FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chicken Samples and Lipid Fraction Separation
2.2. Materials and Reagents
2.3. Gas Chromatography–Mass Spectrometry (GC–MS)
2.4. Preparation of the β-Cyclodextrin/Lipid Complexes
2.5. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.6. Thermogravimetry–Differential Thermogravimetry (TG–DTG)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Principal Component Analysis (PCA)
3. Results and Discussion
3.1. Analysis of the Raw and Processed Chicken Lipids
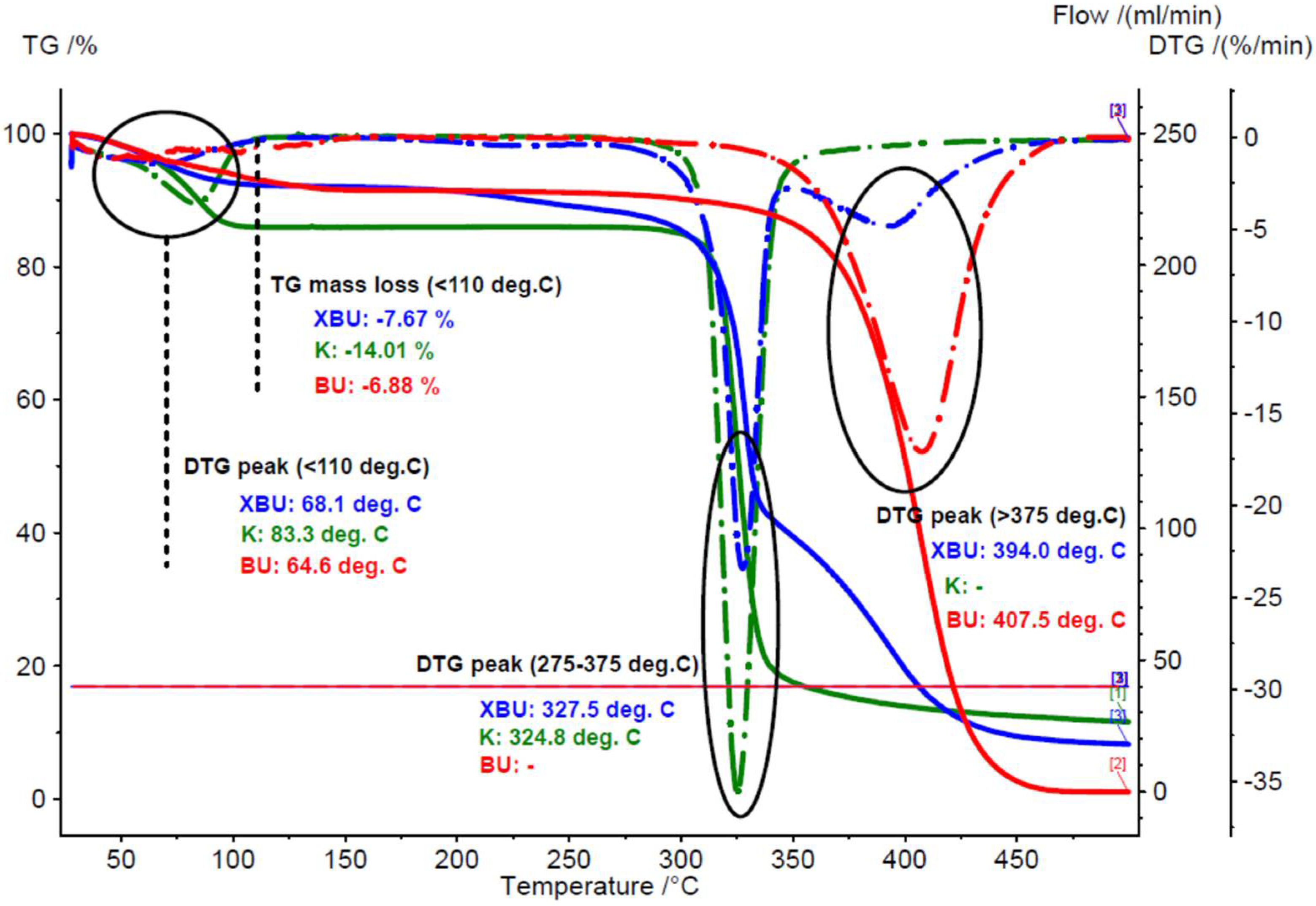
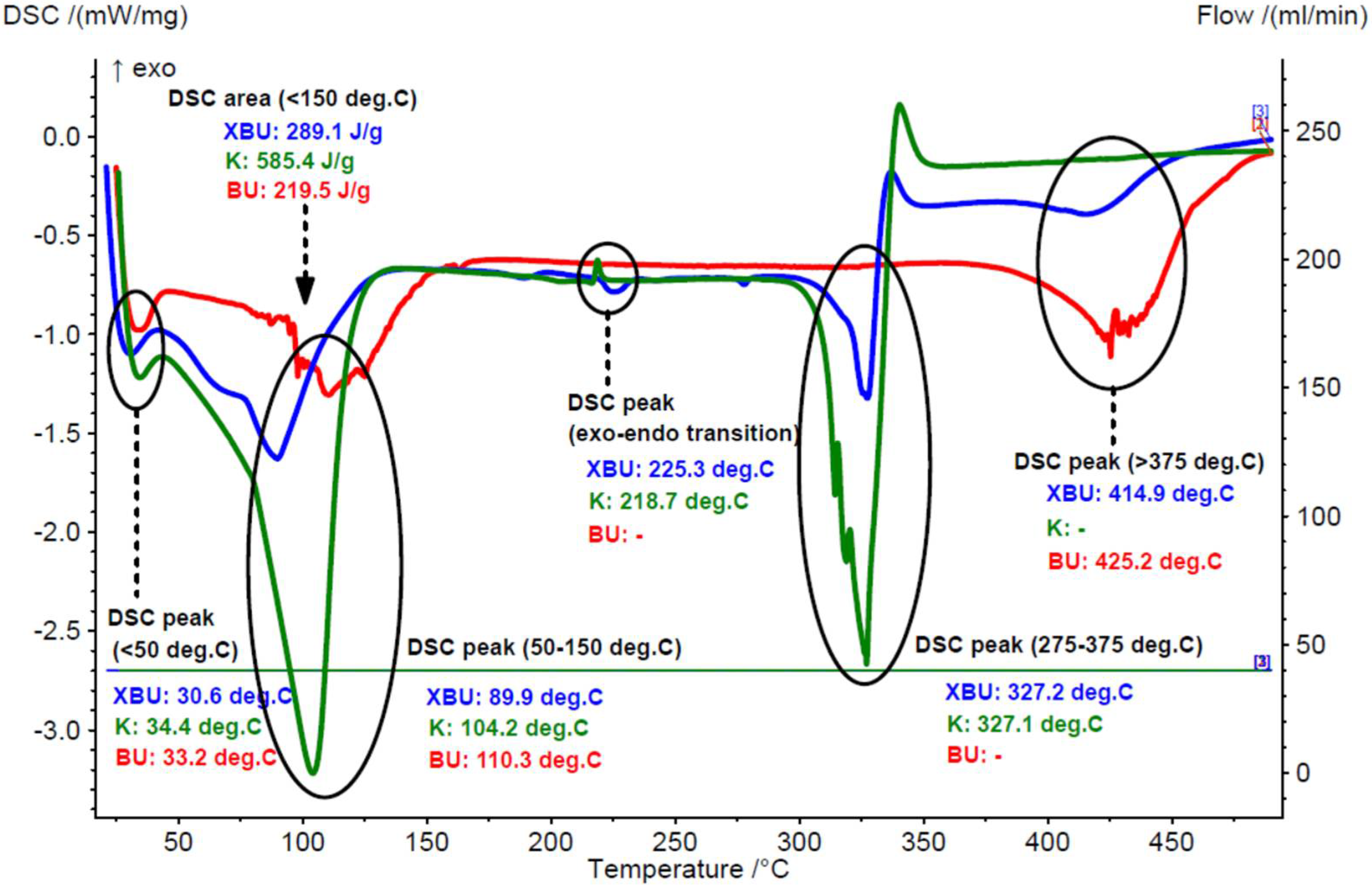
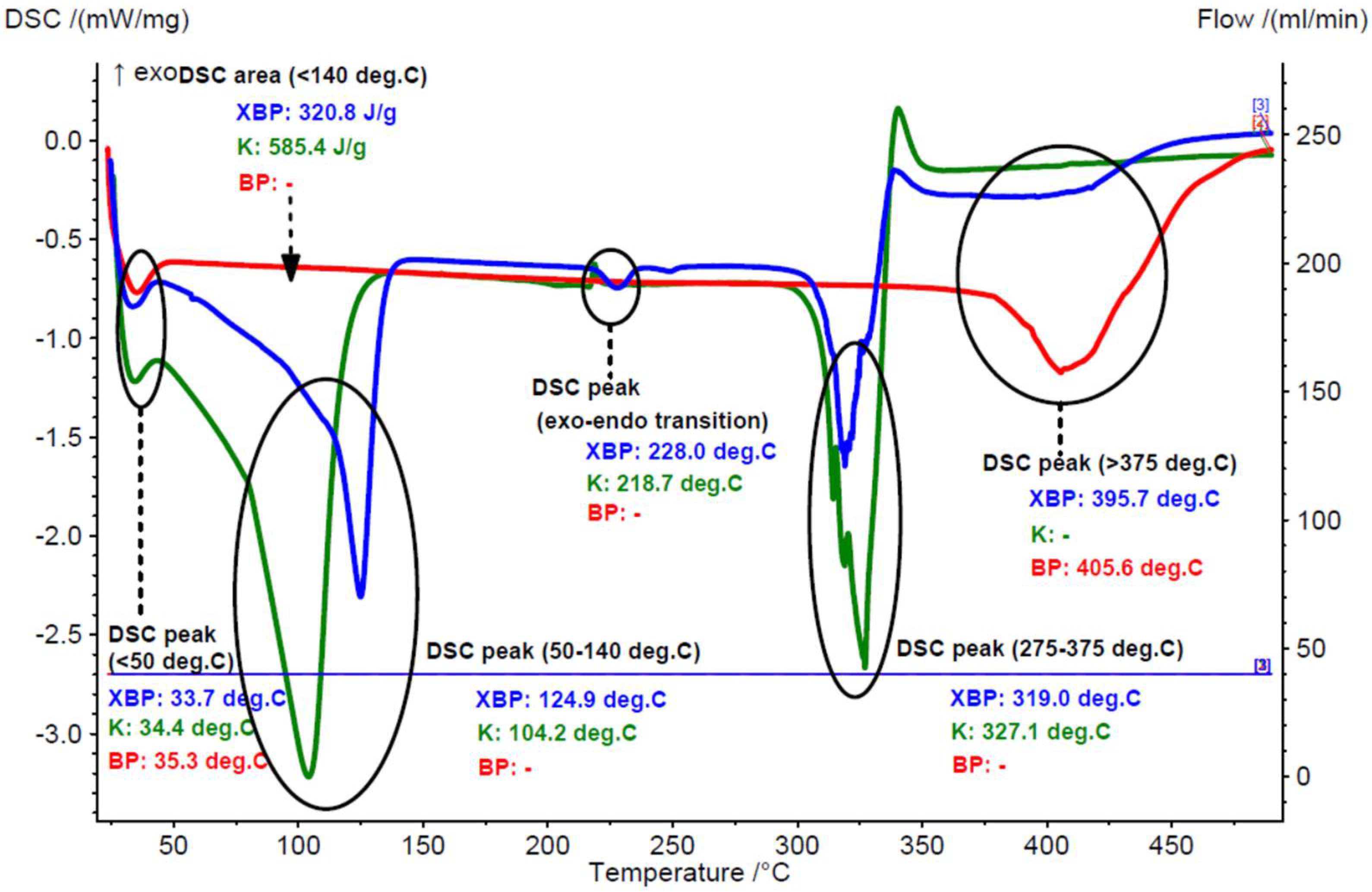
3.1.1. Thermal Analyses of the Chicken Lipids
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the Chicken Lipids
3.2. Preparation and Analysis of the β-Cyclodextrin/Chicken Lipid Complexes
3.2.1. Preparation of the β-Cyclodextrin/Chicken Lipid Complexes by Kneading
3.2.2. Thermal Analyses of the β-Cyclodextrin/Chicken Lipid Complexes
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the β-Cyclodextrin/Chicken Lipid Complexes
