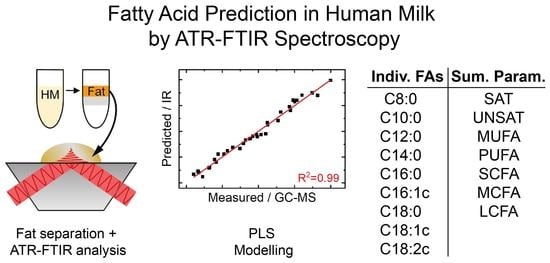
Solvent-Free Lipid Separation and Attenuated Total Reflectance Infrared Spectroscopy for Fast and Green Fatty Acid Profiling of Human Milk
Abstract
1. Introduction
2. Material and Methods
2.1. Human Milk Samples
2.2. Macronutrient Analysis
2.3. Solvent-Free Lipid Separation
2.4. ATR-FTIR Measurements
2.5. GC-MS Reference Measurements
2.6. Data Analysis
3. Results and Discussion
3.1. Macronutrient Analysis
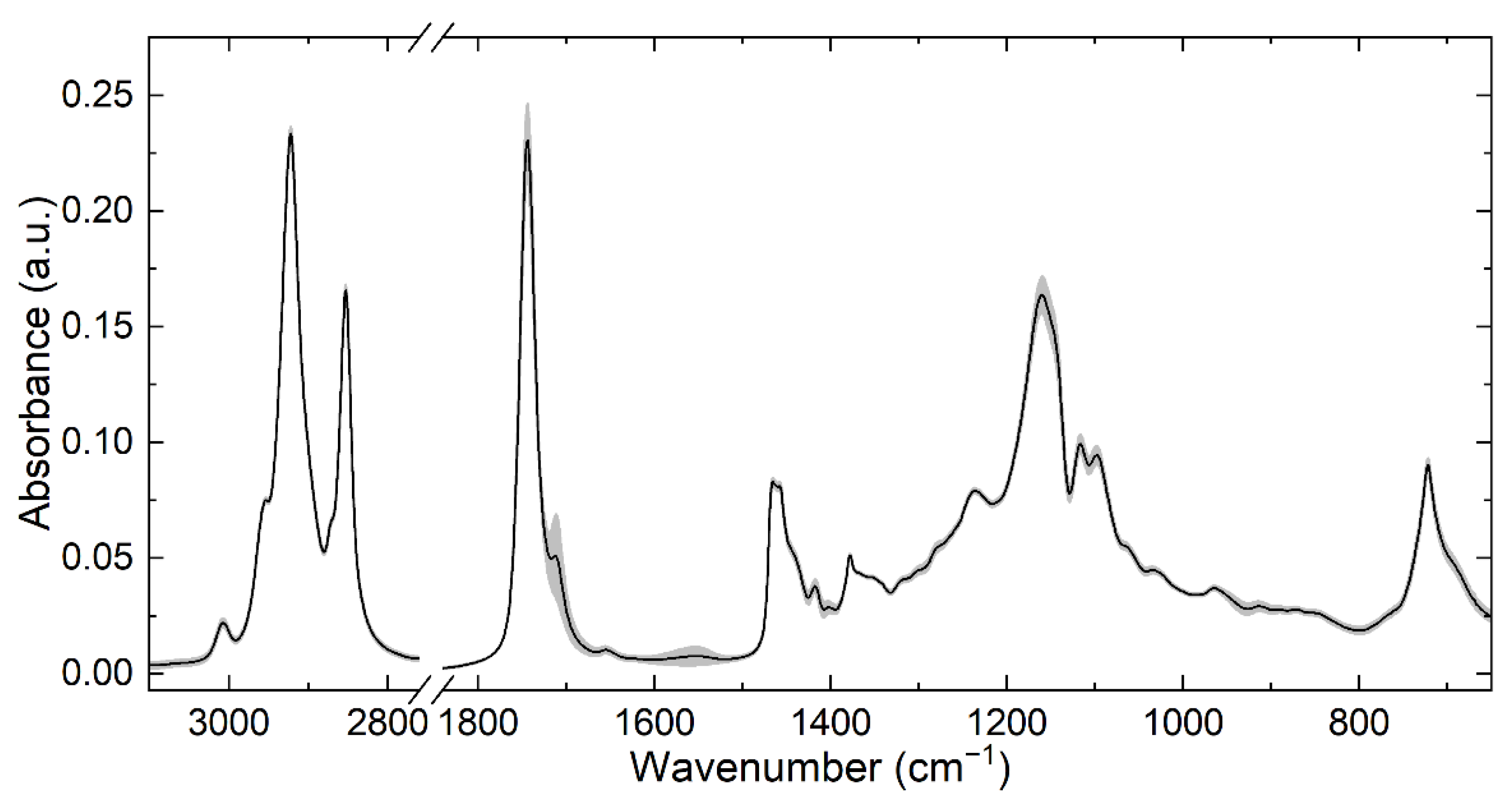
3.2. ATR-FTIR Spectra of Separated HM Fat Fraction
3.3. Mid-IR-Based Determination of the Fatty Acid Profile
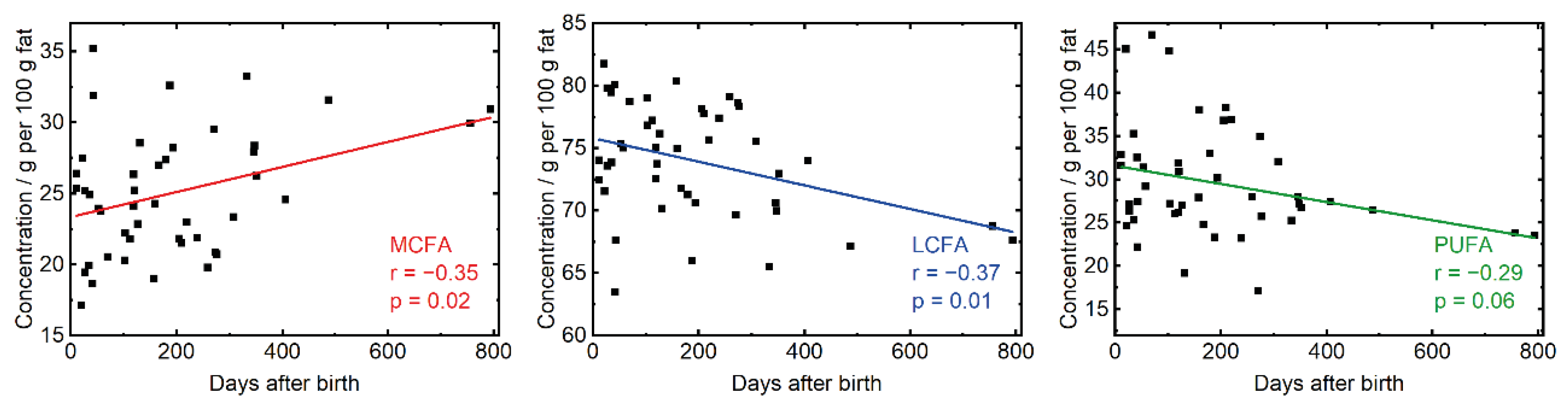
3.4. Investigation of Clinically Relevant Parameters
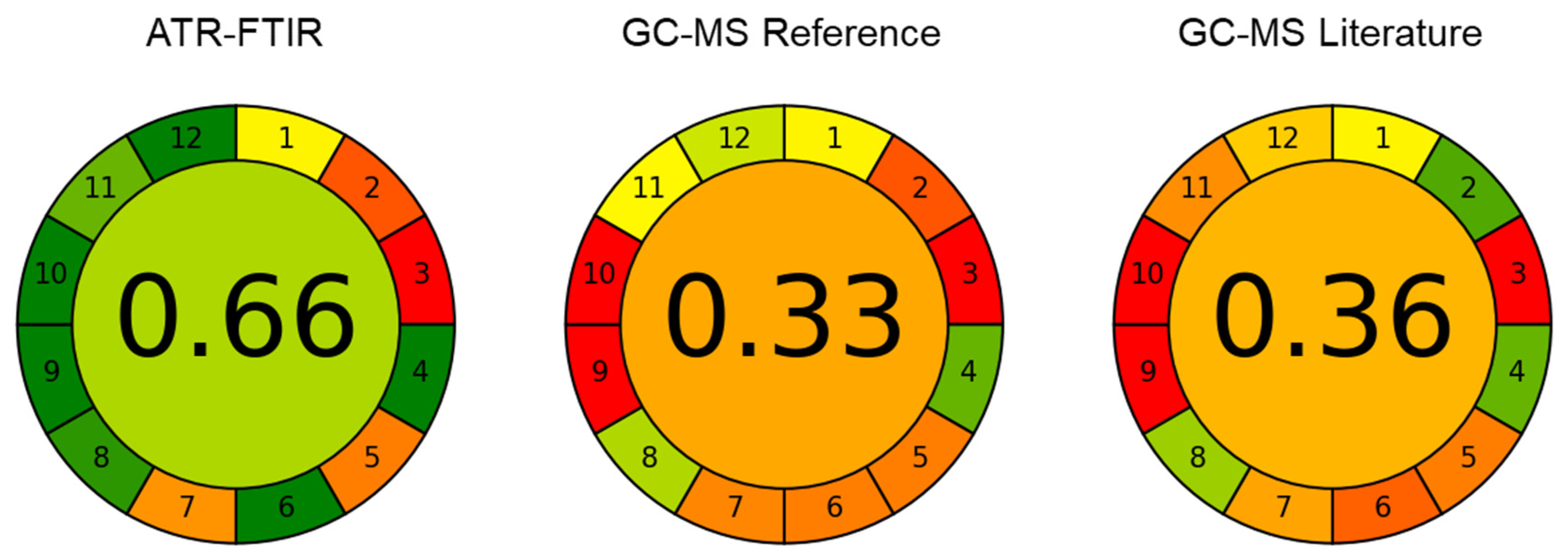
3.5. Greenness Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice. Eur. J. Pediatr. 2011, 170, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- WHO. Resolution WHA54.2, Infant and young child nutrition. In Proceedings of the Fifty-Fourth World Health Assembly, Geneva, Switzerland, 14–22 May 2001. [Google Scholar]
- Chung, M.-Y. Factors Affecting Human Milk Composition. Pediatr. Neonatol. 2014, 55, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G.; Bitman, J.; Carlson, S.E.; Couch, S.C.; Hamosh, M.; Newburg, D.S. Milk Lipids: A. Human Milk Lipids. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 495–542. [Google Scholar]
- de la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero-Marín, M.; Segura, M.T.; Campoy, C.; et al. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Sanders, T.A.B.; Reddy, S. The influence of a vegetarian diet on the fatty acid composition of human milk and the essential fatty acid status of the infant. J. Pediatr. 1992, 120, S71–S77. [Google Scholar] [CrossRef]
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition of human milk in Europe and Africa. J. Pediatr. 1992, 120, S62–S70. [Google Scholar] [CrossRef]
- Boersma, E.R.; Offringa, P.J.; Muskiet, F.A.; Chase, W.M.; Simmons, I.J. Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: An international comparative study. Am. J. Clin. Nutr. 1991, 53, 1197–1204. [Google Scholar] [CrossRef]
