Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. MDDM Manufacturing
2.2. Raw Material (By-Product) and MDDM Quality Parameters
2.2.1. Moisture
2.2.2. Water Activity (aw)
2.2.3. Acidity Index
2.2.4. Oxidative Stability (TBARS)
2.2.5. Total Volatile Basic Nitrogen (TVB-N)
2.2.6. Fatty Acid Profile
2.3. Antioxidant Capacity Evaluation of of Different Antioxidant Compounds
2.3.1. Antioxidant Capacity DPPH
2.3.2. Total Polyphenol Content
2.4. Accelerated Shelf-Life Study -ASLD-
- k: rate constant (frequency of collisions resulting in a reaction)
- A: Pre-exponential factor (an empirical relationship between temperature and rate coefficient)
- Ea: activation energy for the reaction
- R: universal gas constant (8.31 J⋅K−1⋅mol−1)
- T: absolute temperature (Kelvin)
2.5. Effectiveness of the Antioxidant Compound
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity Evaluation of Different Antioxidant Compounds
3.2. Raw Material (By-Product) and MDDM Quality Parameters
3.3. Accelerated Shelf-Life Study -ASLD-
3.4. Effectiveness of the Antioxidant Compound
3.5. Comparative Study in Fatty Acids Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). FAOSTAT. Available online: https://www.fao.org/faostat/es/#home (accessed on 3 February 2022).
- Prato, E.; Biandolino, F. The Contribution of Fish to the Mediterranean Diet. In The Mediterranean Diet: An Evidence-Based Approach; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 165–174. ISBN 9780124079427. [Google Scholar]
- Food and Agriculture Organization (FAO). El Estado Mundial de La Pesca y La Acuicultura 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132756-2. [Google Scholar]
- Asociación Empresarial de Acuicultura en España La Acuicultura En España 2020. 2020. Available online: https://apromar.es/wp-content/uploads/2021/12/La-Acuicultura-en-Espana-2020.pdf (accessed on 3 February 2022).
- Huss, H.H. El Pecado Fresco: Su Calidad y Cambios de Calidad; Manual de capacitaciôn preparado por el Programa de Capacitaciôn FAO/DANIDA en Tecnologîa Pesquera y Control de Calidad; FAO: Rome, Italy, 1988; p. 29. [Google Scholar]
- Prato, E.; Biandolino, F. Total Lipid Content and Fatty Acid Composition of Commercially Important Fish Species from the Mediterranean, Mar Grande Sea. Food Chem. 2012, 131, 1233–1239. [Google Scholar] [CrossRef]
- von Schacky, C.; Weber, P.C. Metabolism and Effects on Platelet Function of the Purified Eicosapentaenoic and Docosahexaenoic Acids in Humans. J. Clin. Investig. 1985, 76, 2446–2450. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive Activity of Fish Protein Hydrolysates and Its Peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- AECOC SHOPPERVIEW; MAPAMA. Análisis Del No Consumidor Joven de Productos Del Mar. 2016. Available online: https://www.mapa.gob.es/es/pesca/temas/mercados-economia-pesquera/analisisdeljovennoconsumidordeproductospesqueros_tcm30-291634.pdf (accessed on 3 February 2022).
- EUMOFA. Las Noticias Destacadas Del Mes. No 8/2021. 2021. Available online: https://www.eumofa.eu/es/market-analysis (accessed on 22 February 2022).
- Seijas, J. Establecimiento de Una Línea de Procesado de Subproductos de Pescado Para La Elaboración de Productos de Valor Añadido Tipo Hamburguesas. Master’s Thesis, Universidad de Cádiz, Cádiz, Spain, 2017. [Google Scholar]
- AZTI. Guía de Valorización de Subproductos de La Acuicultura. 2018. Available online: https://www.azti.es/wp-content/uploads/2018/12/AZTI_guia_VALACUI101218online.pdf (accessed on 25 February 2022).
- FCH. Alternativas y Usos Para Los Residuos de Pescado. Available online: https://fch.cl/noticianoticia-destacadanoticia-antigua/alternativas-usos-los-residuos-pescado/ (accessed on 23 May 2022).
