Elem. Res. 2018, 186, 554–561. [Google Scholar] [CrossRef]
- Gajdosechova, Z.; Palmer, C.H.; Dave, D.; Jiao, G.; Zhao, Y.; Tan, Z.; Chisholm, J.; Zhang, J.; Stefanova, R.; Hossain, A.; et al. Arsenic speciation in sea cucumbers: Identification and quantitation of water-extractable species. Environ. Pollut. 2020, 266, 115190. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Røsjø, H.; Eide, I.A.; Vigen, T.; Ihle-Hansen, H.; Orstad, E.B.; Rønning, O.M.; Lyngbakken, M.N.; Berge, T.; Schmidt, E.B.; et al. Plasma marine n-3 polyunsaturated fatty acids and cardiovascular risk factors: Data from the ACE 1950 study. Eur. J. Nutr. 2020, 59, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djousse, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation 2018, 138, 35–47. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. Omega-3 fatty acids and cardiovascular diseases: Effects, Mechanisms and dietary relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine omega-3 (n-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption. Available online: https://apps.who.int/iris/handle/10665/44666 (accessed on 12 December 2012).
- FAO/WHO/UNU. Protein and Amino acid Requirements in Human Nutrition. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 10 September 2020).
- Bechtel, P.J.; Oliveira, A.C.M.; Demir, N.; Smiley, S. Chemical composition of the giant red sea cucumber, Parastichopus californicus, commercially harvested in Alaska. Food Sci. Nutr. 2013, 1, 63–73. [Google Scholar] [CrossRef]
- Wen, J.; Hu, C. Elemental composition of commercial sea cucumbers (holothurians). Food Addit. Contam. Part B 2010, 3, 246–252. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Chang, Y.; Xue, C.; Li, Z. Investigation of structural proteins in sea cucumber (Apostichopus japonicus) body wall. Sci. Rep. 2020, 10, 18744. [Google Scholar] [CrossRef] [PubMed]
- Sroyraya, M.; Hanna, P.J.; Siangcham, T.; Tinikul, R.; Jattujan, P.; Poomtong, T.; Sobhon, P. Nutritional components of the sea cucumber Holothuria scabra. Funct. Foods Health Dis. 2017, 7, 168–181. [Google Scholar] [CrossRef]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, M.; Leape, J.; Micheli, F.; Allison, E.H.; Basurto, X.; Bennett, A.; Bush, S.R.; Cao, L.; Cheung, W.W.L.; Crona, B.; et al. The vital roles of blue foods in the global food system. Glob. Food Secur. 2022, 33, 100637. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Contribution of Fish and Seafood to Global Food and Feed Supply: An Analysis of the FAO Food Balance Sheet for 2019. Rev. Fish. Sci. Aquac. 2022. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for selenium. Efsa J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Jayasinghe, G.D.T.M.; Pohl, P.; Fowler, S.W. Mitigating the impact of mercury contaminants in fish and other seafood—A review. Mar. Pollut. Bull. 2021, 171, 112710. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef]
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007.
- Hay, I.; Hynes, K.L.; Burgess, J.R. Mild-to-Moderate Gestational Iodine Deficiency Processing Disorder. Nutrients 2019, 11, 1974. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition Allergies. Scientific Opinion on Dietary Reference Values for iodine. Efsa J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vázquez, R.; Regueiro, L.; Atanassova, M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Noriega Fernández, E. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- EC. Commission Regulation EU. 1259/2011 Setting Maximum Levels of Dioxins and PCBs in Food. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32011R1259 (accessed on 14 September 2022).
- EC. Annex II to Regulation (EC) 854/2004 and Annex I to Regulation (EC) No. 2073/2005 on Microbiological Criteria for Foodstuffs, 2285/2015/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R2285 (accessed on 14 September 2022).