- Floris, L.M.; Stahl, B.; Abrahamse-Berkeveld, M.; Teller, I.C. Human milk fatty acid profile across lactational stages after term and preterm delivery: A pooled data analysis. Prostaglandins Leukot. Essent. Fat. Acids 2020, 156, 102023. [Google Scholar] [CrossRef]
- Poulimeneas, D.; Bathrellou, E.; Antonogeorgos, G.; Mamalaki, E.; Kouvari, M.; Kuligowski, J.; Gormaz, M.; Panagiotakos, D.B.; Yannakoulia, M. Feeding the preterm infant: An overview of the evidence. Int. J. Food Sci. Nutr. 2021, 72, 4–13. [Google Scholar] [CrossRef]
- Colaizy, T.T. Effects of milk banking procedures on nutritional and bioactive components of donor human milk. Semin. Perinatol. 2021, 45, 151382. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Miller, J.; Middleton, P.F.; Huang, Y.-C.; McPhee, A.J.; Gibson, R.A. Changes to breast milk fatty acid composition during storage, handling and processing: A systematic review. Prostaglandins Leukot. Essent. Fat. Acids 2019, 146, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ten-Doménech, I.; Ramos-Garcia, V.; Moreno-Torres, M.; Parra-Llorca, A.; Gormaz, M.; Vento, M.; Kuligowski, J.; Quintás, G. The effect of Holder pasteurization on the lipid and metabolite composition of human milk. Food Chem. 2022, 384, 132581. [Google Scholar] [CrossRef]
- Cruz-Hernandez, C.; Goeuriot, S.; Giuffrida, F.; Thakkar, S.K.; Destaillats, F. Direct quantification of fatty acids in human milk by gas chromatography. J. Chromatogr. A 2013, 1284, 174–179. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Brandstetter, M.; Lendl, B. Quantum cascade lasers (QCLs) in biomedical spectroscopy. Chem. Soc. Rev. 2017, 46, 5903–5924. [Google Scholar] [CrossRef]
- Fusch, G.; Rochow, N.; Choi, A.; Fusch, S.; Poeschl, S.; Ubah, A.O.; Lee, S.-Y.; Raja, P.; Fusch, C. Rapid measurement of macronutrients in breast milk: How reliable are infrared milk analyzers? Clin. Nutr. 2015, 34, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ramer, G.; Lendl, B. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy. In Encyclopedia of Analytical Chemistry; Meyers, R., Meyers, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited review: Mid-infrared spectroscopy as phenotyping tool for milk traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef]