- Stori, F. Proposta de Reaproveitamento Dos Resíduos Das Indústrias de Beneficiamento de Pescado de Santa Catarina Com Base Num Sistema Gerencial de Bolsa de Resíduos. In Responsabilidade Social das Empresas. A Contribuiçao Das Universidades; Instituto Ethos de Empresas e Responsabilidade Social: Sao Paolo, Brazil, 2002; ISBN 8585663669. [Google Scholar]
- Nirmal, N.P.; Santivarangkna, C.; Benjakul, S.; Maqsood, S. Fish Protein Hydrolysates as a Health-Promoting Ingredient—Recent Update. Nutr. Rev. 2022, 80, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive Peptides of Plant Origin: Distribution, Functionality, and Evidence of Benefits in Food and Health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Adeleke, R.O.; Odedeji, J.O. Acceptability Studies on Bread Fortified with Tilapia Fish Flour. Pak. J. Nutr. 2010, 9, 531–534. [Google Scholar] [CrossRef]
- Marengoni, N.G.; Soares, M.; Pozza, S.; Castilha, L. Caracterização Microbiológica, Sensorial e Centesimal de Fishburgers de Carne de Tilápia Mecanicamente Separada. Rev. Bras. Saúde Produção Anim. 2009, 10, 168–176. [Google Scholar]
- de Oliveira, I.S.; Lourenço, L.d.F.H.; Sousa, C.L.; Peixoto Joele, M.R.; Ribeiro, S.C.A. Composition of MSM from Brazilian Catfish and Technological Properties of Fish Flour. Food Control 2015, 50, 38–44. [Google Scholar] [CrossRef]
- Desai, A.; Brennan, M.A.; Brennan, C.S. The Effect of Semolina Replacement with Protein Powder from Fish (Pseudophycis Bachus) on the Physicochemical Characteristics of Pasta. LWT 2018, 89, 52–57. [Google Scholar] [CrossRef]
- Calanche, J.; Beltrán, H.; Marquina, P.; Roncalés, P.; Beltrán, J.A. Eating Fish in Another Way: Development of Functional Pasta with Added Concentrates of Farmed Sea Bass (Dicentrarchus Labrax). Cereal Chem. 2019, 96, 856–865. [Google Scholar] [CrossRef]
- Phongthai, S.; D’Amico, S.; Schoenlechner, R.; Homthawornchoo, W.; Rawdkuen, S. Effects of Protein Enrichment on the Properties of Rice Flour Based Gluten-Free Pasta. LWT 2017, 80, 378–385. [Google Scholar] [CrossRef]
- Bakare, A.H.; Adeola, A.A.; Otesile, I.; Obadina, A.O.; Afolabi, W.A.; Adegunwa, M.O.; Akerele, R.A.; Bamgbose, O.O.; Alamu, E.O. Nutritional, Texture, and Sensory Properties of Composite Biscuits Produced from Breadfruit and Wheat Flours Enriched with Edible Fish Meal. Food Sci. Nutr. 2020, 8, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Bonelli, A.; Parisi, G. Effect of Mechanical Separation Process on Lipid Oxidation in European Aquacultured Sea Bass, Gilthead Sea Bream, and Rainbow Trout Products. Food Control 2016, 67, 75–81. [Google Scholar] [CrossRef]
- Bernavent, M. Efecto Del Tiempo de Conservación y de La Temperatura En Los Parámetros Oxidativos de Carne de Potro. Master′s Thesis, Universidad Pública de Navarra, Pamplona, Spain, 2016. [Google Scholar]
- Méndez, C.; Masson, L.; Jiménez, P. Estabilización de Aceite de Pescado Por Medio de Antioxidantes Naturales. Aceites Y Grasas 2010, 3, 270–278. [Google Scholar]
- Mira, M. Uso de Extractos de Romero y Otras Fuentes Vegetales Como Antioxidantes Tecnológicos En Aceites Alimentarios. Estudio Comparativo de Eficacia Frente a Antioxidantes Sintéticos. Relación Estructura Actividad. Ph.D. Thesis, Universidad Católica de Murcia, Murcio, Spain, 2019. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the Re-Evaluation of Butylated Hydroxytoluene BHT (E 321) as a Food Additive. EFSA J. 2012, 10, 2588. [Google Scholar] [CrossRef]
- Ramachandra, C.T.; Rao, P.S. Shelf-Life and Colour Change Kinetics of Aloe Vera Gel Powder under Accelerated Storage in Three Different Packaging Materials. J. Food Sci. Technol. 2013, 50, 747–754. [Google Scholar] [CrossRef]
- Rao, Q.; Rocca-Smith, J.R.; Schoenfuss, T.C.; Labuza, T.P. Accelerated Shelf-Life Testing of Quality Loss for a Commercial Hydrolysed Hen Egg White Powder. Food Chem. 2012, 135, 464–472. [Google Scholar] [CrossRef]
- Amodio, M.L.; Derossi, A.; Mastrandrea, L.; Colelli, G. A Study of the Estimated Shelf Life of Fresh Rocket Using a Non-Linear Model. J. Food Eng. 2015, 150, 19–28. [Google Scholar] [CrossRef]
- Guzmán, L.E.; Martínez, D.P.; Martínez, D.E.; Acevedo, D.; Montero, P.M. Estudio de la vida útil de jamón de cerdo mediante pruebas aceleradas. Interciencia 2016, 41, 488–491. [Google Scholar]
- Sothornvit, R.; Kiatchanapaibul, P. Quality and Shelf-Life of Washed Fresh-Cut Asparagus in Modified Atmosphere Packaging. LWT Food Sci. Technol. 2009, 42, 1484–1490. [Google Scholar] [CrossRef]
- Aínsa, A. Vida Útil y Evolución Del Perfil de Ácidos Grasos En Pastas Alimenticias Suplementadas Con Concentrado de Pescado. Master′s Thesis, University of Zaragoza, Zaragoza, Spain, 2019. [Google Scholar]
- Copant NTE INEN 0038: Grasas y Aceites Comestibles Determinación de La Acidez. Available online: https://www.normalizacion.gob.ec/buzon/normas/38.pdf (accessed on 25 March 2022).