- Galimberti, C.; Corti, I.; Cressoni, M.; Moretti, V.M.; Menotta, S.; Galli, U.; Cambiaghi, D. Evaluation of mercury, cadmium and lead levels in fish and fishery products imported by air in North Italy from extra-European Union Countries. Food Control 2016, 60, 329–337. [Google Scholar] [CrossRef]
- Chiocchetti, G.; Jadán-Piedra, C.; Vélez, D.; Devesa, V. Metal(loid) contamination in seafood products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Committee., Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. Efsa J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- Choi, J.Y.; Habte, G.; Khan, N.; Nho, E.Y.; Hong, J.H.; Choi, H.; Park, K.S.; Kim, K.S. Determination of toxic heavy metals in Echinodermata and Chordata species from South Korea. Food Addit. Contam. Part B 2014, 7, 295–301. [Google Scholar] [CrossRef]
- Chatzi, L.; Ierodiakonou, D.; Margetaki, K.; Vafeiadi, M.; Chalkiadaki, G.; Roumeliotaki, T.; Fthenou, E.; Pentheroudaki, E.; McConnell, R.; Kogevinas, M.; et al. Associations of Prenatal Exposure to Cadmium With Child Growth, Obesity, and Cardiometabolic Traits. Am. J. Epidemiol. 2019, 188, 141–150. [Google Scholar] [CrossRef]
- Kippler, M.; Bottai, M.; Georgiou, V.; Koutra, K.; Chalkiadaki, G.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Fthenou, E.; Vassilaki, M.; et al. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. Eur. J. Epidemiol. 2016, 31, 1123–1134. [Google Scholar] [CrossRef]
- FAO/WHO. Safety Evaluation of Certain Contaminants in Food, Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/handle/10665/44520 (accessed on 14 September 2020).
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Heavy metals in sediment, microplastic and sea cucumber Apostichopus japonicus from farms in China. Mar. Pollut. Bull. 2019, 143, 42–49. [Google Scholar] [CrossRef]
- IARC. Arsenic, Metals, Fibres and Dusts. Lyon (FR): International Agency for Research on Cancer. Working Group on the Evaluation of Carcinogenic Risks to Humans. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304375/ (accessed on 10 September 2020).
- EC. Commission Regulation (EU) 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015R1006 (accessed on 24 October 2022).
- Afonso, C.; Matos, J.; Guarda, I.; Gomes-Bispo, A.; Gomes, R.; Cardoso, C.; Gueifão, S.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Bioactive and nutritional potential of Alaria esculenta and Saccharina latissima. J. Appl. Phycol. 2021, 33, 501–513. [Google Scholar] [CrossRef]
- Coelho, J. Arsenic speciation in algae: Case studies in Europe. Compr. Anal. Chem. 2019, 85, 179–198. [Google Scholar] [CrossRef]
- Song, Z.; Li, H.; Wen, J.; Zeng, Y.; Ye, X.; Zhao, W.; Xu, T.; Xu, N.; Zhang, D. Consumers’ attention on identification, nutritional compounds, and safety in heavy metals of Canadian sea cucumber in Chinese food market. Food Sci. Nutr. 2020, 8, 5962–5975. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristaldi, A.; Copat, C. Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts: A health risk assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef]
- Petursdottir, A.H.; Sloth, J.J.; Feldmann, J. Introduction of regulations for arsenic in feed and food with emphasis on inorganic arsenic, and implications for analytical chemistry. Anal. Bioanal. Chem. 2015, 407, 8385–8396. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Lead in Food. Efsa J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Mohammadizadeh, M.; Bastami, K.D.; Ehsanpour, M.; Afkhami, M.; Mohammadizadeh, F.; Esmaeilzadeh, M. Heavy metal accumulation in tissues of two sea cucumbers, Holothuria leucospilota and Holothuria scabra in the northern part of Qeshm Island, Persian Gulf. Mar. Pollut. Bull. 2016, 103, 354–359. [Google Scholar] [CrossRef]
- Dolmatova, L.S.; Slinko, E.N.; Kolosova, L.F. Variations in the Heavy Metal Contents in Tissues of the Sea Cucumber Eupentacta fraudatrix in the Coastal Waters of the Sea of Japan: The Influence of Physiological and Anthropogenic Factors. Oceanology 2020, 60, 446–457. [Google Scholar] [CrossRef]
- McAloon, K.M.; Mason, R.P. Investigations into the bioavailability and bioaccumulation of mercury and other trace metals to the sea cucumber, Sclerodactyla briareus, using in vitro solubilization. Mar. Pollut. Bull. 2003, 46, 1600–1608. [Google Scholar] [CrossRef]
- Malisch, R.; Kotz, A. Dioxins and PCBs in feed and food—Review from European perspective. Sci. Total Environ. 2014, 491, 2–10. [Google Scholar] [CrossRef]
- Larsen, J.C. Risk assessments of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in food. Mol. Nutr. Food Res. 2006, 50, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- van den Berg, M.; Kypke, K.; Kotz, A.; Tritscher, A.; Lee, S.Y.; Magulova, K.; Fiedler, H.; Malisch, R. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Arch. Toxicol. 2017, 91, 83–96. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. Efsa J. 2018, 16, e05333. [Google Scholar] [CrossRef]
- EC. Commission Regulation EU. 2017/644 Laying down Methods of Sampling and Analysis for the Control of Levels of Dioxins, Dioxin-Like PCBs and Non-Dioxin-Like PCBs in Certain Foodstuffs and Repealing REGULATION (EU) No 589/2014. Available online: http://data.europa.eu/eli/reg/2017/644/oj (accessed on 10 September 2020).
