Role of Stingray (Himantura signifier) Non-Protein Nitrogenous Fraction on the Oxidative Stability of Lipid and Myoglobin
Abstract
1. Introduction
2. Materials and Methods
2.1. NPN Fractionation from Stingray Muscle
2.2. Effect of Fish NPN on the Oxidative Stability of Lipid in a Lecithin Liposome Model System
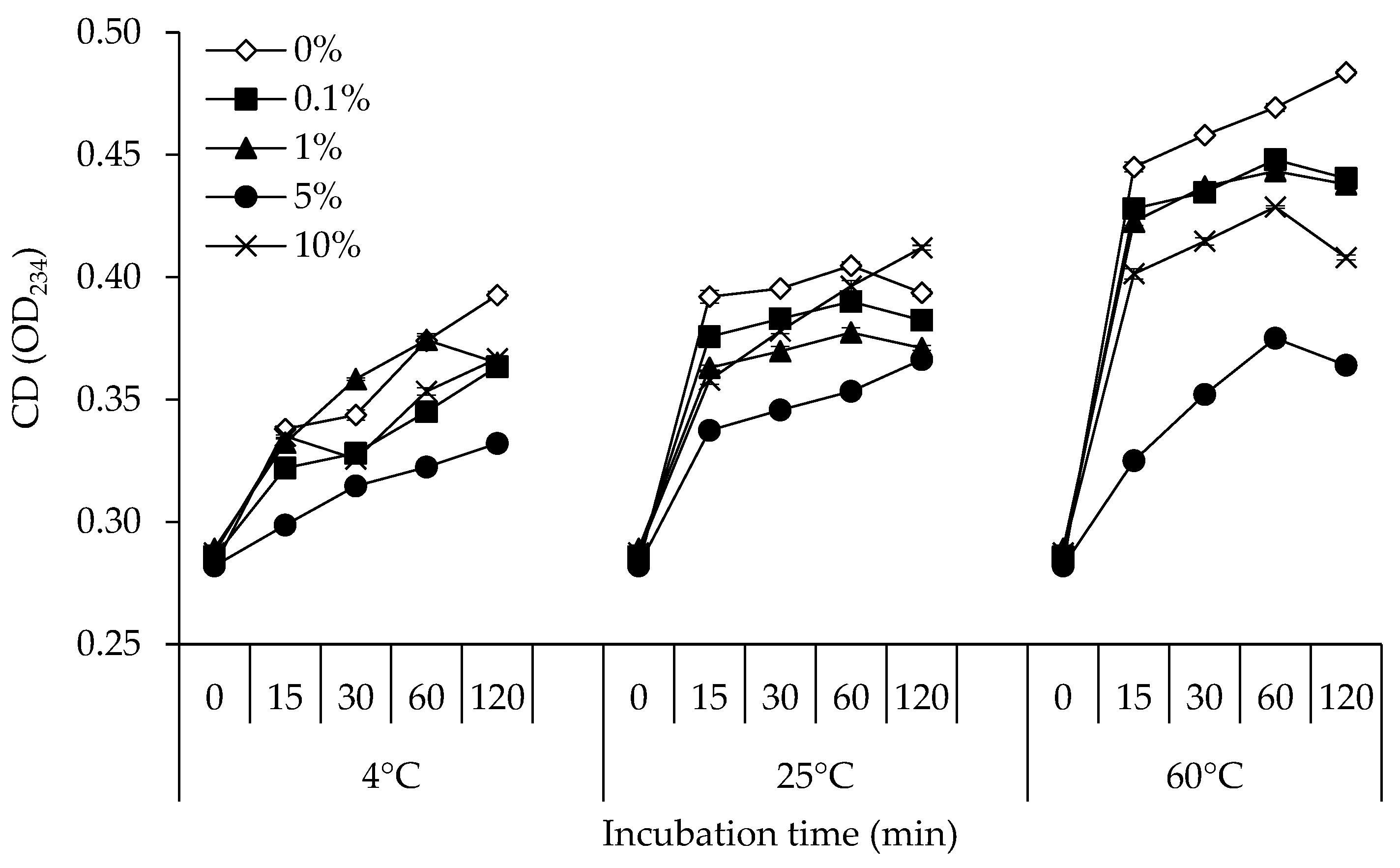
2.2.1. Determination of CD
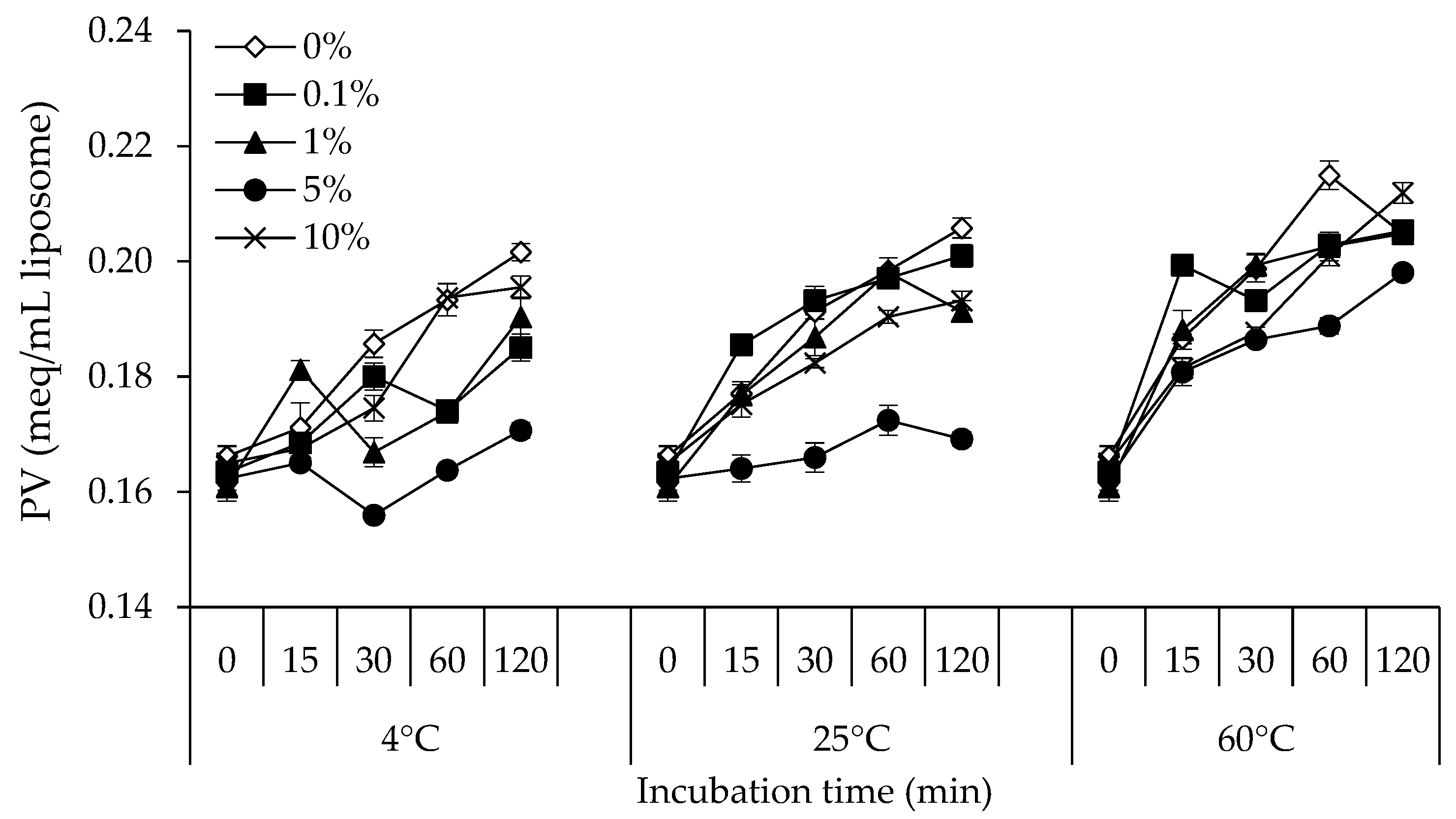
2.2.2. Determination of PV
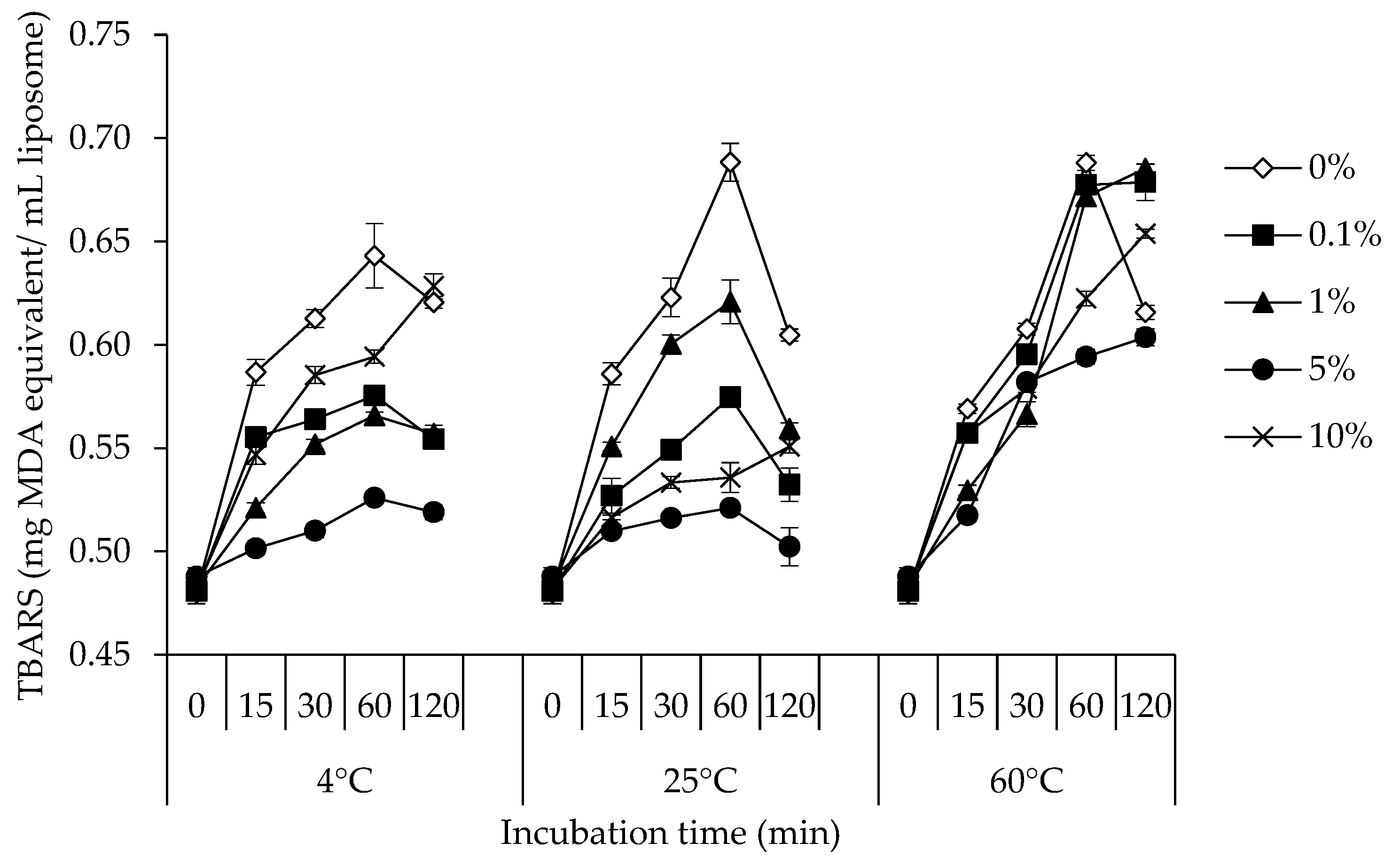
2.2.3. Determination of TBARS
2.3. Effect of Fish NPN on the Redox Stability of Myoglobin in an Oxymyoglobin Model System
2.3.1. Determination of Oxymyoglobin and Metmyoglobin
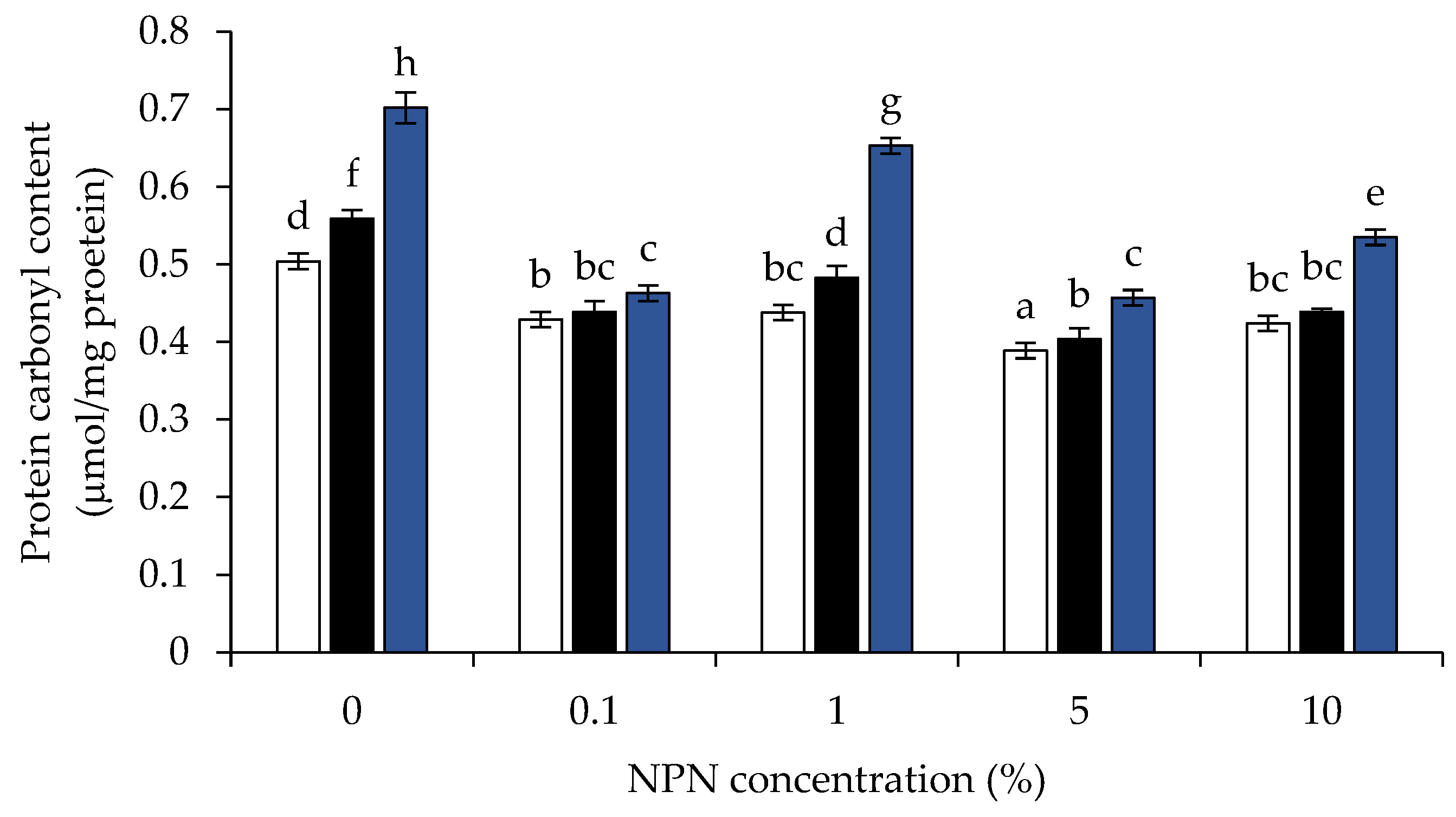
2.3.2. Determination of Protein Carbonyl
2.4. Statistical Analysis
3. Results and Discussion
3.1. CD
3.2. PV
3.3. TBARS
3.4. Myoglobin Oxidation
3.5. Protein Carbonyl Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikorski, Z.E. The contents of proteins and other nitrogenous compounds in marine animals. In Seafood Proteins, 1st ed.; Sikorski, Z.E., Pan, B.S., Shahidi, F., Eds.; Chapman & Hall: New York, NY, USA, 1994; pp. 6–12. [Google Scholar]
- Limsuwanmanee, J.; Chaijan, M.; Manurakchinakorn, S.; Panpipat, W.; Klomklao, S.; Benjakul, S. Antioxidant activity of Maillard reaction products derived from stingray (Himantura signifier) non-protein nitrogenous fraction and sugar model systems. LWT 2014, 57, 718–724. [Google Scholar] [CrossRef]
- Chaijan, M.; Klomklao, S.; Benjakul, S. Characterisation of muscles from Frigate mackerel (Auxis thazard) and catfish (Clarias macrocephalus). Food Chem. 2013, 139, 414–419. [Google Scholar] [CrossRef]