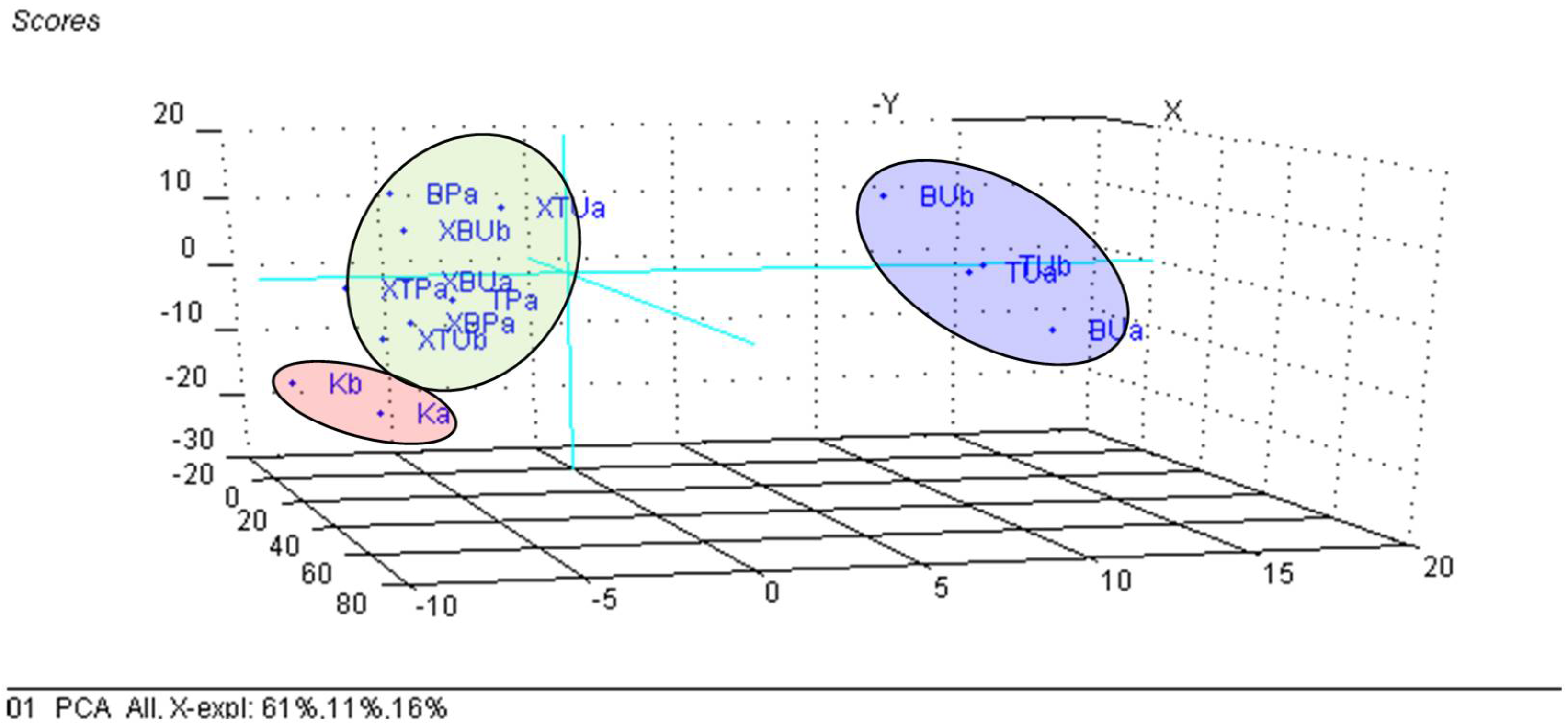
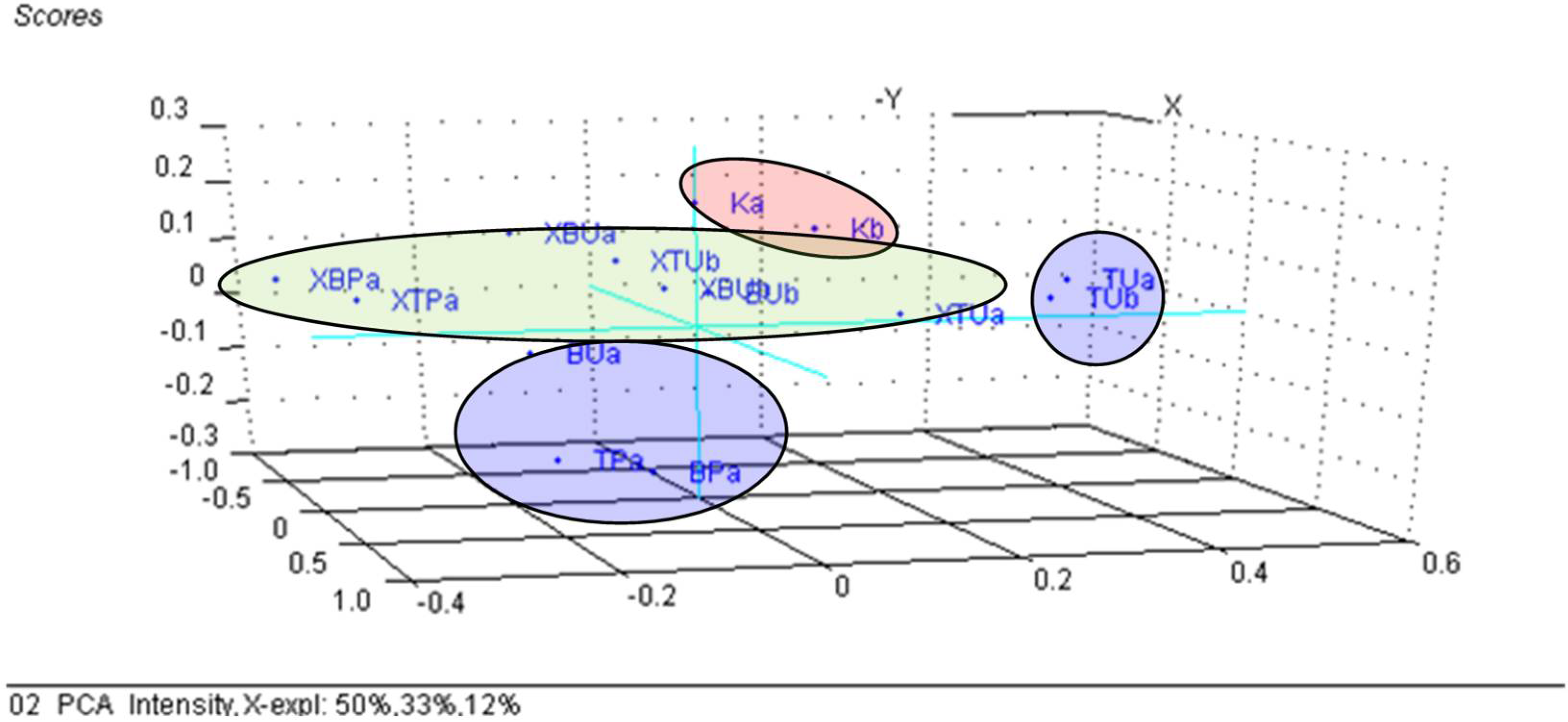
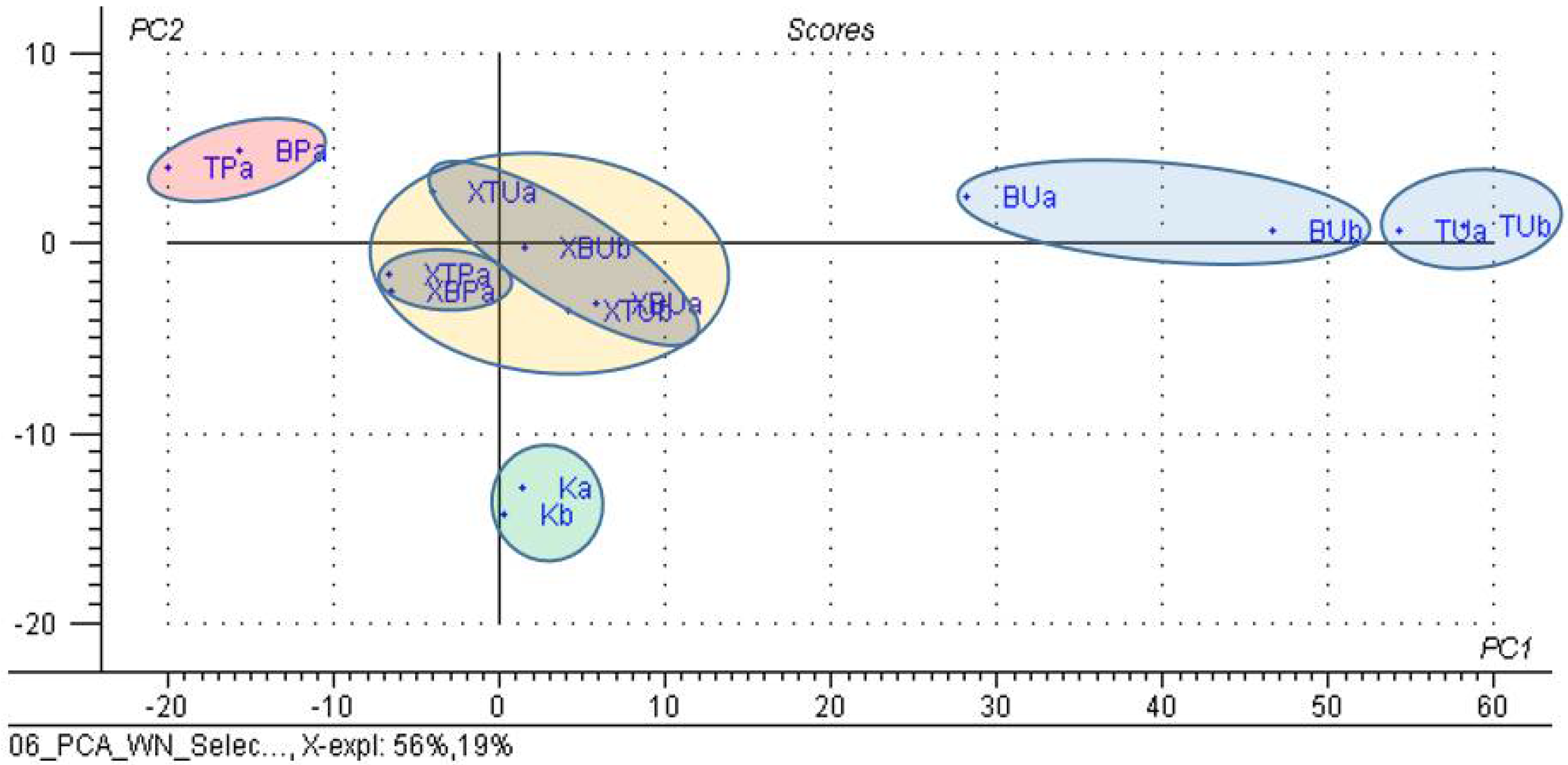
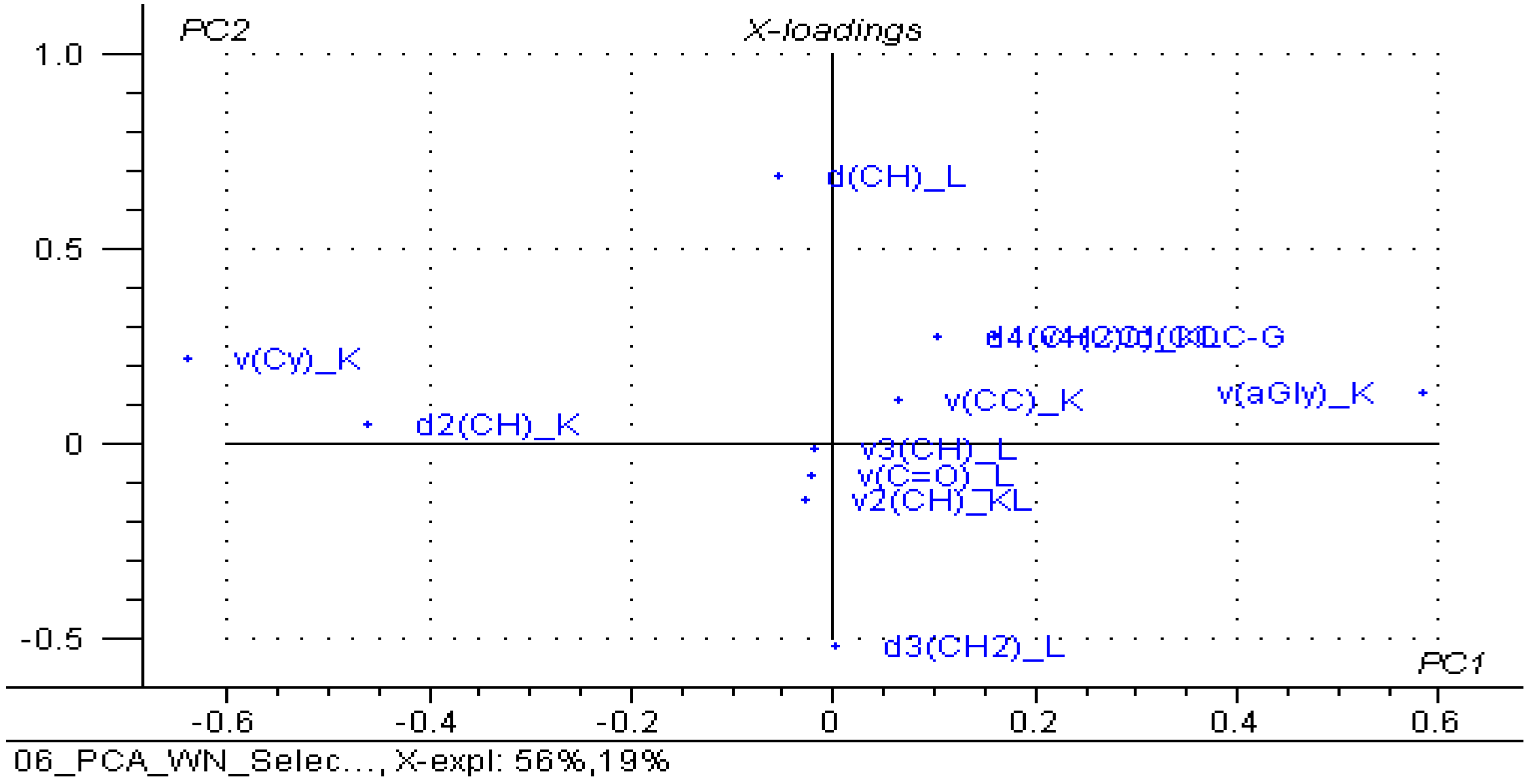
3.3. Principal Component Analysis (PCA) on the β-Cyclodextrin/Chicken Lipid Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EU Market Situation for Poultry; Committee for the Common Organisation of the Agricultural Markets: Bruxelles, Belgium, 2019; p. 19.
- FAOSTAT. Crops and Livestock Products; Food and Agriculture Organization: Rome, Italy, 2022; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 31 August 2022).
- Handbook of Poultry Science and Technology. Primary Processing; Guerrero-Legarreta, I., Hui, Y.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 788. [Google Scholar]
- Bracke, M.; de-Jong, I.; Gerritzen, M.; Jacobs, L.; Nalon, E.; Nicol, C.; O’Connell, N.; Porta, F. The Welfare of Broiler Chickens in the EU; Eurogroup for Animals: Brussels, Belgium, 2020; p. 92. Available online: https://www.eurogroupforanimals.org/ (accessed on 31 August 2022).