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Alpha.-Tocopherol Contents and Lipid Oxidation in Pork Muscle and Adipose Tissue during Storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EC) No 2074/2005 of 5 December 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2074&qid=1668511847677&from=ES (accessed on 24 March 2022).
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Lettuce and Chicory Byproducts as a Source of Antioxidant Phenolic Extracts. J. Agric. Food Chem. 2004, 52, 5109–5116. [Google Scholar] [CrossRef]
- Ordoñez, E.; Leon-Arevalo, A.; Rivera-Rojas, H.; Vargas, E. Quantification of Total Polyphenols and Antioxidant Capacity in Skins and Seeds from Cacao (Theobroma Cacao L.), Tuna (Opuntia Ficus Indica Mill), Grape (Vitis Vinífera) and Uvilla (Pourouma Cecropiifolia). Sci. Agropecu. 2019, 10, 175–183. [Google Scholar] [CrossRef]
- García Baldizón, C.; Molina Córdoba, M.E. Estimación De La Vida Útil De Una Mayonesa Mediante Pruebas Aceleradas. Rev. Ing. 2008, 18, 57–64. [Google Scholar] [CrossRef][Green Version]
- Cao, J.; Li, H.; Xia, X.; Zou, X.-G.; Li, J.; Zhu, X.-M.; Deng, Z.-Y. Effect of Fatty Acid and Tocopherol on Oxidative Stability of Vegetable Oils with Limited Air. Int. J. Food. Prop. 2015, 18, 808–820. [Google Scholar] [CrossRef]
- Callisaya, J.C.; Alvarado, J.A. Oxidative Stability in Almonds of Bertholletia Excelsa; Accelerated Deterioration of the Dry Fruits under Thermal Conditions. Rev. Boliv. Química 2016, 33, 62–71. [Google Scholar]
- Castro, P.L. Estudio de La Calidad y Vida Útil de Doradas y Lubinas Criadas En Las Islas Canarias Influencia de La Sustitución Del Aceite de Pescasdo Por Aceite de Lino En La Dieta. Doctoral Thesis, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2011. [Google Scholar]
- Wang, Y.-Z.; Fu, S.-G.; Wang, S.-Y.; Yang, D.-J.; Wu, Y.-H.S.; Chen, Y.-C. Effects of a Natural Antioxidant, Polyphenol-Rich Rosemary (Rosmarinus Officinalis L.) Extract, on Lipid Stability of Plant-Derived Omega-3 Fatty-Acid Rich Oil. LWT Food Sci. Technol. 2018, 89, 210–216. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of Biological Molecules. The Antioxidant Activity of Vitamin E and Related Chain-Breaking Phenolic Antioxidants in Vitro. J. Am. Chem. Soc 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- BEDCA. BEDCA, Base Espñola de Composición de Alimentos. Available online: https://www.bedca.net/bdpub/index.php (accessed on 12 March 2022).
- Villanueva, J.C. Determinación de La Influencia de La Oxidación Lipídica En Los Procesos de Elaboración de Pescado Congelado, Salado y Ahumado. Bachelor’s Thesis, Universidad Nacional de San Agustín, Arequipa, Perú, 2015. [Google Scholar]
- Badui, S. Química de Los Alimentos, 4th ed.; Quintanar, E., Ed.; Pearson Educación: Naucalpan de Juárez, Mexico, 2006. [Google Scholar]
- Tribuzi Estudio de La Efectividad de Agua Electrolizada Ácida En La Vida Útil de Lubina Fresca. Master′s Thesis, Universitat Politècnica de Valencia, Valencia, Spain, 2011.