- Finne, G. Non-protein nitrogen compounds in fish and shellfish. In Advance in Seafood Biochemistry, 1st ed.; Flick, G.J., Martin, R.E., Eds.; Technomic Publishing Inc.: New York, NY, USA, 1992; pp. 393–401. [Google Scholar]
- Ikeda, S. Other organic components. In Advances in Fish Science and Technology, 1st ed.; Connell, J.J., Ed.; Fishing News Books: West Byfleet, UK, 1979; pp. 111–124. [Google Scholar]
- Hiemori-Kondo, M.; Shinya, D.; Ueta, R. Development of a quantitative method for analyzing three imidazole dipeptides using high-performance liquid chromatography and its application for meat and fish. J. Food Compost. Anal. 2022, 106, 104323. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.E.; Cavaliere, C.; Laganà, A.; Montone, C.M.; Piovesana, S.; Chiozzi, R.Z.; Capriotti, A.L. Comprehensive identification of native medium-sized and short bioactive peptides in sea bass muscle. Food Chem. 2021, 343, 128443. [Google Scholar] [CrossRef]
- Ahmed, Z.; Donkor, O.; Street, W.A.; Vasiljevic, T. Calpains-and cathepsins-induced myofibrillar changes in postmortem fish: Impact on structural softening and release of bioactive peptides. Trends Food Sci. Technol. 2015, 45, 130–146. [Google Scholar] [CrossRef]
- Chaijan, M.; Rodsamai, T.; Charoenlappanit, S.; Roytrakul, S.; Panya, A.; Phonsatta, N.; Cheong, L.Z.; Panpipat, W. Antioxidant activity and stability of endogenous peptides from farmed hybrid catfish (Clarias macrocephalus × Clarias gariepinus) muscle. Int. J. Food Sci. Technol. 2022, 57, 1083–1092. [Google Scholar] [CrossRef]