- Council Directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production, Amended in 2017 and 2019. Off. J. Eur. Union EUR-Lex 2007, L182, 19–28. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02007L0043-20191214 (accessed on 31 August 2022).
- Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91, Amended in 2008 and 2013, Repealed in 2018. Off. J. Eur. Union EUR-Lex 2007, L189, 1–39. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2007.189.01.0001.01.ENG&toc=OJ%3AL%3A2007%3A189%3ATOC (accessed on 31 August 2022).
- Commission Regulation (EC) No 543/2008 of 16 June 2008 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 as regards the marketing standards for poultry meat, Amended in 2008, 2009, 2010, 2011, 2012 and 2013. Off. J. Eur. Union EUR-Lex 2008, L157, 46–87. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2008.157.01.0046.01.ENG&toc=OJ%3AL%3A2008%3A157%3ATOC (accessed on 31 August 2022).
- Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control, Amended in 2008–2021. Off. J. Eur. Union EUR-Lex 2008, L250, 1–84. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R0889-20210101 (accessed on 31 August 2022).
- Ravindran, V. Main ingredients used in poultry feed formulations. In Poultry Development Review; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; pp. 67–69. [Google Scholar]
- Abbasi, F.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.; Shams-Shargh, M. Production of omega-3 fatty acid-enriched broiler chicken meat by the application of nanoencapsultsed flaxseed oil prepared via ultrasonication. J. Funct. Foods 2019, 57, 373–381. [Google Scholar] [CrossRef]
- Kartikasari, L.R.; Hughes, R.J.; Geier, M.S.; Makrides, M.; Gibson, R.A. Dietary alpha-linolenic acid enhances omega-3 long chain polyunsaturated fatty acid levels in chicken tissues. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Buta, A.; Oprea, O.; Sevciuc, P.; Daradics, Z.; Spătariu, S.; Ognean, L. Analysis of the influence of a nutraceutical supplement with probiotic effects on health index and productive performance in broiler chicken. AgroLife Sci. J. 2020, 9, 54–63. [Google Scholar]
- Turcu, R.P.; Panaite, T.D.; Șoica, C.; Olteanu, M.; Vlaicu, P.A. Effect of using flax meal in broiler diets on meat fatty acids content. AgroLife Sci. J. 2020, 9, 347–352. [Google Scholar]
- Soriano-Santos, J. Chemical composition and nutritional content of raw poultry meat. In Handbook of Poultry Science and Technology. Primary Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 467–489. [Google Scholar]
- Chmiel, M.; Słowiński, M.; Dasiewicz, K. Application of computer vision systems for estimation of fat content in poultry meat. Food Control 2011, 22, 1424–1427. [Google Scholar] [CrossRef]
- De-Marchi, M.; Riovanto, R.; Penasa, M.; Cassandro, M. At-line prediction of fatty acid profile in chicken breast using near infrared reflectance spectroscopy. Meat Sci. 2012, 90, 653–657. [Google Scholar] [CrossRef]
- Riovanto, R.; De-Marchi, M.; Cassandro, M.; Penasa, M. Use of near infrared transmittance spectroscopy to predict fatty acid composition of chicken meat. Food Chem. 2012, 134, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Feddern, V.; Kupski, L.; Cipolatti, E.P.; Giacobbo, G.; Mendes, G.L.; Badiale-Furlong, E.; de-Souza-Soares, L.A. Physico-chemical composition, fractionated glycerides and fatty acid profile of chicken skin fat. Eur. J. Lipid Sci. Technol. 2010, 112, 1277–1284. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Rymer, C.; Givens, D.I. Fatty acid composition of cooked chicken meat and chicken meat products as influenced by price range at retail. Food Chem. 2013, 138, 1749–1756. [Google Scholar] [CrossRef]
- Dalziel, C.J.; Kliem, K.E.; Givens, D.I. Fat and fatty acid composition of cooked meat from UK retail chickens labelled as from organic and non-organic production systems. Food Chem. 2015, 179, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Zhao, S.; House, J.D. Performance and tissue fatty acid profile of broiler chickens and laying hens fed hemp oil and HempOmegaTM. Poult. Sci. 2017, 96, 1809–1819. [Google Scholar] [CrossRef]
- Puvača, N.; Lukač, D.; Ljubojević, D.; Stanaćev, V.; Beuković, M.; Kostadinović, L.; Plavša, N. Fatty acid composition and regression prediction of fatty acid concentration in edible chicken tissues. World’s Poult. Sci. J. 2014, 70, 585–592. [Google Scholar] [CrossRef]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of dietary incorporation of n-3 polyunsaturated fatty acids rich oil sources on fatty acid profile, keeping quality and sensory attributes of broiler chicken meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef]
- Chirilă, C.A.; Szakal, R.N.; Hădărugă, N.G. Classical versus modern analysis of poultry lipids—A review. J. Agroaliment. Processes Technol. 2020, 26, 60–73. [Google Scholar]
- Joseph, P. Oxidative Stability and Shelf Life of Bulk Animal Fats and Poultry Fats. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 233–249. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Holser, R.A. Oxidative Stability of Fatty Acids; Cherian, G., Poureslami, R., Eds.; Context Products Ltd.: Nottingham, UK, 2012; pp. 85–98. [Google Scholar]
- O’Sullivan, M.G. The Stability and Shelf Life of Meat and Poultry. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 297, pp. 521–543. [Google Scholar]