- Ucar, Y.; Ozogul, Y.; Ozogul, F.; Durmus, M.; Kösker, A.R.; Kuley Boga, E. The Impact of Different Levels of Nisin as a Biopreservative Agent on the Chemical, Sensory and Microbiological Quality of Vacuum-Packed Sea Bass (Dicentrarchus Labrax) Fillets Stored at 4 ± 2 °C. Grasas Aceites 2021, 72, e401. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-Life Extension of Refrigerated Seabass Slices under Modified Atmosphere Packaging. J. Sci. Food Agric. 2002, 82, 873–880. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EC) No 1022/2008 of 17 October 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1022&qid=1668511928214&from=ES (accessed on 25 March 2022).
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Zhou, J.; Barba, F.J.; Lorenzo, J.M. Nutritional Characterization of Sea Bass Processing By-Products. Biomolecules 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Baki, B.; Gonener, S.; Kaya, D. Comparison of Food, Amino Acid and Fatty Acid Compositions of Wild and Cultivated Sea Bass (Dicentrarchus Labrax L.,1758). Turk. J. Fish Aquat. Sci. 2015, 15, 175–179. [Google Scholar] [CrossRef]
- Grigorakis, K.; Alexis, M.N.; Anthony Taylor, K.D.; Hole, M. Comparison of Wild and Cultured Gilthead Sea Bream (Sparus Aurata); Composition, Appearance and Seasonal Variations. Int. J. Food Sci. Technol. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Rombenso, A.N.; Trushenski, J.T.; Jirsa, D.; Drawbridge, M. Successful Fish Oil Sparing in White Seabass Feeds Using Saturated Fatty Acid-Rich Soybean Oil and 22:6n-3 (DHA) Supplementation. Aquaculture 2015, 448, 176–185. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Torres, A.; Guerra, M.; Rosquete, Y. Estimación de la vida útil de una fórmula dietética en función de la disminución de lisina disponible. Ciência Tecnol. Aliment. 2001, 21, 129–133. [Google Scholar] [CrossRef]
- Wada, S.; Fang, X. The Synergistic Antioxidant Effect of Rosemary Extract and α-Tocopherol in Sardine Oil Model System and Frozen-Crushed Fish Meat. J. Food Process. Preserv. 1992, 16, 263–274. [Google Scholar] [CrossRef]
- González-Baceiro, J.; Moreno, V.R.; del Monte, A. Las Lipasas: Enzimas Con Potencial Para El Desarrollo de Biocatalizadores Inmovilizados Por Adsorción 45 Interfacial. Rev. Colomb. Biotecnol. 2010, 12, 124–140. [Google Scholar]
- Veselý, V.; Chudožilov, L.K. Sur Les Acides Gadoléique et Sélacholéique Synthétiques. Collect. Czechoslov. Chem. Commun. 1930, 2, 95–107. [Google Scholar] [CrossRef]
- Stéphan, G.; Guillaume, J.; Lamour, F. Lipid Peroxidation in Turbot (Scophthalmus Maximus) Tissue: Effect of Dietary Vitamin E and Dietary n − 6 or n − 3 Polyunsaturated Fatty Acids. Aquaculture 1995, 130, 251–268. [Google Scholar] [CrossRef]
- Pirini, M.; Gatta, P.P.; Testi, S.; Trigari, G.; Monetti, P.G. Effect of Refrigerated Storage on Muscle Lipid Quality of Sea Bass (Dicentrarchus Labrax) Fed on Diets Containing Different Levels of Vitamin E. Food Chem. 2000, 68, 289–293. [Google Scholar] [CrossRef]