- Ishihara, K.; Watanabe, R.; Kato, T.; Seko, T.; Matsuda, T.; Omura; Y.; Shigemura, Y.; Kawabata, Y.; Maegawa, T. Isolation of balenine from opah (Lampris megalopsis) muscle and comparison of antioxidant and iron-chelating activities with other major imidazole dipeptides. Food Chem. 2021, 364, 130343. [Google Scholar] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Løvaas, E. Antioxidative effects of polyamines. J. Am. Oil Chem. Soc. 1991, 68, 353–358. [Google Scholar] [CrossRef]
- Kanner, J.; German, J.B.; Kinsella, J.E.; Hultin, H.O. Initiation of lipid peroxidation in biological systems. Crit. Rev. Food Sci. Nutr. 1987, 25, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants, 1st ed.; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 1–37. [Google Scholar]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Ohta, T.; Decker, E.A. Antioxidant activity of water-soluble fractions of salmon spermary tissue. J. Agric. Food Chem. 1996, 44, 1682–1686. [Google Scholar] [CrossRef]
- Undeland, I.; Hultin, H.O.; Richards, M.P. Aqueous extracts from some muscles inhibit hemoglobin-mediated oxidation of cod muscle membrane lipids. J. Agric. Food Chem. 2003, 51, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Sannaveerappa, T.; Carlsson, N.G.; Sandberg, A.S.; Undeland, I. Antioxidative properties of press juice from herring (Clupea harengus) against hemoglobin (Hb) mediated oxidation of washed cod mince. J. Agric. Food Chem. 2007, 55, 9581–9591. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Undeland, I. Glazing herring (Clupea harengus) fillets with herring muscle press juice: Effect on lipid oxidation development during frozen storage. Int. J. Food Sci. Technol. 2017, 52, 1229–1237. [Google Scholar] [CrossRef]
- Raizi, P.; Vareltzis, P.; Petridis, D. Processing and digestion of press juices from different fish muscles; temperature and lyophilization effects on their anti-oxidative properties. J. Aquat. Food Prod. Technol. 2019, 28, 519–530. [Google Scholar] [CrossRef]