- Pawar, D.P.; Boomathi, S.; Hathwar, S.C.; Rai, A.K.; Modi, V.K. Effect of conventional and pressure frying on lipids and fatty acid composition of fried chicken and oil. J. Food Sci. Technol. 2013, 50, 381–386. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Yang, Y.; Nie, S.; Yang, X.; Wang, Y.; Yang, M.; Li, C.; Xie, M. The analysis of trans fatty acid profiles in deep frying palm oil and chicken fillets with an improved gas chromatography method. Food Control 2014, 44, 191–197. [Google Scholar] [CrossRef]
- Dillon, G.P.; Yiannikouris, A.; Brandl, W.; Cardinall, C.; Yuan, W.; Moran, C.A. Fitness for purpose and stability assessment of long-chain polyunsaturated fatty acids in chicken tissues. Regul. Toxicol. Pharmacol. 2019, 103, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Roszko, M.; Adamczak, L.; Florowski, T.; Pietrzak, D. Influence of storage and packaging method on chicken breast meat chemical composition and fat oxidation. Poult. Sci. 2019, 98, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.; Guardiola, F.; Grimpa, S.; Barroeta, A.C.; Codony, R. Oxidative Stability of Dark Chicken Meat through Frozen Storage: Influence of Dietary Fat and α-Tocopherol and Ascorbic Acid Supplementation. Poult. Sci. 2001, 80, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Grimpa, S.; Guardiola, F.; Barroeta, A.C.; Codony, R. Effects of Various Fat Sources, α-Tocopheryl Acetate, and Ascorbic Acid Supplements on Fatty Acid Composition and α-Tocopherol Content in Raw and Vacuum-Packed, Cooked Dark Chicken Meat. Poult. Sci. 2006, 85, 1472–1481. [Google Scholar] [CrossRef]
- Barido, F.H.; Jang, A.; Pak, J.I.; Kim, Y.J.; Lee, S.K. Combined effects of processing method and black garlic extract on quality characteristics, antioxidative, and fatty acid profile of chicken breast. Poult. Sci. 2022, 101, 101723. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kaczmarek, A. Chemometric Analysis of Fatty Acid Composition of Raw Chicken, Beef, and Pork Meat with Plant Extract Addition during Refrigerated Storage. Molecules 2021, 26, 4952. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Păunescu, V.; Tatu, C.; Ordodi, V.L.; Bandur, G.; Lupea, A.X. Bioactive Nanoparticles (6). Thermal Stability of Linoleic Acid/α and β Cyclodextrin Complexes. Food Chem. 2006, 99, 500–508. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, E. Cyclodextrin-Lipid Complexes: Cavity Size Matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Medeleanu, M.A.; Hădărugă, D.I.; Muntean, C.V.; Popescu, G.; Rada, M.; Hegheş, A.; Zippenfening, S.E.; Lucan-(Banciu), C.A.; Velciov, A.B.; Bandur, G.N.; et al. Structure-property relationships on recrystallized β-cyclodextrin solvates: A focus on X-ray diffractometry, FTIR and thermal analyses. Carbohydr. Polym. 2021, 265, 118079. [Google Scholar] [CrossRef] [PubMed]
- Paramita, V.; Novia, S.F.; Ariyanto, H.D.; Pramudono, B.; Yoshii, H.; Kusumayanti, H.; Amalia, R. The influence of α-cyclodextrin on the stability and fatty acids of medium-chain triglycerides high oil load nanoemulsion. Mater. Today: Proc. 2022, 63, S312–S317. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Birău (Mitroi), C.L.; Gruia, A.T.; Păunescu, V.; Bandur, G.N.; Hădărugă, N.G. Moisture evaluation of β-cyclodextrin/fish oils complexes by thermal analyses: A data review on common barbel (Barbus barbus L.), Pontic shad (Alosa immaculata Bennett), European wels catfish (Silurus glanis L.), and common bleak (Alburnus alburnus L.) living in Danube river. Food Chem. 2017, 236, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Szakal, R.N.; Chirilă, C.A.; Lukinich-Gruia, A.T.; Păunescu, V.; Muntean, C.; Rusu, G.; Bujancă, G.; Hădărugă, D.I. Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2020, 303, 125419. [Google Scholar] [CrossRef] [PubMed]
- Ünlüsayin, M.; Hădărugă, N.G.; Rusu, G.; Gruia, A.T.; Păunescu, V.; Hădărugă, D.I. Nano-encapsulation competitiveness of omega-3 fatty acids and correlations of thermal analysis and Karl Fischer water titration for European anchovy (Engraulis encrasicolus L.) oil/beta-cyclodextrin complexes. LWT-Food Sci. Technol. 2016, 68, 135–144. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Ünlüsayin, M.; Gruia, A.T.; Birău-(Mitroi), C.; Rusu, G.; Hădărugă, N.G. Thermal and oxidative stability of Atlantic salmon oil (Salmo salar L.) and complexation with β-cyclodextrin. Beilstein J. Org. Chem. 2016, 12, 179–191. [Google Scholar] [CrossRef]
- Choi, M.-J.; Ruktanonchai, U.; Min, S.-G.; Chun, J.-Y.; Soottitantawat, A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion-diffusion method. Food Chem. 2010, 119, 1694–1703. [Google Scholar] [CrossRef]
- Choi, M.-J.; Ruktanonchai, U.; Soottitantawat, A.; Min, S.-G. Morphological characterization of encapsulated fish oil with β-cyclodextrin and polycaprolactone. Food Res. Int. 2009, 42, 989–997. [Google Scholar] [CrossRef]
- Lee, C.-M.; Kim, M.-H.; Na, H.-S.; Kim, J.; Lee, K.-Y. The Effect of Caseinate on Inclusion Complexes of γ-Cyclodextrin for Oxidative Stabilization of Fish Oil. Biotechnol. Bioprocess Eng. 2013, 18, 507–513. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Wang, F.; Regenstein, J.M.; Liu, R. Optimization of Microencapsulation of Fish Oil with Gum Arabic/Casein/Beta-Cyclodextrin Mixtures by Spray Drying. J. Food Sci. 2015, 80, C1445–C1452. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-S.; Kim, J.-N.; Kim, J.-M.; Lee, K.-Y. Encapsulation of Fish Oil Using Cyclodextrin and Whey Protein Concentrate. Biotechnol. Bioprocess Eng. 2011, 16, 1077–1082. [Google Scholar] [CrossRef]