- Scaife, J.R.; Onibi, G.E.; Murray, I.; Fletcher, T.C.; Houlihan, D.F. Influence of Alpha-Tocopherol Acetate on the Short-and Long-Term Storage Properties of Fillets from Atlantic Salmon Salmo Salar Fed a High Lipid Diet. Aquac. Nutr. 2000, 6, 65–71. [Google Scholar] [CrossRef]
- Ruff, N.; FitzGerald, R.D.; Cross, T.; Hamre, K.; Kerry, J.P. Assessment of Dietary Vitamins E and C on Market-Size Turbot (Scophthalmus Maximus) Fillet Quality. Aquac. Nutr. 2003, 9, 91–103. [Google Scholar] [CrossRef]
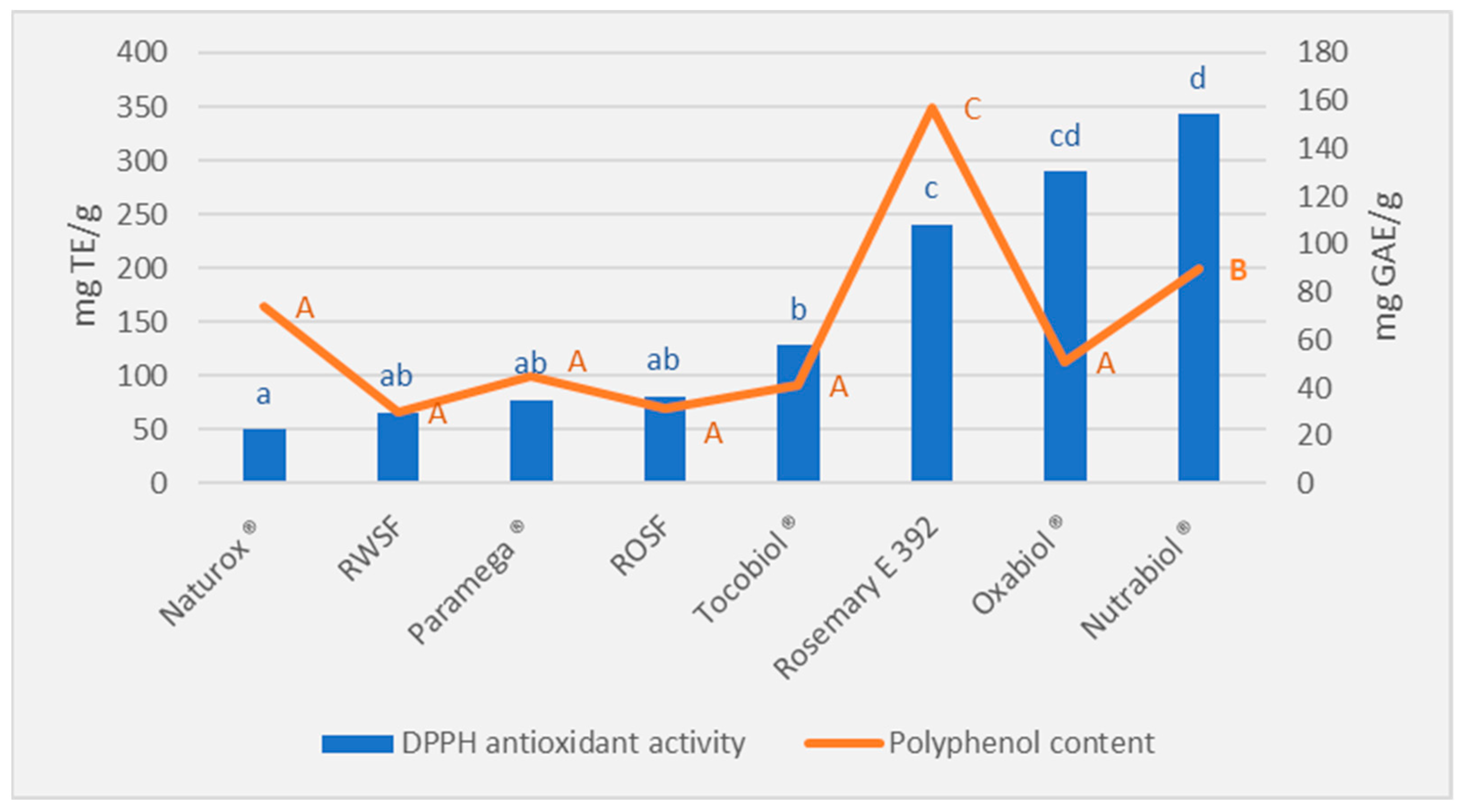


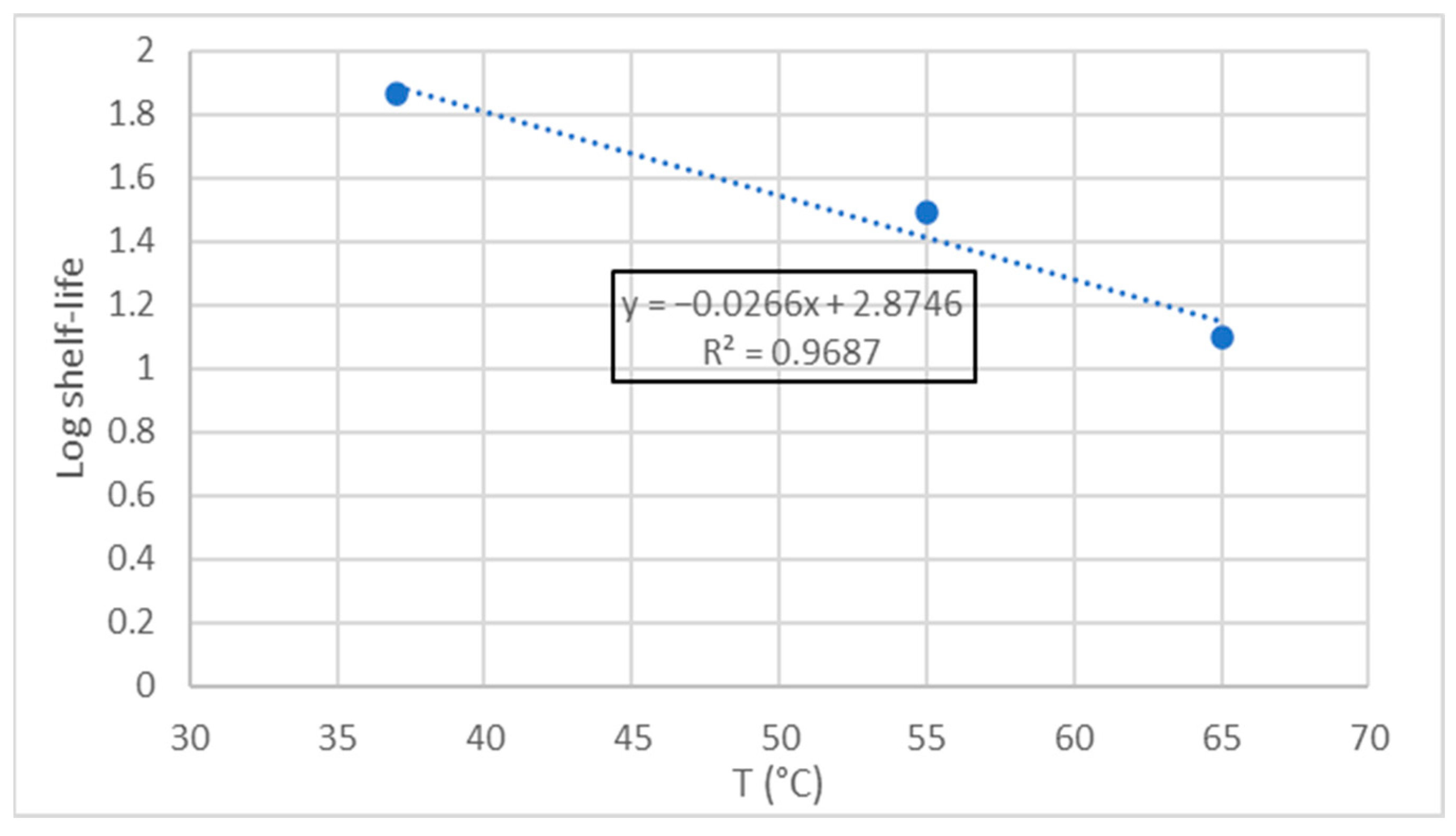
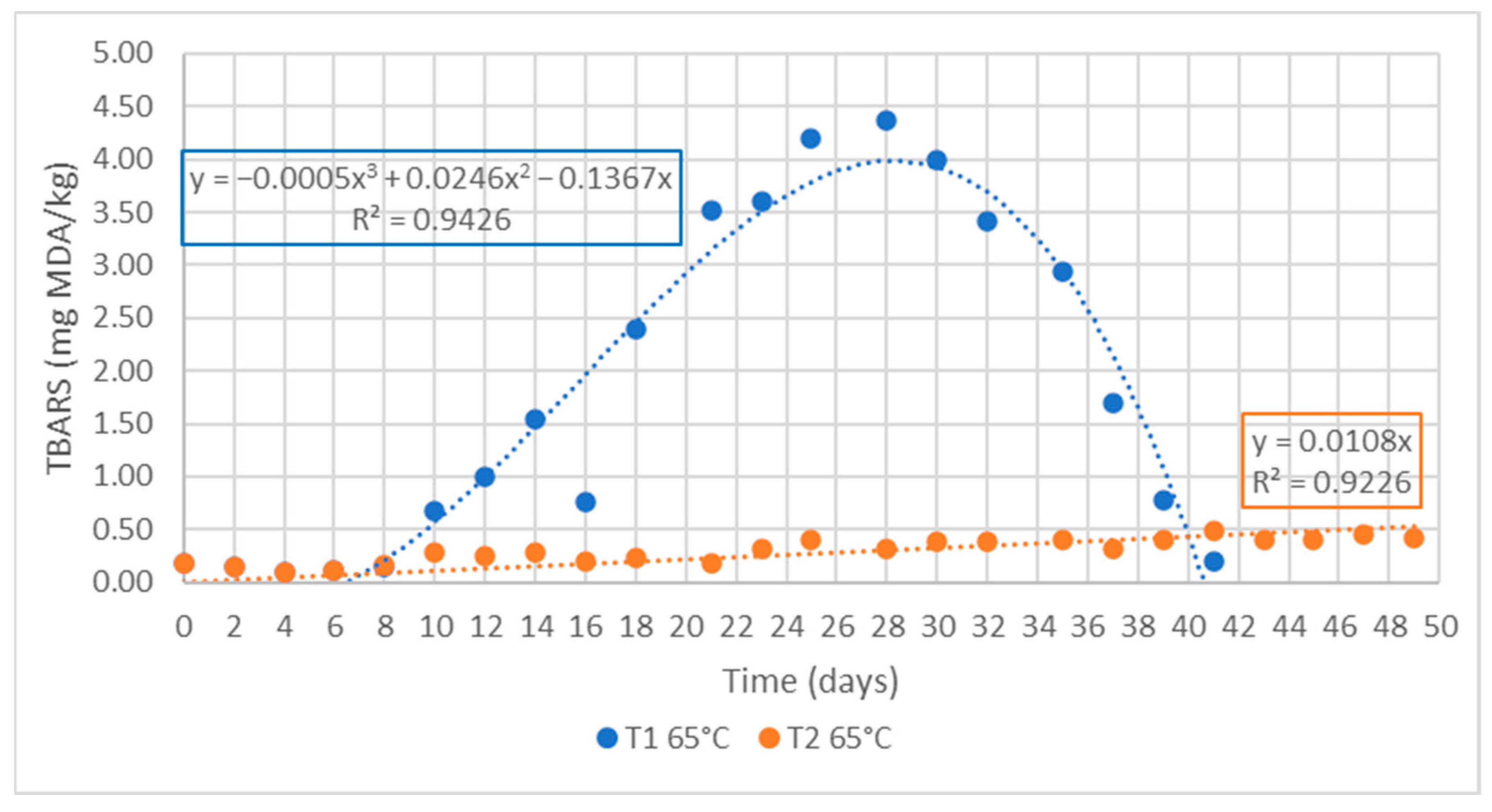
| Antioxidant | Composition |
|---|---|
| Rosemary E 392 (Marbys, Barcelona, Spain) | Rosmarinic Acid (8% min.), Carnosic acid (5% min.) |
| Rosemary water soluble fraction (RWSF) (Evesa, Cádiz, Spain) | Carnosic acid (5.5% min.) |
| Rosemary oil soluble fraction (ROSF) (Evesa, Cádiz, Spain) | Carnosic acid (5.7% min) |
| Tocobiol® (BTSA, Madrid, Spain) | Tocopherols E 306 (18.5%), Sterols (<10%), Squalene (<5%), Monoglycerides (19%) |
| Paramega® * (Kemin, Des Moines, IA, USA) | Tocopherols E 306, Botanical origin oils |
| Naturox® * (Kemin, Des Moines, IA, USA) | Vegetable oil Tocopherols E 306, Lecithin, Rosemary extract E 392 |
| Oxabiol® * (BTSA, Madrid, Spain) | Tocopherols E 306 (50%), α-tocopherol (5% min.), β + γ-tocopherol (55% min.), δ-tocopherol 18% min.) |
| Nutrabiol® (BTSA, Madrid, Spain) | Tocopherols E 306 (50%) α-tocopherol (13.8%), β + γ-tocopherol (58.4% min.), δ-tocopherol (27.8% min.) |