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Polycyclic Aromatic Hydrocarbons in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. Efsa J. 2008, 6, 724. [Google Scholar] [CrossRef]
- EC. Commission Regulation EU. 835/2011 Amending Regulation (EC) 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:En:PDF (accessed on 14 September 2022).
- Khazaali, A.; Kunzmann, A.; Bastami, K.D.; Baniamam, M. Baseline of polycyclic aromatic hydrocarbons in the surface sediment and sea cucumbers (Holothuria leucospilota and Stichopus hermanni) in the northern parts of Persian Gulf. Mar. Pollut. Bull. 2016, 110, 539–545. [Google Scholar] [CrossRef]
- EFSA. Panel on Contaminants in the Food Chain Risk to human health related to the presence of perfluoroalkyl substances in food. Efsa J. 2020, 18, e06223. [Google Scholar] [CrossRef]
- Martín, J.; Hidalgo, F.; García-Corcoles, M.T.; Ibáñez-Yuste, A.J.; Alonso, E.; Vilchez, J.L.; Zafra-Gómez, A. Bioaccumulation of perfluoroalkyl substances in marine echinoderms: Results of laboratory-scale experiments with Holothuria tubulosa Gmelin, 1791. Chemosphere 2019, 215, 261–271. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific support for preparing an EU position in the 48th Session of the Codex Committee on Pesticide Residues (CCPR). Efsa J. 2016, 14, e04571. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Saravi, S.S.; Yazdi, Z. Lindane residues in cultivated cucumber and in the most consumed fish in Caspian Sea (Iran). Toxicol. Ind. Health 2009, 25, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Lee, J.-J.; Kim, B.-S.; Choi, S.H. Whole-Body Microbiota of Sea Cucumber (Apostichopus japonicus) from South Korea for Improved Seafood Management. J. Microbiol. Biotechnol. 2017, 27, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Dang, H.; Wang, L.; Fu, S.; Ding, J. Comparison of whole genome sequences of three Bacillus cereus strains reveals the food safety risks of Apostichopus japonicus in China. Aquac. Rep. 2021, 20, 100649. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, H.; Chen, J.; Xie, H.; Du, J. Investigation of antibiotics in sea cucumbers: Occurrence, pollution characteristics, and human risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 32081–32087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, W.; Quan, W.; Jiang, J.; Qu, B. Occurrence and levels of nitrofuran metabolites in sea cucumber from Dalian, China. Food Addit. Contam. Part A 2016, 33, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Gao, W.; Jiao, J.; Wang, D.; Fan, M. Highly sensitive SERS detection of residual nitrofurantoin and 1-amino-hydantoin in aquatic products and feeds. Luminescence 2022, 37, 82–88. [Google Scholar] [CrossRef] [PubMed]
- EC. Commission Regulation EU. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2073&from=EN (accessed on 14 September 2022).