- Ferrand-Calmels, M.; Palhière, I.; Brochard, M.; Leray, O.; Astruc, J.M.; Aurel, M.R.; Barbey, S.; Bouvier, F.; Brunschwig, P.; Caillat, H.; et al. Prediction of fatty acid profiles in cow, ewe, and goat milk by mid-infrared spectrometry. J. Dairy Sci. 2014, 97, 17–35. [Google Scholar] [CrossRef]
- Eskildsen, C.; Rasmussen, M.; Engelsen, S.; Larsen, L.; Poulsen, N.A.; Skov, T. Quantification of individual fatty acids in bovine milk by infrared spectroscopy and chemometrics: Understanding predictions of highly collinear reference variables. J. Dairy Sci. 2014, 97, 7940–7951. [Google Scholar] [CrossRef]
- Akhgar, C.K.; Nürnberger, V.; Nadvornik, M.; Velik, M.; Schwaighofer, A.; Rosenberg, E.; Lendl, B. Fatty Acid Prediction in Bovine Milk by Attenuated Total Reflection Infrared Spectroscopy after Solvent-Free Lipid Separation. Foods 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.; Juárez, M.; Fuente, M.A. Validation of a Rapid Milk Fat Separation Method to Determine the Fatty Acid Profile by Gas Chromatography. J. Dairy Sci. 2005, 88, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Akhgar, C.K.; Nürnberger, V.; Nadvornik, M.; Ramos-Garcia, V.; Ten-Doménech, I.; Kuligowski, J.; Schwaighofer, A.; Rosenberg, E.; Lendl, B. Fatty Acid Determination in Human Milk Using Attenuated Total Reflection Infrared Spectroscopy and Solvent-Free Lipid Separation. Appl. Spectrosc. 2022, 76, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Feng, S.; Lock, A.L.; Garnsworthy, P.C. Technical Note: A Rapid Lipid Separation Method for Determining Fatty Acid Composition of Milk. J. Dairy Sci. 2004, 87, 3785–3788. [Google Scholar] [CrossRef] [PubMed]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Kaylegian, K.E.; Lynch, J.M.; Fleming, J.R.; Barbano, D. Influence of fatty acid chain length and unsaturation on mid-infrared milk analysis. J. Dairy Sci. 2009, 92, 2485–2501. [Google Scholar] [CrossRef]