| Parameter | Fresh by Product | Mddm |
|---|---|---|
| Moisture (%) * | 82.20 b | 23.08 a |
| aw * | 0.9985 b | 0.9135 a |
| Acidity index (g oleic acid/100 g) * | 0.092 a | 0.63 b |
| TBARS mg MDA/kg | 0.11 a | 0.19 a |
| TVB-N (mg/100 g) * | 23.51 a | 56.71 b |
| Fatty Acid | FSB | T0 | T1 | T2 |
|---|---|---|---|---|
| C14 | 2.35 a | 2.39 a | 3.59 b | 2.56 a |
| C15 * | 0.25 a | 0.24 a | 0.35 b | 0.25 a |
| C16 * | 15.37 a | 15.27 a | 21.94 b | 15.76 a |
| C17 * | 0.28 b | 0.22 a | 0.37 c | 0.27 ab |
| C18 | 3.25 a | 3.45 b | 4.77 c | 3.46 b |
| C19 | 0.07 a | 0.07 a | 0.06 a | 0.04 a |
| C20 * | 0.20 a | 0.23 b | 0.31 c | 0.23 b |
| C21* | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| C22 * | 0.03 a | 0.08 a | 0.06 a | 0.04 a |
| ∑ SFA | 21.79 a | 21.95 a | 31.45 b | 22.62 a |
| C14:1 | 0.02 a | 0.03 a | 0.00 a | 0.00 a |
| C16:1 * | 3.85 a | 3.86 a | 4.96 c | 4.10 b |
| C17:1 | 0.34 ab | 0.27 ab | 0.19 a | 0.49 b |
| tC18:1 n-9 * | 0.24 a | 0.31 a | 0.43 b | 0.32 a |
| C18:1 n-11 * | 3.06 b | 2.95 a | 3.75 c | 3.05 b |
| C18:1 n-9 * | 34.60 a | 34.67 a | 43.23 c | 35.26 b |
| tC18:1 n-7 | 0.11 ab | 0.10 a | 0.13 c | 0.11 b |
| C20:1 | 6.45 c | 6.43 c | 0.33 b | 0.25 a |
| C22:1 n-9 * | 0.33 b | 0.29 a | 0.38 c | 0.27 a |
| C24:1 | 0.07 a | 0.07 a | 0.17 a | 0.03 a |
| ∑ MUFA | 49.06 b | 48.97 b | 53.56 c | 43.88 a |
| C18:3 n-3 ALA * | 6.45 b | 6.43 b | 4.17 a | 6.32 b |
| C18:3 n-6 * | 0.23 a | 0.23 a | 0.03 a | 0.23 a |
| tC18:2 n-6 * | 0.30 b | 0.30 b | 0.16 a | 0.31 b |