- Hashimoto, K.; Watabe, S.; Kono, M.; Shiro, K. Muscle protein composition of sardine and mackerel. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 1435–1441. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Yi, O.S.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of grape extracts in a lecithin liposome system. J. Am. Oil Chem. Soc. 1997, 74, 1301–1307. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Prior, E.; Aeschbach, R. Evaluation of antioxidant activity of rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J. Sci. Food Agric. 1996, 72, 201–208. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1989. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Brown, W.D.; Mebine, L.B. Autoxidation of oxymyoglobins. J. Biol. Chem. 1969, 244, 6696–6701. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.J.; Sereika, H.E. Factors affecting color stability of prepackaged frozen fresh beef in display cases. J. Illum. Eng. Soc. 1969, 64, 620–624. [Google Scholar]
- Liu, G.; Xiong, Y.L.; Butterfield, D.A. Chemical, physical, and gel-forming properties of oxidized myofibrils and whey-and soy-protein isolates. J. Food Sci. 2000, 65, 811–818. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem. 2006, 99, 83–91. [Google Scholar] [CrossRef]
- Nissen, L.R.; Byrne, D.V.; Bertelsen, G.; Skibsted, L.H. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004, 68, 485–495. [Google Scholar] [CrossRef]
- Wu, H.; Richards, M.P.; Undeland, I. Lipid oxidation and antioxidant delivery systems in muscle food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1275–1299. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Zhang, J.; Yu, X.; Wang, J.; Huang, F. Effects of temperature and NaCl percentage on lipid oxidation in pork muscle and exploration of the controlling method using response surface methodology (RSM). Food Chem. 2012, 131, 817–825. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of storage conditions on the quality of arbequina extra virgin olive oil and the impact on the composition of flavor-related compounds (phenols and volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Aidos, I.; van der Padt, A.; Luten, J.B.; Boom, R.M. Seasonal changes in crude and lipid composition of herring fillets, byproducts, and respective produced oils. J. Agric. Food Chem. 2002, 50, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Ferreira, M.J.P.; dos Santos, D.Y. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Shahidi, F.; Amarowicz, R. Antioxidant activity of protein hydrolyzates from aquatic species. J. Am. Oil Chem. Soc. 1996, 73, 1197–1199. [Google Scholar] [CrossRef]
- Ahn, D.U.; Lee, E.J. Production of off-odor volatiles from liposome-containing amino acid homopolymers by irradiation. J. Food Sci. 2002, 67, 2659–2665. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Characterisation of myoglobin from sardine (Sardinella gibbosa) dark muscle. Food Chem. 2007, 100, 156–164. [Google Scholar] [CrossRef]
- Wongwichian, C.; Klomklao, S.; Panpipat, W.; Benjakul, S.; Chaijan, M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015, 174, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Promeyrat, A.; Sayd, T.; Laville, E.; Chambon, C.; Lebret, B.; Gatellier, P. Early post-mortem sarcoplasmic proteome of porcine muscle related to protein oxidation. Food Chem. 2011, 127, 1097–1104. [Google Scholar] [CrossRef]