- De Ruig, W.G.; Dijkstra, G. Characterization and Identification of Triglycerides by Infrared Spectroscopy. Fette Seifen Anstrichm. 1975, 77, 211–216. [Google Scholar] [CrossRef]
- Kohler, A.; Afseth, N.; Jørgensen, K.; Randby, Å.; Martens, H. Quality Analysis of Milk by Vibrational Spectroscopy. In Handbook of Vibrational Spectroscopy; Jim, C., Peter, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.-F.; Genot, C. Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Ismail, A.A.; van de Voort, F.R.; Emo, G.; Sedman, J. Rapid quantitative determination of free fatty acids in fats and oils by fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1993, 70, 335–341. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Permanyer, M.; Castellote, A.I.; López-Sabater, M.C. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- Delgado, F.J.; Cava, R.; Delgado, J.; Ramírez, R. Tocopherols, fatty acids and cytokines content of holder pasteurised and high-pressure processed human milk. Dairy Sci. Technol. 2014, 94, 145–156. [Google Scholar] [CrossRef]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.A.; Alashmali, S.M.; Hopperton, K.E.; Unger, S.; Pouliot, Y.; Doyen, A.; O’Connor, D.L.; Bazinet, R.P. Oxylipin concentration, but not fatty acid composition, is altered in human donor milk pasteurised using both thermal and non-thermal techniques. Br. J. Nutr. 2019, 122, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]

| g/100 g Fat | ||||||||
|---|---|---|---|---|---|---|---|---|
| Training Set (n = 35) | Validation Set (n = 15) | |||||||
| Fatty Acid | LVs | Range | RMSEC | R2 | RMSECV | R2CV | RMSEP | R2P |
| SAT | 6 | 21–40 | 0.42 | 0.99 | 0.68 | 0.98 | 0.54 | 0.97 |
| MONO | 5 | 29–51 | 1.1 | 0.96 | 1.4 | 0.94 | 1.3 | 0.94 |
| PUFA | 4 | 16–47 | 1.3 | 0.96 | 1.5 | 0.95 | 1.4 | 0.93 |
| UNSAT | 6 | 60–79 | 0.42 | 0.99 | 0.68 | 0.98 | 0.54 | 0.97 |
| SCFA | 4 | 0.40–1.7 | 0.13 | 0.78 | 0.18 | 0.62 | 0.15 | 0.25 |
| MCFA | 4 | 17–36 | 0.99 | 0.96 | 1.3 | 0.93 | 0.65 | 0.94 |