| C18:2 n-6 * | 15.03 b | 15.95 c | 8.77 a | 16.04 c |
| C20:2 n-6 * | 1.18 c | 1.08 b | 0.61 a | 1.07 c |
| C20:3 n-3 | 0.29 b | 0.30 b | 0.00 a | 0.44 b |
| C20:3 n-6 * | 0.23 b | 0.22 b | 0.03 a | 0.21 b |
| C22:2 n-6 * | 0.57 b | 0.52 b | 0.13 a | 0.30 ab |
| C20:4 n-6 * | 0.44 bc | 0.46 c | 0.03 a | 0.39 b |
| C22:6 n-3 DHA * | 5.47 d | 4.93 c | 0.38 a | 3.94 b |
| C20:5 n-3 EPA * | 3.57 c | 3.59 c | 0.39 a | 3.10 b |
| C22:5 n-3 * | 1.60 d | 1.26 c | 0.28 a | 1.10 b |
| Ω3 | 17.38 d | 16.51 cd | 5.21 a | 14.91 b |
| Ω6 | 17.98 b | 18.77 c | 9.76 a | 18.57 bc |
| ∑ PUFA | 35.37 c | 35.28 c | 14.98 a | 33.48 b |
| ∑ Total UFA | 84.42 c | 84.25 c | 68.53 a | 77.35 b |
| ∑ Total UFA + SFA | 106.21 | 106.20 | 99.98 | 99.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honrado, A.; Rubio, S.; Beltrán, J.A.; Calanche, J. Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life. Foods 2022, 11, 3656. https://doi.org/10.3390/foods11223656
Honrado A, Rubio S, Beltrán JA, Calanche J. Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life. Foods. 2022; 11(22):3656. https://doi.org/10.3390/foods11223656
Chicago/Turabian StyleHonrado, Adrián, Sara Rubio, José Antonio Beltrán, and Juan Calanche. 2022. "Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life" Foods 11, no. 22: 3656. https://doi.org/10.3390/foods11223656
APA StyleHonrado, A., Rubio, S., Beltrán, J. A., & Calanche, J. (2022). Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life. Foods, 11(22), 3656. https://doi.org/10.3390/foods11223656