- David, I.; Orboi, M.D.; Simandi, M.D.; Chirilă, C.A.; Megyesi, C.I.; Rădulescu, L.; Lukinich-Gruia, A.T.; Muntean, C.; Hădărugă, D.I.; Hădărugă, N.G. Fatty acid profile of Romanian’s common bean (Phaseolus vulgaris L.) lipid fractions and their complexation ability by β-cyclodextrin. PLoS ONE 2019, 14, e0225474. [Google Scholar] [CrossRef] [PubMed]
- Hamoudi, M.C.; Bourasset, F.; Domergue-Dupont, V.; Gueutin, C.; Nicolas, V.; Fattal, E.; Bochot, A. Formulations based on alpha cyclodextrin and soybean oil: An approach to modulate the oral release of lipophilic drugs. J. Control. Release 2012, 161, 861–867. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Yoshikiyo, K.; Yoshioka, Y.; Oe, Y.N.S.; Kawahara, H.; Kurata, K.; Shimizu, H.; Yamamoto, T. Thermal stability and bioavailability of inclusion complexes of perilla oil with γ-cyclodextrin. Food Chem. 2019, 294, 56–59. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Gazza, L.; Taddei, F.; Nocente, F.; De-Benedetto, G.E.; De-Caroli, M.; Piro, G.; Mita, G. Bioactive composition and sensory evaluation of innovative spaghetti supplemented with free or α-cyclodextrin chlatrated pumpkin oil extracted by supercritical CO2. Food Chem. 2019, 294, 112–122. [Google Scholar] [CrossRef]
- Corpaş, L.; Hădărugă, N.G.; David, I.; Pîrşan, P.; Hădărugă, D.I.; Isengard, H.-D. Karl Fischer Water Titration-Principal Component Analysis Approach on Wheat Flour. Food Anal. Methods 2014, 7, 1353–1358. [Google Scholar] [CrossRef]
- Iordănescu, O.A.; Băla, M.; Iuga, A.C.; Gligor-(Pane), D.; Dascălu, I.; Bujancă, G.S.; David, I.; Hădărugă, N.G.; Hădărugă, D.I. Antioxidant Activity and Discrimination of Organic Apples (Malus domestica Borkh.) Cultivated in the Western Region of Romania: A DPPH Kinetics-PCA Approach. Plants 2021, 10, 1957. [Google Scholar] [CrossRef]
- Petroman, C.; Popescu, G.; Szakal, R.-N.; Păunescu, V.; Drăghia, L.P.; Bujancă, G.S.; Chirilă, C.A.; Hădărugă, D.I.; Văduva, L.; Hădărugă, N.G.; et al. Fatty Acid Profile of Lipid Fractions of Mangalitza (Sus scrofa domesticus) from Northern Romania: A GC-MS-PCA Approach. Foods 2021, 10, 242. [Google Scholar] [CrossRef]
- Popescu, G.; Radulov, I.; Iordănescu, O.A.; Orboi, M.D.; Radulescu, L.; Drugă, M.; Bujancă, G.S.; David, I.; Hădărugă, D.I.; Lucan-(Banciu), C.A.; et al. Karl Fischer Water Titration-Principal Component Analysis Approach on Bread Products. Appl. Sci. 2020, 10, 6518. [Google Scholar] [CrossRef]
- Akin, G.; Elmas, Ş.N.K.; Arslan, F.N.; Yılmaz, İ.; Kenar, A. Chemometric classification and quantification of cold pressed grape seed oil in blends with refined soybean oils using attenuated total reflectance-mid infrared (ATR–MIR) spectroscopy. LWT-Food Sci. Technol. 2019, 100, 126–137. [Google Scholar] [CrossRef]
- Herman-Lara, E.; Tejeda-Paz, M.; Martínez-Sánchez, C.E.; Rodríguez-Miranda, J.; Ramírez-Rivera, E.J.; Hernández-Santos, B.; Juárez-Barrientos, J.M. Differential scanning calorimetry coupled with chemometric tools for determining adulteration with vegetable fat in fresh cheeses. LWT-Food Sci. Technol. 2017, 85, 269–274. [Google Scholar] [CrossRef]
- Karacaglar, N.N.Y.; Bulat, T.; Boyaci, I.H.; Topcu, A. Raman spectroscopy coupled with chemometric methods for the discrimination of foreign fats and oils in cream and yogurt. J. Food Drug Anal. 2019, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Poddighe, M.; Garroni, S.; Malfatti, L. Application of IR and UV–VIS spectroscopies and multivariate analysis for the classification of waste vegetable oils. Resour. Conserv. Recycl. 2022, 178, 106088. [Google Scholar] [CrossRef]
- Nunes, C.A. Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Res. Int. 2014, 60, 255–261. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Pravdova, V.; Boucon, C.; de-Jong, S.; Walczak, B.; Massart, D.L. Three-way principal component analysis applied to food analysis: An example. Anal. Chim. Acta 2002, 462, 133–148. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Gálvez, L.; Quezada, C.; Navarro, R. Principal Component Analysis as an exploration tool for kinetic modeling of food quality: A case study of a dried apple cluster snack. J. Food Eng. 2013, 119, 229–235. [Google Scholar] [CrossRef]
- Gandhi, K.; Sharma, R.; Seth, R.; Mann, B. Detection of coconut oil in ghee using ATR-FTIR and chemometrics. Appl. Food Res. 2022, 2, 100035. [Google Scholar] [CrossRef]
- Jin, H.; Li, H.; Yin, Z.; Zhu, Y.; Lu, A.; Zhao, D.; Li, C. Application of Raman spectroscopy in the rapid detection of waste cooking oil. Food Chem. 2021, 362, 130191. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Wei, W.; Wang, X. Characterisation of bovine and buffalo anhydrous milk fat fractions along with infant formulas fat: Application of differential scanning calorimetry, Fourier transform infrared spectroscopy, and colour attributes. LWT-Food Sci. Technol. 2020, 129, 109542. [Google Scholar] [CrossRef]
- Ejeahalaka, K.K.; On, S.L.W. Chemometric studies of the effects of milk fat replacement with different proportions of vegetable oils in the formulation of fat-filled milk powders: Implications for quality assurance. Food Chem. 2019, 295, 198–205. [Google Scholar] [CrossRef]