| LCFA | 4 | 63–82 | 0.87 | 0.97 | 1.1 | 0.95 | 0.7 | 0.94 |
| C8:0 | 2 | 0.0-0.15 | 0.022 | 0.59 | 0.026 | 0.52 | 0.017 | 0.29 |
| C10:0 | 4 | 0.40–1.5 | 0.12 | 0.79 | 0.16 | 0.63 | 0.15 | 0.2 |
| C12:0 | 6 | 1.1–7.2 | 0.18 | 0.99 | 0.3 | 0.96 | 0.19 | 0.96 |
| C14:0 | 6 | 1.5–7.9 | 0.26 | 0.98 | 0.44 | 0.94 | 0.63 | 0.71 |
| C16:0 | 6 | 12–22 | 0.78 | 0.92 | 1.1 | 0.84 | 1.1 | 0.88 |
| C16:1cis | 4 | 0.0–1.2 | 0.29 | 0.32 | 0.42 | 0.017 | 0.31 | 0.19 |
| C18:0 | 4 | 1.8–5.9 | 0.41 | 0.77 | 0.55 | 0.6 | 0.47 | 0.75 |
| C18:1cis | 6 | 28–51 | 1.1 | 0.96 | 1.6 | 0.92 | 1.4 | 0.92 |
| C18:2cis | 4 | 16–47 | 1.2 | 0.97 | 1.5 | 0.95 | 1.5 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhgar, C.K.; Ramos-Garcia, V.; Nürnberger, V.; Moreno-Giménez, A.; Kuligowski, J.; Rosenberg, E.; Schwaighofer, A.; Lendl, B. Solvent-Free Lipid Separation and Attenuated Total Reflectance Infrared Spectroscopy for Fast and Green Fatty Acid Profiling of Human Milk. Foods 2022, 11, 3906. https://doi.org/10.3390/foods11233906
Akhgar CK, Ramos-Garcia V, Nürnberger V, Moreno-Giménez A, Kuligowski J, Rosenberg E, Schwaighofer A, Lendl B. Solvent-Free Lipid Separation and Attenuated Total Reflectance Infrared Spectroscopy for Fast and Green Fatty Acid Profiling of Human Milk. Foods. 2022; 11(23):3906. https://doi.org/10.3390/foods11233906
Chicago/Turabian StyleAkhgar, Christopher Karim, Victoria Ramos-Garcia, Vanessa Nürnberger, Alba Moreno-Giménez, Julia Kuligowski, Erwin Rosenberg, Andreas Schwaighofer, and Bernhard Lendl. 2022. "Solvent-Free Lipid Separation and Attenuated Total Reflectance Infrared Spectroscopy for Fast and Green Fatty Acid Profiling of Human Milk" Foods 11, no. 23: 3906. https://doi.org/10.3390/foods11233906
APA StyleAkhgar, C. K., Ramos-Garcia, V., Nürnberger, V., Moreno-Giménez, A., Kuligowski, J., Rosenberg, E., Schwaighofer, A., & Lendl, B. (2022). Solvent-Free Lipid Separation and Attenuated Total Reflectance Infrared Spectroscopy for Fast and Green Fatty Acid Profiling of Human Milk. Foods, 11(23), 3906. https://doi.org/10.3390/foods11233906