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Presence of microplastics and nanoplastics in food, with particular focus on seafood. Efsa J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Ryder, J.; Elvevoll, E.O.; Olsen, R.L. Microplastics in Fish and Shellfish—A Threat to Seafood Safety? J. Aquat. Food Prod. Technol. 2020, 29, 417–425. [Google Scholar] [CrossRef]
| Species | Water | Ash | Protein | Fat | Ref |
|---|---|---|---|---|---|
| Fresh sea cucumber | |||||
| Apostichopus japonicus | 90.7 ± 0.35 | 3.2 ± 0.4 | 3.4 ± 0.1 | 0.1 ± 0.1 | Rasyid et al. [23] |
| Cucumaria frondosa | 90.5 | 0.8 | 5.5 | 3.5 | Hossain et al. [24] |
| Holothuria poli | 81.2 ± 0.4 | 7.9 ± 0.9 | 8.7 ± 1.2 | 0.2 ± 0.0 | Rasyid et al. [23] |
| Holothuria scabra | 84.5–87.2 | 3.59–11.1 | 5.8–9.5 | 0.2–0.8 | Özer et al., Rasyid et al. [23,44] |
| Holothuria tubulosa | 81.4–86.5 | 4.1–6.1 | 8.0–10.2 | 1.6–1.9 | Künili & Çolakoğlu, Rasyid et al. [23,45] |
| Dried sea cucumber | |||||
| Actinopyga caerulea | 0.81 ± 0.03 | 28.4 ± 0.3 | 56.9 ± 0.4 | 10.1 ± 0.25 | Wen et al. [43] |
| Actinopyga mauritana | 11.6 ± 0.31 | 15.4 ± 0.2 | 63.3 ± 0.4 | 1.4 ± 0.02 | Wen et al. [43] |
| Bohadschia argus | 13.0 ± 0.26 | 17.7 ± 0.2 | 62.1 ± 0.4 | 1.1 ± 0.01 | Wen et al. [43] |
| Holothuria fuscogilva | 11.6 ± 0.28 | 26.4 ± 0.3 | 57.8 ± 0.4 | 0.3 ± 0.01 | Wen et al. [43] |
| Holothuria fuscopunctata | 7.0 ± 0.14 | 39.6 ± 0.2 | 50.1 ± 0.4 | 0.3 ± 0.01 | Wen et al. [43] |
| Holothuria polii | 22.0 ± 3.1 | 48.2 ± 1.1 | 37.0 ± 0.6 | 0.6 ± 0.1 | Rasyid et al. [23] |
| Holothuria scabra | 9.1 ± 0.3 | 5.8 ± 0.4 | 72.3 ± 0.6 | 2.0 ± 0.1 | Rasyid et al. [23] |
| Holothuria tubulosa | 16.2 ± 1.5 | 46.4 ± 0.5 | 44.6 ± 1.0 | 0.7 ± 0.1 | Rasyid et al. [23] |
| Stichopus herrmanni | 10.2 ± 0.32 | 37.9 ± 0.3 | 47.0 ± 0.4 | 0.8 ± 0.02 | Wen et al. [43] |
| Theleonata ananas | 15.1 ± 0.29 | 25.1 ± 0.3 | 55.2 ± 0.4 | 1.9 ± 0.01 | Wen et al. [43] |
| Theleonata anax | 1.2 ± 0.06 | 39.2 ± 0.3 | 40.7 ± 0.3 | 9.9 ± 0.27 | Wen et al. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvevoll, E.O.; James, D.; Toppe, J.; Gamarro, E.G.; Jensen, I.-J. Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers. Foods 2022, 11, 3992. https://doi.org/10.3390/foods11243992
Elvevoll EO, James D, Toppe J, Gamarro EG, Jensen I-J. Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers. Foods. 2022; 11(24):3992. https://doi.org/10.3390/foods11243992
Chicago/Turabian StyleElvevoll, Edel Oddny, David James, Jogeir Toppe, Esther Garrido Gamarro, and Ida-Johanne Jensen. 2022. "Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers" Foods 11, no. 24: 3992. https://doi.org/10.3390/foods11243992
APA StyleElvevoll, E. O., James, D., Toppe, J., Gamarro, E. G., & Jensen, I.-J. (2022). Food Safety Risks Posed by Heavy Metals and Persistent Organic Pollutants (POPs) related to Consumption of Sea Cucumbers. Foods, 11(24), 3992. https://doi.org/10.3390/foods11243992