- Estévez, M.; Heinonen, M. Effect of phenolic compounds on the formation of α-aminoadipic and γ-glutamic semialdehydes from myofibrillar proteins oxidized by copper, iron, and myoglobin. J. Agric. Food Chem. 2010, 58, 4448–4455. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Xiong, Y.L.; Alderton, A.L.; Ooizumi, T. Biochemical changes in myofibrillar protein isolates exposed to three oxidizing systems. J. Agric. Food Chem. 2006, 54, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Kejriwal, A. Non-heme iron coordination complexes for alkane oxidation using hydrogen peroxide (H2O2) as powerful oxidant. J. Coord. Chem. 2022, 75, 937–971. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panpipat, W.; Limsuwanmanee, J.; Cheong, L.-Z.; Chaijan, M. Role of Stingray (Himantura signifier) Non-Protein Nitrogenous Fraction on the Oxidative Stability of Lipid and Myoglobin. Foods 2023, 12, 274. https://doi.org/10.3390/foods12020274
Panpipat W, Limsuwanmanee J, Cheong L-Z, Chaijan M. Role of Stingray (Himantura signifier) Non-Protein Nitrogenous Fraction on the Oxidative Stability of Lipid and Myoglobin. Foods. 2023; 12(2):274. https://doi.org/10.3390/foods12020274
Chicago/Turabian StylePanpipat, Worawan, Jutaporn Limsuwanmanee, Ling-Zhi Cheong, and Manat Chaijan. 2023. "Role of Stingray (Himantura signifier) Non-Protein Nitrogenous Fraction on the Oxidative Stability of Lipid and Myoglobin" Foods 12, no. 2: 274. https://doi.org/10.3390/foods12020274
APA StylePanpipat, W., Limsuwanmanee, J., Cheong, L.-Z., & Chaijan, M. (2023). Role of Stingray (Himantura signifier) Non-Protein Nitrogenous Fraction on the Oxidative Stability of Lipid and Myoglobin. Foods, 12(2), 274. https://doi.org/10.3390/foods12020274