- Berhe, D.T.; Eskildsen, C.E.; Lametsch, R.; Hviid, M.S.; van-den-Berg, F.; Engelsen, S.B. Prediction of total fatty acid parameters and individual fatty acids in pork backfat using Raman spectroscopy and chemometrics: Understanding the cage of covariance between highly correlated fat parameters. Meat Sci. 2016, 111, 18–26. [Google Scholar] [CrossRef]
- Chirilă, C.A.; Guran, A.; Mitroi, C.L.; Hădărugă, D.I.; Hădărugă, N.G. Evaluation of the similarity/dissimilarity of poultry lipid profiles by Fourier transform infrared spectroscopy. In Proceedings of the 2nd International Conference on Life Sciences, Timişoara, Romania, 23–24 May 2019; Filodiritto Publisher: Bologna, Italy; pp. 194–207. [Google Scholar]
- Chirilă, C.A.; Szakal, R.N.; Hădărugă, D.I.; Hădărugă, N.G. Influence of growing and processing factors on the fatty acid profile of poultry lipids. J. Agroaliment. Processes Technol. 2020, 26, 74–81. [Google Scholar]
- Jahan, K.; Paterson, A. Lipid composition of retailed organic, free-range and conventional chicken breasts. Int. J. Food Sci. Technol. 2007, 42, 251–262. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ran, J.; Yang, C.; Lin, Z.; Liu, Y. LC/MS-based lipidomics to characterize breed-specific and tissue-specific lipid composition of chicken meat and abdominal fat. LWT-Food Sci. Technol. 2022, 163, 113611. [Google Scholar] [CrossRef]
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Sikorska, E. Near and mid infrared spectroscopy and multivariate data analysis in studies of oxidation of edible oils. Food Chem. 2015, 187, 416–423. [Google Scholar] [CrossRef]
- Ondo, D. Thermodynamic study on complexation of long-chain fatty acid anions with α-cyclodextrin in water. J. Mol. Liq. 2020, 311, 113172. [Google Scholar] [CrossRef]

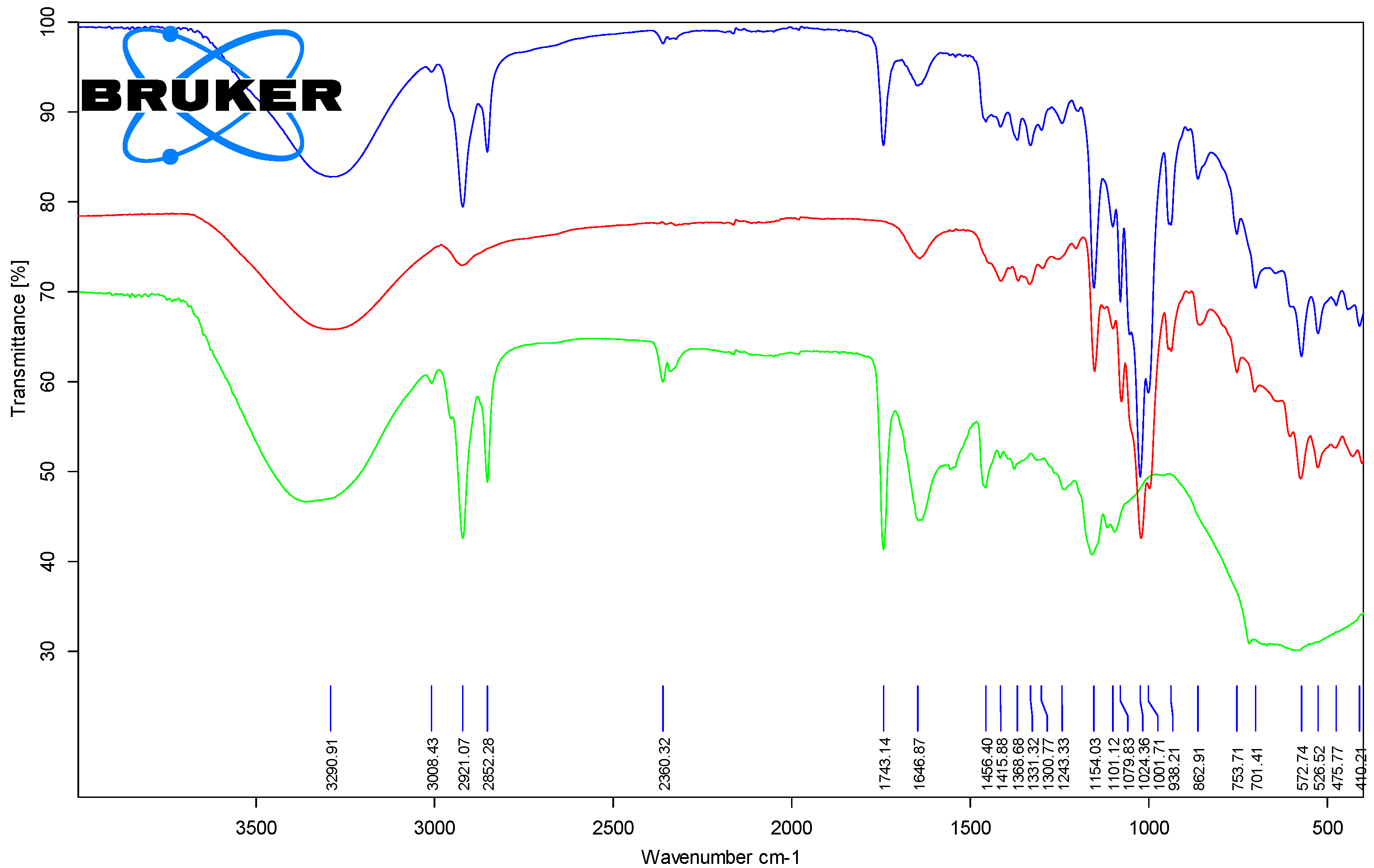

| Band Assignments for the Lipid Fractions (cm−1) | Band Assignments for the β-CD/Lipid Complexes (cm−1) | Description |
|---|---|---|
| 3287.4–3357.8 | 3290.4–3309.2 | νO-H, stretching vibration |
| 3007.9–3009.1 | 3007.8–3011.0 | νs=CH, symmetric stretching vibration |
| 2952.8–2957.4 | 2951.8–2954.0 | νasC-H, asymmetric stretching vibration |
| 2917.2–2921.9 | 2921.1–2922.7 | νasC-H, asymmetric stretching vibration |
| 2850.4–2852.7 | 2852.3–2853.5 | νasCH, asymmetric stretching vibration |
| 1741.2–1743.5 | 1743.1–1744.4 | νC=O, stretching vibration of the ester groups from triglycerides |
| 1634.6–1652.1 | 1644.4–1650.1 | νC=C, stretching vibration of the cis R1HC=CHR2 groups |
| 1455.7–1463.0 | 1455.9–1460.7 | δCH, bending vibration in the CH2 and CH3 groups |
| 1412.4–1417.52 | 1415.9–1418.0 | δrk=CH, bending vibration (rocking) of the cis R1HC=CHR2 groups |
| 1376.5–1379.4 | 1366.1–1370.6 | δCH2, bending vibration |
| 1237.4–1241.6 | 1239.0–1243.3 | δCH2, bending vibration |
| 1154.4–1162.9 | 1152.6–1154.4 | δCH2, bending vibration |
| 1079.9–1098.7 | 1078.0–1080.2 | νC-O, stretching vibration |
| 1052.7–1054.6 | 1051.4–1054.0 | νC-O, stretching vibration |
| 1022.3–1034.6 | 1023.0–1025.1 | νC-O, stretching vibration |
| 946.0–946.7 | 938.2–946.1 | δC=C, bending vibration of the trans R3HC=CHR4 groups |
| Band Assignments for the β-CD (cm−1) | Band Assignments for the β-CD/Lipid Complexes (cm−1) | Description |
|---|---|---|
| 3292.8–3294.3 | 3290.4–3309.2 | νO-H, stretching vibration |
| 2923.6–2924.5 | 2921.1–2922.7 | νasC-H, asymmetric stretching vibration |
| 1455.7–1463.0 | 1455.9–1460.7 | δCH, bending vibration in the CH2 and CH3 groups |
| 1415.1–1416.3 | 1415.9–1418.0 | δOH, in-plan bending vibration |
| 1365.7–1365.9 | 1366.1–1370.6 | δCH2, bending vibration |
| 1334.1–1334.6 | 1331.3–1340.7 | δCH, bending vibration |
| 1152.2–1152.3 | 1152.6–1154.4 | δC-O-C, stretching vibration for the glucosidic bonds |
| 1076.9–1077.0 | 1078.0–1080.2 | νC-O, stretching vibration/νC-C, stretching vibration |
| 1021.4–1021.8 | 1023.0–1025.1 | νC-O, stretching vibration |
| 937.8–937.8 | 938.2–946.1 | νring, stretching vibration of the cyclodextrin ring |
| 857.2–858.6 | 854.8–863.0 | να(1→4), stretching vibration of the α(1→4) glucosidic bond |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hădărugă, N.G.; Chirilă, C.A.; Szakal, R.N.; Gălan, I.M.; Simandi, M.D.; Bujancă, G.S.; David, I.; Riviş, A.; Stanciu, S.M.; Hădărugă, D.I. FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin. Foods 2022, 11, 3632. https://doi.org/10.3390/foods11223632
Hădărugă NG, Chirilă CA, Szakal RN, Gălan IM, Simandi MD, Bujancă GS, David I, Riviş A, Stanciu SM, Hădărugă DI. FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin. Foods. 2022; 11(22):3632. https://doi.org/10.3390/foods11223632
Chicago/Turabian StyleHădărugă, Nicoleta Gabriela, Cosmina Andrea Chirilă, Raymond Nandy Szakal, Iulia Maria Gălan, Marius Daniel Simandi, Gabriel Stelian Bujancă, Ioan David, Adrian Riviş, Sorin Mihai Stanciu, and Daniel Ioan Hădărugă. 2022. "FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin" Foods 11, no. 22: 3632. https://doi.org/10.3390/foods11223632
APA StyleHădărugă, N. G., Chirilă, C. A., Szakal, R. N., Gălan, I. M., Simandi, M. D., Bujancă, G. S., David, I., Riviş, A., Stanciu, S. M., & Hădărugă, D. I. (2022). FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin. Foods, 11(22), 3632. https://doi.org/10.3390/foods11223632






